Abstract
Self-assembled capsules are nanoscale structures made up of multiple synthetic subunits held together by weak intermolecular forces. They act as host structures that can completely surround small molecule guests of the appropriate size, shape and chemical surface. Like their biological counterparts, multimeric enzymes and receptors, the subunits of the capsules are generally identical, and lead to homomeric assemblies of high symmetry. In both biological and synthetic systems small variations in structures are tolerated and lead to heteromeric assemblies with slightly different recognition properties. The synthetic capsules are dynamic, with lifetimes from milliseconds to hours, and allow the direct spectroscopic observation of smaller molecules inside, under ambient conditions at equilibrium in solution. We report here the assembly of hybrid capsules made up of 2 very different structures, both capable of forming their own homomeric capsules through hydrogen bonding. These hybrids exhibit host properties that differ markedly from the parent capsules, and suggest that other capsules may emerge from seemingly unrelated modules that have curved surfaces and are rich in hydrogen bonding capabilities.
Reversible encapsulation complexes are supramolecular structures in which guest molecules are completely surrounded by a self-assembled host structure (1). The complexes provide access to isolated species that cannot be seen in bulk solution (2); they act as nanometric reaction chambers (3), as means to stabilize reagents (4,5), and as spaces where new forms of stereochemistry can emerge (6). The forces holding the assembly together can be hydrophobic effects (7), salt bridges (8), metal/ligand interactions (9,10), and hydrogen bonds. The capsules form when, and only when, suitable guests are present to fill the space inside. For example, 6 molecules of resorcinarene 1 (Fig. 1) and 8 molecules of water form a hexameric capsule 16 in the solid state through a seam of 60 hydrogen bonds (11). The capsule also self-assembles in solution with wet solvents such as chloroform and benzene inside (12,13) or in the presence of large quaternary ammonium guests (14,15). The resorcinarene can be elaborated to the cavitand 2, which dimerizes in chloroform to capsule 22 through a seam of 8 bifurcated hydrogen bonds. When dissolved in chloroform, the capsule forms around 3 solvent molecules. Alternatively, a hybrid structure 1.2 forms immediately on mixing the 2 homomeric capsules 16 and 22 and all 3 capsule assemblies coexist in the same solution (16). The exchange of partners was surprising, because “self-assembly” implies a distinction between self and nonself, with some selection, correction and sorting at work (17,18), but these capsules are related because they share resorcinarene modules that fix their dimensions and symmetries. Here, we report a new example of hybrid formation from 2 unrelated capsules.
Fig. 1.
Line drawings of the subunits and energy minimized structures: The hexameric capsule 16; cylindrical capsule 22; hybrid capsule 1.2; tennis ball 32; hybrid capsule 2.3. Peripheral alkyl and aryl groups (R = R′ = C11H23, Ar = pC6H4-N(Bu)2) in the calculated structures have been removed.
Results
The capsule 32 is a notional “tennis ball” (19) that self-assembles through hydrogen bonding around small molecules such as methane. At first glance, the formation of a hybrid between 22 and 32 appears unlikely, given the different sizes, hydrogen bonding patterns, and symmetries of the modules, but modeling gives cause for optimism (Fig. 1). Slight flexing of 3 at its 7-membered rings and 2 at its 9-membered rings brings about a match of most of the hydrogen bond donors and acceptors; only 4 imide oxygens are left without hydrogen bond partners. In the experiment, a new assembly emerges immediately and irreversibly upon the addition of 32 to 22 in CDCl3. The resulting NMR signals indicated complete disappearance of 22 (Fig. 2f); the homomeric capsules do not coexist in chloroform. The hybrid capsule 2.3 also forms in tetrachloroethane and p-xylene (see SI).
Fig. 2.
Partial 1H NMR spectra of capsular assemblies. (a) Resorcinarene 1 (2 mM) in chloroform; the formation of hexamer 16 occurs with 6–8 encapsulated solvent molecules. (b) Two micromolar 2 in chloroform yields the cylindrical capsule 22 with 3 surrounded solvent molecules. (c) Mixing 2 (2 mM) with 1 (2.5 mM) results in the formation of hybrid capsule 1.2; the original homomeric capsules are also present. (d) Tennis ball 32 (2 mM) in chloroform containing dissolved atmospheric gases. (e) Mixing of 22 (2 mM) and 32 (2 mM) gives the new hybrid capsule 2.3. Complete NMR peaks assignment of 2.3 are given in the SI.
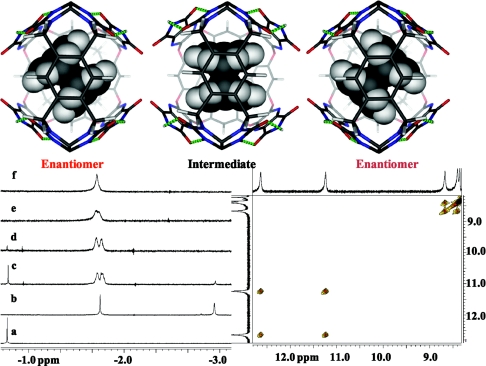
We examined a variety of guests expected to be of appropriate size and shape for 2.3. Ethane is well-accommodated inside 32 (Fig. 3a), and is coencapsulated with 2,2-paracyclophane in 22 (Fig. 3b). The 2,2-paracyclophane alone is not bound in 22–a single molecule fills too little of the space whereas 2 molecules fill too much–but the combination of these 2 guests in their encapsulation complexes in the solvent mesitylene-d12 gives only the hybrid capsule 2.3 with the 2,2-paracyclophane inside (Fig. 3 c and d). The snug fit of this guest in 2.3 is also shown; if the guest is not allowed to rotate freely in the cavitand, the symmetry planes are lost. The NMR spectrum shows this to be the case in the hybrid capsule. Two enantiomeric complexes are present as shown and, with gentle heating, they interconvert (ΔG‡ = 15.3 kcal/mole at coalescence T = 312 K;) through the rotation of the guest along its long axis. The imide hydrogens show 2 signals in the NMR spectrum with a Δδ >1 ppm; the 2D ROESY spectroscopy showed exchange cross-peaks between them at room temperature and provided ΔG‡ = 16.2 kcal/mol calculated for the guest rotation. Diffusion ordered spectroscopy (DOSY) (20) was performed on the sample of 2,2-paracyclophane in 2.3. Diffusion coefficients confirmed that all 3 components diffuse as a single species and therefore exist in the same complex (see SI). The packing coefficient of 2,2-paracyclophane in 1.2 is unusually high (70%), and resembles the value for closely packed spheres in the solid state (74%). Along with data from other hybrid capsules and coencapsulation (21) experiments with 2,2-paracyclophane in 22, the value for solid guests in reversible encapsulation complexes appears to be ≈70%. The corresponding value for gases in such capsules is ≈40% (22).
Fig. 3.
Rotation of p-cyclophane in the hybrid capsule. (Upper) A view into the complex of 2.3 with p-cyclophane; rotation of the guest gives an intermediate with planes of symmetry and interconverts the magnetic environments of the cavitand's walls. (Lower) (Left) Upfield regions of the 1H NMR spectra (600 MHz, mesitylene-d12) of ethane in 32 (4 mM) (a), coencapsulation of ethane and 2,2-paracyclophane in 22 (4 mM) (b), immediately after mixing a and b (c), c after 15 min d, d at 310 K (e), d at 320 K (f). The signals at −1.57 ≈ −1.62 ppm are those of the methylenes of 2,2-paracyclophane located at the tapered end of 2.3. (Right) 2D ROESY spectrum of encapsulated 2,2-paracyclophane in 2.3; mixing time 300 ms. Cross-peaks indicate the exchange of environments among the imide and ureido NH signals, as 2,2-paracyclophane slowly rotates inside the capsule at 300 K.
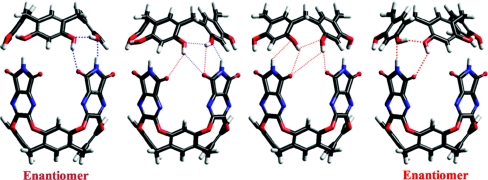
Bulky guests such as 2,2-paracyclophane are also taken up by the other hybrid 1.2. Surprisingly, the number and location of the upfield NMR signals revealed rapid rotation of this guest within that capsule at ambient temperature. It appears that hybrid 1.2 features a wider space inside, forced apart by the diverging OH groups of the resorcinarene “lid.” Hybrid capsule 1.2 is also chiral, because the head-to-tail arrangement of these OH groups can be either clockwise or counterclockwise. The 2 enantiomers are shown in Fig. 4, but they interconvert rapidly even at low temperatures. A mechanism that makes new hydrogen bonds as old ones are broken is proposed and features a slight rotation of the lid. The alternate process involving dissociation and reassembly to interconvert the enantiomers would be energetically much more costly.
Fig. 4.
Racemization of hybrid capsules 1.2. A slight rotation of the resorcinarene and rearrangement of the hydrogen bond seam causes interconversion of enantiomeric capsules.
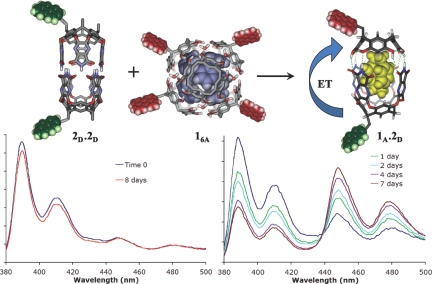
The dynamics of hybrid assembly are not accessible by NMR studies; they assemble instantly and completely at millimolar concentrations. They can be studied in real time at the micro- to nanomolar concentration ranges using fluorescence resonance energy transfer (FRET) (23). We recently reported the synthesis of labeled versions of resorcinarenes and cavitand monomers (24,25). Perylene labeled resorcinarene 1A (acceptor fluorophore) was added to pyrene labeled cavitand 2D (donor fluorophore) in mesitylene with both components at 250 nM. No FRET signal was observed, indicating that the hybrid capsule 1.2 had not formed (Fig. 5). This was expected as there was no suitable guest present to template the formation of any capsule. When 2,2-paracyclophane was added as the guest, a large FRET signal developed after a few days (Fig. 5). The large increase in the acceptor fluorescence was accompanied by a commensurate decrease in the donor fluorescence as 1.2 assembles.
Fig. 5.
(Upper) Representation of the donor dye-labeled cylindrical capsule 2D2 and the acceptor dye-labeled hexamer 1A6 and the hybrid capsule 1A.2D. FRET occurs only upon formation of the hybrid assembly. (Lower Left) Fluorescence spectrum of a mixture of pyrene labeled monomer 2D and perylene labeled resorcinarene 1A initially, and after 8 days showing no FRET increase. (Lower Right) Development of FRET with time upon addition of 2,2-paracyclophane as the nucleating guest.
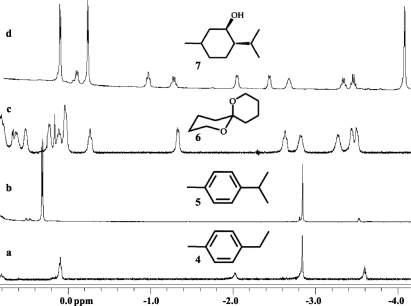
The aromatic rings of the hosts provide strong magnetic shielding for encapsulated guest molecules and the guest proton resonances can be shifted upfield as much as 5 ppm (26). We examined guest species that were not well accomodated by either of 22 or 33 but were of appropriate size and shape for the hybrid assemblies. All of the guests reported to fit in the hybrid 1.2 fit in the new hybrid assembly as well, but 2.3 accepted a broader range of guests. Two different complexes–carceroisomers (27)–are formed with unsymmetrical guests like p-ethyl toluene 4 (Fig. 6). This guest is too long to “tumble” within the hybrid and shows 2 orientations. The favored isomer (67%) positions the tolyl methyl deep in the tapered end for strong C-H/π interactions (28,29). With p-cymene 5 the single methyl in the tapered end of the cavitand was even more favorable (92%), because the isopropyl group does not fit into this space comfortably. Chiral guests such 1,7-dioxaspiro[5.5]undecane 6 or menthol 7 are encapsulated in 2.3, and they rotate freely inside because no diastereomeric complexes appear in their NMR spectra (see SI).
Fig. 6.
Upfield regions of 1H NMR spectra show signals for encapsulation of p-ethyl toulene (a), p-cymene (b), racemic 1,7-dioxaspiro[5.5]undecane (c), (±) menthol in the hybrid capsule 3.2 (mesitylene-d12, T = 300 K, 600 MHz) (d).
Midsize alkanes such as 2-methyl heptane 8 or 2,2-dimethyl hexane 9 are also taken up in 2.3; in both cases the carceroisomer with ω-methyl group at the tapered end is slightly favored (57% and 61%, respectively). These alkanes appear too long to tumble freely in the hybrid, but 2D ROESY spectroscopy shows otherwise (Fig. 7). The cross-peaks show the carceroisomers interconvert on the NMR timescale at room temperature. Two different motions are possible: In one of them, 3 completely dissociates from 2 to make more room for the tumbling of the alkane. Evidence to support this mechanism is from 2D spectra that shows exchange of 2.3 with the excess of 32 in the solution. The other possible mechanism involves “breathing” along the seam of hydrogen bonds to make enough space for guest rotation. The ΔG‡'s for the tumbling motions of 8 and 9 in 2.3 were calculated as 16.6 kcal/mol and 16.3 kcal/mol, respectively.
Fig. 7.
Upfield regions of 2D ROESY spectra (mesitylene-d12, T = 300 K, 600 MHz). Cross-peaks show exchange between 2 carceroisomers (shown in a different color) of 8 in 3.2 (Upper) and 9 (Lower).
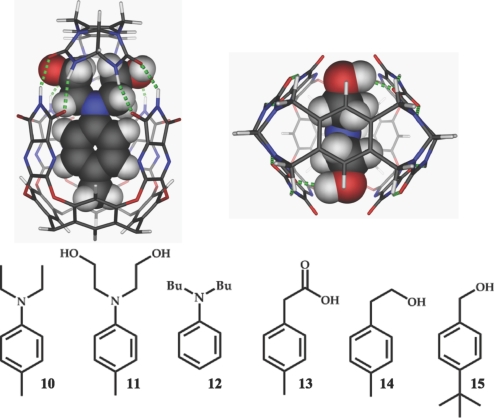
The openings and seams of hydrogen bonds at the “upper” part of 2.3 favor bulky, polar guest features, a preference evident in the spectra with 10-15. The complex with 11 is modeled in Fig. 8, where the hydroxyl groups of the guest have a number of hydrogen-bonding opportunities. This structure resembles the encapsulation complexes of Raymond (30), that feature “dangling arms.”
Fig. 8.
(Upper) Semiemperical energy-minimized complex of 11 in 2.3 (Peripheral alkyl and aryl groups in the modeled structures have been removed). (Lower) Line drawings of the some suitable guest for hybrid capsule 2.3.
Discussion
The homomeric capsules have either strong hydrogen bond donors (imide N-H's in 22) or strong hydrogen bond acceptors (ureido carbonyls in 33), and these complement each other optimally in the hybrid 2.3. A downfield shift of 2 ppm is observed for the appropriate N-H signal in the NMR spectrum, indicating stronger hydrogen bonding in the hybrid capsule. The entropy changes of the hybridization process is hard to estimate in CDCl3 as the occupancy of 32 is unknown. There are 3 solvent molecules in 22 and 2 in the hybrid capsule 2.3. Once formed, the hybrid 2.3 thwarts attempts at self-sorting, unlike hybrid 1.2. For example, addition of p,p′-dimethyl stilbene, one of the best occupants of 22 has no effect on the 2,2-paracyclophane complex with 2.3. In short, the hybrid capsule 1.2, assembles even in direct competition with its respective homomeric capsules. Heteromeric species that assemble in preference to homomeric ones are known, especially when the modules have close structural relationships (31,32). In contrast, many proteins (33) and the much smaller resorcin[4]arenes and pyrogallol[4]arenes show different behavior. The latter differ marginally yet strict self-sorting of their hexameric capsules occurs in solution (34). In the gas phase the whole range of heteromeric species is observed (35). Attempts to form the remaining possible hybrid combination (1.3) showed no evidence of hybridization between the components; instead, self-sorting to 16 and 32 was observed. However, cavitand 2 exhibits a remarkable affinity for glycolurils beyond the tennis ball (36,37). Perhaps the rich array of hydrogen bond donors and acceptors of glycolurils (38) is responsible for their promiscuity with other modules of the proper scale and curvature. Whatever the reason, the hybrid capsule 2.3 is an exception to self-sorting phenomena and expands the repertoire of new encapsulated species.
Materials and Methods
All reagents were obtained from commercial suppliers and used without further purification. NMR spectra were recorded on a Bruker DRX-600 spectrometer. The NMR samples were prepared as follows. A 2 mM concentration of 1 and/or 2 and/or 3 and 1–20 mM concentrations of the appropriate guest were mixed with 0.6 mL of mesitylene-d12 in a NMR tube. The tube was placed in an ultrasonic bath (230 W) and sonicated for 5–10 min. 1H NMR and 2D spectra were recorded on a Bruker DRX-600 spectrometer with a 5-mm QNP probe. Proton (1H) chemical shifts are reported in parts per million (δ) with respect to tetramethylsilane (TMS, δ = 0) and referenced internally with respect to the protio solvent impurity. The ROESY spectra were recorded at 300 K at 600 MHz with the phase-sensitive ROESY pulse sequence supplied with the Bruker software. Each of the 512 F1 increments was the accumulation of 40 scans with a 300-ms mixing time.
Fluorescence measurements were obtained using a Fluorolog-3 Model FL3–21 spectrofluometer. Mesitylene was distilled before use to remove fluorescent impurities. All measurements were conducted at 25.0 ± 0.1 °C. Solutions of each capsule 16 (D) and 22 (A) were prepared at 7.7 × 10−6 M and allowed to equilibrate for at least 48 h. Equimolar amounts of each solution were then mixed, and an aliquot of the mixture was diluted into 3 mL of mesitylene so that the concentration of each capsule was 250 nM. The fluorescence spectrum was then recorded with an excitation wavelength of 350 nm.
Supplementary Material
Acknowledgments.
This work was supported by The Skaggs Institute. J-L.H. and E.S.B. are Skaggs Postdoctoral Fellows and T.J.D is a Skaggs Predoctoral Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809903106/DCSupplemental.
References
- 1.Conn MM, Rebek J., Jr Self-assembling capsules. Chem Reviews. 1997;97:1647–1668. doi: 10.1021/cr9603800. [DOI] [PubMed] [Google Scholar]
- 2.Kusukawa T, Fujita M. Ship-in-a-bottle“ formation of stable hydrophobic dimers of cis-azobenzene and -stilbene derivatives in a self-assembled coordination nanocage. J Am Chem Soc. 1999;121:1397–1398. [Google Scholar]
- 3.Fiedler D, Bergman RG, Raymond KN. Supramolecular catalysis of a unimolecular transformation: Aza-cope rearrangement within a self-assembled host. Angew Chem Int Ed. 2004;43:6748–6751. doi: 10.1002/anie.200461776. [DOI] [PubMed] [Google Scholar]
- 4.Yoshizawa M, Kusukawa T, Fujita M, Yamaguchi K. Cavity-directed synthesis of otherwise labile silanol oligomers within self-assembled coordination cages. J Am Chem Soc. 201;123:10454–10459. doi: 10.1021/ja010875t. [DOI] [PubMed] [Google Scholar]
- 5.Dong VM, Fiedler D, Carl B, Bergman RG, Raymond KN. Molecular recognition and stabilization of iminium ions in water. J Am Chem Soc. 2006;128:14464–14465. doi: 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivanyuk A, Rebek J., Jr Social isomers in encapsulation complexes. J Am Chem Soc. 2002;124:12074–12075. doi: 10.1021/ja020607a. [DOI] [PubMed] [Google Scholar]
- 7.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. A hydrophobic nanocapsule controls the photophysics of aromatic molecules by suppressing their favored solution pathways. J Am Chem Soc. 2005;127:3674–3675. doi: 10.1021/ja0425381. [DOI] [PubMed] [Google Scholar]
- 8.Corbellini F, Di Constanzo L, Crego-Calama M, Geremia S, Reinhoudt DR. Guest encapsulation in a water-soluble molecular capsule based on ionic interactions. J Am Chem Soc. 2003;125:9946–9947. doi: 10.1021/ja034535e. [DOI] [PubMed] [Google Scholar]
- 9.Fujita M, et al. Molecular paneling via coordination. Chem Comm. 2001:509–518. [Google Scholar]
- 10.Fiedler D, Leung DH, Bergman RG, Raymond KN. Selective molecular recognition, C—H bond activation, and catalysis in nanoscale reaction vessels. Acc Chem Res. 2005;38:349–358. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]
- 11.MacGillivray LR, Atwood JL. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature. 1997;389:469–472. [Google Scholar]
- 12.Avram L, Cohen Y. Spontaneous formation of hexameric resorcinarene capsule in chloroform solution as detected by diffusion NMR. J Am Chem Soc. 2002;124:15148–15149. doi: 10.1021/ja0272686. [DOI] [PubMed] [Google Scholar]
- 13.Shivanyuk A, Rebek J., Jr Assembly of resorcinarene capsules in wet solvents. J Am Chem Soc. 2003;125:3432–3433. doi: 10.1021/ja027982n. [DOI] [PubMed] [Google Scholar]
- 14.Shivanyuk A, Rebek J., Jr Recognition of guests in solution by self-assembling resorcinarene subunits. Proc Natl Acad Sci USA. 2001;98:7662–7665. doi: 10.1073/pnas.141226898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivanyuk A, Rebek J., Jr Reversible encapsulation of multiple, neutral guests in hexameric resorcinarene hosts. Chem Comm. 2001:2424–2425. doi: 10.1039/b109464p. [DOI] [PubMed] [Google Scholar]
- 16.Ajami D, Schramm MP, Volonterio A, Rebek J., Jr Assembly of hybrid synthetic capsules. Angew Chem Int Ed Engl. 2006;46:242–244. doi: 10.1002/anie.200603421. [DOI] [PubMed] [Google Scholar]
- 17.Wu AX, Isaacs L. Self-sorting: The exception or the rule? J Am Chem Soc. 2003;125:4831–4835. doi: 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]
- 18.Valdés C, Spitz UP, Toledo L, Kubik S, Rebek J., Jr Synthesis and self-assembly of pseudo-spherical homo-and heterodimeric capsules. J Am Chem Soc. 1995;117:12733–12745. [Google Scholar]
- 19.Wyler R, de Mendoza J, Rebek J., Jr A synthetic cavity assembles through self-complementary hydrogen bonds. Angew Chem Int Ed Eng. 1993;32:1699–1701. [Google Scholar]
- 20.Avram L, Cohen Y. Diffusion measurements for molecular capsules: Pulse sequences effect on water signal decay. J Am Chem Soc. 2005;127:5714–5719. doi: 10.1021/ja043985j. [DOI] [PubMed] [Google Scholar]
- 21.Scarso A, Onagi H, Rebek J., Jr Mechanically regulated rotation of a guest in a nanoscale host. J Am Chem Soc. 2004;126:12728–12729. doi: 10.1021/ja046703o. [DOI] [PubMed] [Google Scholar]
- 22.Ajami D, Rebek J., Jr Gas behavior in self-assembled capsules. Angew Chemie Intl Ed Engl. 2008;47:6059–6061. doi: 10.1002/anie.200802023. [DOI] [PubMed] [Google Scholar]
- 23.Castellano RK, Craig SL, Nuckolls C, Rebek J., Jr Detection and mechanistic studies of multi-component assembly by fluorescence resonance energy transfer. J Am Chem Soc. 2000;122:7876–7882. [Google Scholar]
- 24.Barrett ES, Dale TJ, Rebek J., Jr Synthesis and assembly of monofunctionalized pyrogallolarene capsules monitored by fluorescence resonance energy transfer. Chem Commun. 2007:4224–4226. doi: 10.1039/b712713h. [DOI] [PubMed] [Google Scholar]
- 25.Barrett ES, Dale TJ, Rebek J., Jr Assembly and exchange of resorcinarene capsules monitored by fluorescence resonance energy transfer. J Am Chem Soc. 2007;129:3818–3819. doi: 10.1021/ja0700956. [DOI] [PubMed] [Google Scholar]
- 26.Ajami D, Iwasawa T, Rebek J., Jr Experimental and computational probes of the space in a self-assembled capsule. Proc Natl Acad Sci USA. 2006;103:8934–8936. doi: 10.1073/pnas.0602781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmerman P, Verboom W, van Veggel FCJM, van Duynhovan JPM, Reinhoudt DN. A novel type of stereoisomerism in calix[4]arene-based carceplexes. Angew Chem Int Ed Eng. 1994;33:2345–2348. [Google Scholar]
- 28.Nishio M, Hirota M, Umezawa Y. The C-H/π Interaction: Evidence, Nature, and Consequences. Stereochemistry of Organic Compounds. New York: Wiley; 1998. [Google Scholar]
- 29.Grotzfeld RM, Branda N, Rebek J., Jr Reversible encapsulation of disc-shaped guests by a synthetic, self-assembled host. Science. 1996;271:487–489. doi: 10.1126/science.271.5248.487. [DOI] [PubMed] [Google Scholar]
- 30.Tiedemann BDF, Raymond KN. Dangling arms: A tetrahedral supramolecular host with partially encapsulated guests. Angew Chem Int Ed. 2006;45:83–86. doi: 10.1002/anie.200502209. [DOI] [PubMed] [Google Scholar]
- 31.Rivera JM, Martin T, Rebek J., Jr Structural rules governing self-assembly emerge from new molecular capsules. J Am Chem Soc. 1998;120:819–820. [Google Scholar]
- 32.Castellano RK, Rebek J., Jr Formation of discrete, functional assemblies and informational polymers through the hydrogen bonding preferences of calixarene aryl and sulfonyl tetraureas. J Am Chem Soc. 1998;120:3657–3663. [Google Scholar]
- 33.Goodsell DS, Olson AJ. Structural symmetry and protein function. Annu Rev Biophys Biomol Struct. 2000;29:105–153. doi: 10.1146/annurev.biophys.29.1.105. [DOI] [PubMed] [Google Scholar]
- 34.Avram L, Cohen Y. Self-recognition, structure, stability, and guest affinity of pyrogallol[4]arene and resorcin[4]arene capsules in solution. J Am Chem Soc. 2004;126:11556–11563. doi: 10.1021/ja047698r. [DOI] [PubMed] [Google Scholar]
- 35.Beyeh NK, Kogej M, Aahman A, Rissanen K, Schalley CA. Flying capsules: Mass spectrometric detection of pyrogallarene and resorcinarene hexamers. Angew Chem Int Ed. 2006;45:5214–5218. doi: 10.1002/anie.200600687. [DOI] [PubMed] [Google Scholar]
- 36.Ajami D, Rebek J., Jr Expanded capsules with reversibly added spacers. J Am Chem Soc. 2006;128:5314–5315. doi: 10.1021/ja060095q. [DOI] [PubMed] [Google Scholar]
- 37.Ajami D, Rebek J., Jr Adaptations of guest and host in expanded self-assembled capsules. Proc Natl Acad Sci USA. 2007;104:16000–16003. doi: 10.1073/pnas.0707759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowan A, Elemans JAW, Nolte RJM. Molecular and supramolecular objects from glycoluril. Acc Chem Res. 1999;32:995–1006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.