Abstract
The ability to induce durable transplantation tolerance predictably and consistently in the clinic is a highly desired but elusive goal. Progress is hampered by lack of appropriate experimental models in which to study resistance to transplantation tolerance. Here, we demonstrate that T helper 1-associated T box 21 transcription factor (Tbet) KO recipients exhibit allograft tolerance resistance specifically mediated by IL-17-producing CD8 T (T17) cells. Neutralization of IL-17 facilitates long-term cardiac allograft survival with combined T cell co-stimulation (CD28-CD80/86 and CD154-CD40) blockade in Tbet KO recipients. We have used this T17-biased Tbet KO model of allograft tolerance resistance to study the impact of targeting a T cell-co-stimulatory pathway, and demonstrate that targeting T cell Ig and mucin domain-1 (Tim-1) with anti-Tim-1 overcomes this resistance by specifically inhibiting the pathogenic IL-17-producing CD8 T17 cells. These data indicate that in the absence of Th1 immunity, CD8 T17 alloreactivity constitutes a barrier to transplantation tolerance. Targeting TIM-1 provides an approach to overcome resistance to tolerance in clinical transplantation.
Keywords: costimulation, IL-17, rejection, immunosuppression
Development of tolerogenic strategies that could reduce or eliminate the need for maintenance immunosuppressive therapy is important for successful organ transplantation. T cell–costimulation blockade has emerged as one of the promising approaches to tolerance induction in clinical transplantation. While costimulation blockade induces tolerance in less stringent mouse models of transplantation such as vascularized grafts, it is not as effective in more stringent models such as skin transplantation and in non-human primates (1). It has been suggested that, whilst CD4 T cell responses against the allograft are effectively controlled, CD8 T cells contribute to resistance to tolerance induction by co-stimulation blockade (2–6).
Until the recent discovery of the IL-17-producing T helper type, T17 cells, naïve T helper cells were considered to differentiate into two distinct populations, Th1 and Th2, each producing its own set of cytokines and mediating separate effector functions (7). Differentiation of Th1, Th2, and T17 cells is tightly cross-regulated, such that development of one subset is inhibited by cytokines produced by the other (8). In many inflammatory autoimmune diseases, such as type-1 autoimmune diabetes, multiple sclerosis, and rheumatoid arthritis, Th1 cells were believed to be pathogenic while Th2 cells thought to be protective; however, this paradigm was challenged by subsequent studies (9). Further, T17 cells have been implicated to play a critical role in many autoimmune diseases that were traditionally thought to be mediated by Th1 immunity (7). Similarly, it had been generally regarded that Th2 cells promote allograft tolerance whilst Th1 cells initiate allograft rejection, by promoting the development of alloantigen-specific cytotoxic and delayed-type hypersensitivity responses, until growing evidence indicated that each type of T helper cell including Th1, Th2, and most recently T17 cells, can mediate allograft rejection (10, 11). In our recent studies, we noted that, in the absence of Th1-mediated alloimmune responses, CD4 T17 cells mediate an aggressive proinflammatory response, culminating in severe accelerated rejection and vasculopathy in a cardiac allograft model of chronic rejection in Tbet KO recipients (12). Burrell et al. also just reported unconventional CD8 T17-cell mediated CD40-ligand-blockade resistant cardiac allograft rejection in Tbet KO recipients (13). Here we report that targeting the T cell-costimulatory Tim-1:Tim-4 pathway (14–16) overcomes resistance to transplantation tolerance by specifically targeting CD8 T17 cell-mediated alloimmunity.
Results
Tbet Deficiency Accelerates Acute Cardiac Allograft Rejection.
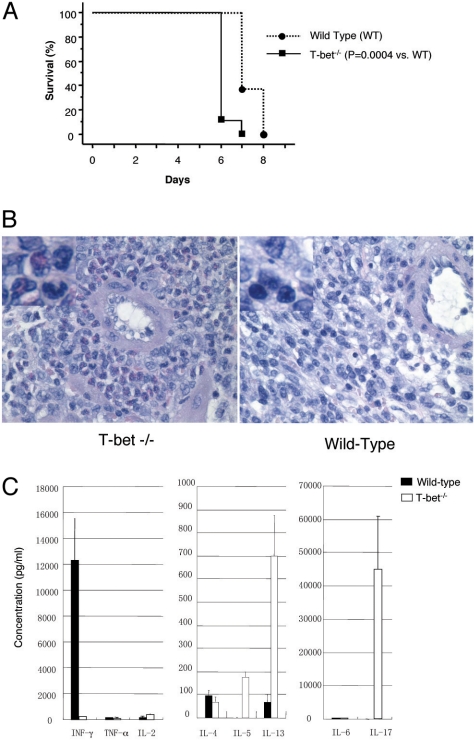
We and others recently reported accelerated and unconventional rejection characterized by massive granulocytic infiltration of the cardiac grafts in Tbet KO recipients (12, 13). In the present study, using a fully allogeneic, cardiac transplant model of acute rejection [BALB/c into C57BL/6(B6)], we found that heart graft survival was slightly, yet consistently and significantly, shorter in the Tbet KO mice [Median survival time (MST): 6 days] compared with wild-type (WT) mice (7 days, P < 0.01) (Fig. 1A). Histopathologic analysis of the grafts harvested from Tbet KO recipients demonstrated conspicuous polymorphonuclear (PMN) interstitial infiltrates containing significant populations of neutrophils and eosinophils (Fig. 1B). Further, in the Tbet KO recipients Th1 cytokine IFN-γ production was significantly reduced compared with the WT control recipients (217.4 ± 11.5 vs. 12,321.3 ± 3198.1 pg/mL respectively; P < 0.001 (Fig. 1C). In contrast, Th2 cytokine IL-5 and IL-13 production was significantly greater in Tbet KO recipients compared with WT controls (IL-5: 178 ± 20 vs. 1.7 ± 0.5 pg/mL, P = 0.001; IL-13: 701.3 ± 171.5 vs. 67.2 ± 37.3 pg/mL, P < 0.005 respectively), confirming a Th2 phenotype in the former animal (Fig. 1C). Taken together, these data indicate that Tbet KO recipients of fully mismatched cardiac allografts mount an aggressive alloimmune response despite a strong Th2 phenotype characterized by increased Th2-associated IgG isotype and cytokines and profound deficiency of the Th1 cytokine IFN-γ. Interestingly, there was a marked increase in the production of proinflammatory cytokine IL-17 (44,966.7 ± 147.84 vs. 6.62 ± 8.71 pg/mL respectively; P < 0.001) in Tbet KO recipients compared to WT controls (Fig. 1C).
Fig. 1.
Tbet deficiency accelerates acute cardiac allograft rejection characterized by intense PMN infiltration and T17/Th2 skewing of alloantigen specific cytokine profile. (A) Kaplan-Meier survival curves of fully mismatched (BALB/c into B6) heart allografts in untreated WT and Tbet KO recipients. Each group had 5 to 7 animals. (B) Representative photomicrograph of an H&E-stained sections of cardiac allografts in WT and Tbet KO recipients. Note the dense perivascular PMN (neutrophils and eosinophils) and intramural inflammatory infiltrates in the Tbet KO recipient. (C) Bar graph illustrating Th1, Th2, and T17 proinflammatory cytokine production by the splenocytes of WT and Tbet KO recipients, 7 days after transplantation. Presented are mean ± SD; representative of at least 3 independent experiments.
Tbet Deficiency Prevents Tolerance Induction by Combined T Cell-Costimulation Blockade.
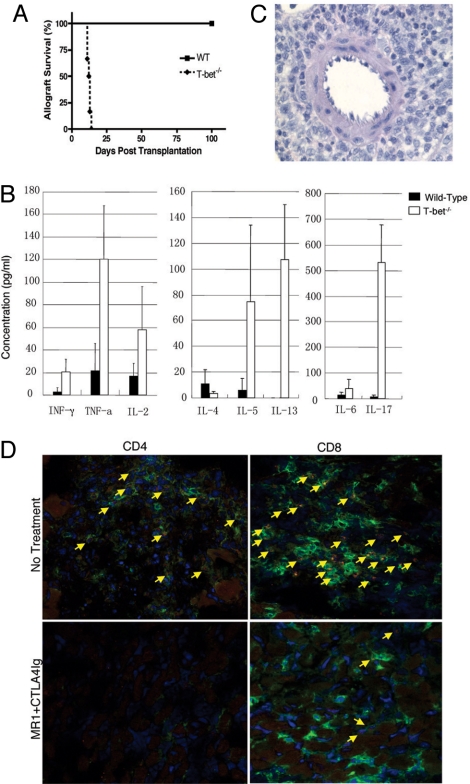
It is recognized that simultaneous blockade of CD28 and CD154 is significantly more effective in promoting long-term allograft survival and tolerance in stringent transplant models, than blockade of either pathway alone (17). Further, CD28 co-stimulation of T cells is thought to be essential for generation of CD4 T17 cells (18). Therefore, next, we examined the effect of combined T cell–co-stimulation (CD28-CD80/86 and CD154-CD40) blockade with CTLA4Ig and MR1 (anti-CD154) in the promotion of allograft tolerance in Tbet KO mice. Again, Tbet KO mice rejected their grafts acutely with a MST of 13 days compared with long-term survival (>100 days) in all of the WT recipients (P < 0.004) (Fig. 2A). In addition, conventional immunosuppression including cyclosporine (CsA) or sirolimus (SRL) did not prolong heart allograft survival. However, unlike heart allografts, there was slight prolongation of skin allograft survival in Tbet KO mice with CTLA4Ig+MR1 (Figs. S2 and S3]. Interestingly, although CTLA4Ig+MR1 almost eliminated IFN-γ, TNF-α, IL-4, IL-5, IL-13, and IL-17 production in WT recipients, there was reduced but persistent IFN-γ, TNF-α, IL-5, IL-13, and particularly IL-17 production in Tbet KO recipients (Fig. 2B). Histopathologic analysis of heart allografts taken from Tbet KO recipients treated with CTLA4Ig+MR1 demonstrated reduced but persistent PMN interstitial infiltrates (Fig. 2C). Immunofluorescence analysis of grafts from untreated Tbet KO recipients demonstrated CD4 and CD8 T cell infiltration and IL-17 production predominantly by the CD8 T cells (Fig. 2D Upper). Whereas, treatment of Tbet KO recipients with CTLA4Ig+MR1 eliminated CD4 T cell infiltration and only diminished the IL-17-producing CD8 T cell infiltration (Fig. 2D Lower). Taken together, these data indicate that Tbet plays a critical role in induction of allograft tolerance by inhibiting differentiation of proinflammatory T17 cells. Further, targeting CD28 and CD154 was not sufficient for controlling CD8 T17 alloimmunity, raising the possibility that there might be other pathways and mechanisms that promote T17 differentiation in the absence of Th1 immunity.
Fig. 2.
Tbet deficiency prevents induction of transplantation tolerance by combined co-stimulation blockade with persistent T17/Th2 skewing of alloantigen specific cytokine profile, and PMN, CD4, and IL-17-producing CD8 T cell infiltration. (A) Kaplan-Meier survival curves of fully mismatched cardiac allografts in WT and Tbet KO recipients treated with CTLA4Ig+MR1. Each group had 5 to 7 animals. (B) Bar graph illustrating Th1, Th2, and T17 proinflammatory cytokine production by the splenocytes of WT and Tbet KO recipients 14 days after transplantation of heart allograft. Presented are mean ± SD; representative of at least 3 independent experiments. (C) Representative photomicrographs of H&E stained sections of cardiac allografts in WT and Tbet KO recipients treated with CTLA4Ig+MR1. Note the persistent perivascular PMN (including neutrophils and eosinophils) and inflammatory infiltrates in the Tbet KO recipient. (D) Representative photomicrographs of immunofluorescence staining and confocal microscopy of heart allografts harvested 2 weeks after transplantation for IL-17 expression by CD4 and CD8 T cells (400× magnification). Green represents either CD4 or CD8; red represents IL-17; yellow in the merged image represents double staining for IL-17 and CD4 or CD8; Blue represent nuclear staining with DAPI. IL-17 expression by most of the CD8 (Top Right) graft infiltrating T cells in the untreated Tbet KO recipients (Upper) is seen in contrast to WT recipients where only a few of the infiltrating T cells express IL-17. Note the diminished but persistent IL-17-producing CD8 T cell infiltrates (Lower Right) in the Tbet KO recipient treated with CTLA4Ig+MR1 (Lower). Presented are results from 1 experiment and are representative of 3 independent experiments.
Accelerated Acute Rejection and Resistance to Allograft Tolerance in Tbet KO Recipients Is Mediated by CD8 T17 Cells.
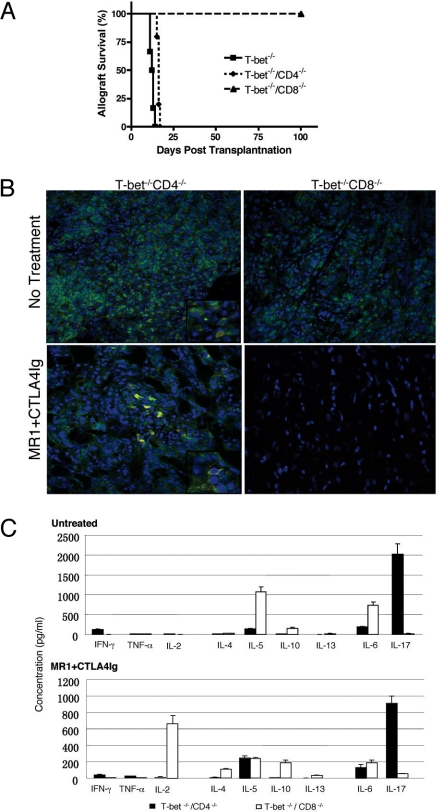
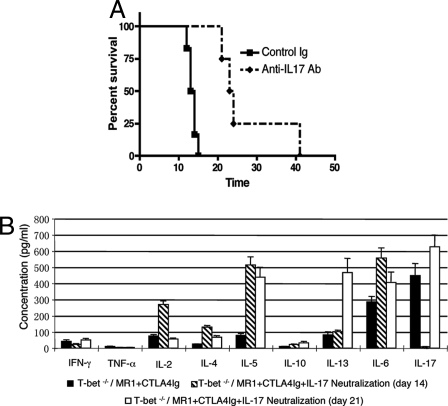
Although IL-17 has been found to be expressed by CD8 T cells (19), γδ-T cells (20), and neutrophils (21), the functional significance of IL-17 expression by these cells is currently unclear. Recently Burrell et al., by depleting CD4 or CD8 T cell populations with mAbs, demonstrated that CD8 T cells are the principle source of IL-17 and mediate costimulation blockade-resistant rejection (13). We confirmed that CD8 depletion results in significant prolongation of allograft survival in Tbet KO recipients treated with CTLA4Ig+MR1 (MST 13 days compared with 44 days; P < 0.01). In addition, CD8/Tbet double knockout (DKO) recipients, accepted the grafts for more than 100 days, whilst CD4/Tbet DKO mice rejected the heart grafts with a MST of 16 days; P < 0.01 (Fig. 3A) confirming that CD8 T cells mediate the resistance to tolerance induction by combined co-stimulation blockade in Tbet KO recipients. Moreover, Tbet/Ig DKO recipients lacking Tbet, mature B cells, and immunoglobulins (Ig) were also resistant to tolerance induction (Fig. S4) and Ig deposition was not seen in rejected grafts. Immunofluorescence analysis of heart allografts from DKO recipients revealed that CD4 and CD8 T cells infiltrate in the CD8/Tbet DKO and CD4/Tbet DKO, respectively, and only CD8 T cells produce IL-17 (Fig. 3B Upper). Further, combined co-stimulation blockade eliminates CD4 T cell infiltration in CD8/Tbet DKO recipients but grafts from CD4/Tbet DKO recipients show reduced but persistent IL-17 producing CD8 T cell infiltration (Fig. 3B Lower). Furthermore, splenocytes from CD8/Tbet DKO recipients, that enjoy long-term graft survival, produce very little IL-17 (50.6 ± 4.0 pg/mL) (Fig. 3C) while IL-17 production by CD4/Tbet DKO recipients, that reject the allografts, is persistent albeit reduced with combined co-stimulation blockade (908.5 ± 89.2 P < 0.001) (Fig. 3C Lower). Similar to our previous observation (12), in vivo neutralization of IL-17 with anti-IL-17 mAb for a brief period along with combined co-stimulation blockade significantly prolongs fully mismatched allograft survival in Tbet KO recipients (Fig. 4A) associated with markedly diminished IL-17 but elevated IL-5, IL-13, and IL-6 production (Fig. 4B). The onset of rejection in these recipients at day 21 was associated with persistent elevation of IL-5, IL-13, and IL-6 and rebound increase in IL-17 levels (Fig. 4B). Taken together, these data indicate that CD8 T17 cells mediate the resistance to tolerance induction independent of CD4 T helper cells and IL-6. Intriguingly, IL-2 production is significantly increased in CD8/Tbet DKO recipients enjoying long-term graft survival with combined co-stimulation blockade (Fig. 3C), suggesting modulation of immunoregulatory networks that promote Treg survival and function (22).
Fig. 3.
CD8 but not CD4 T cells mediate resistance to allograft tolerance in Tbet KO recipients. (A) Survival of fully allogeneic cardiac graft in CD4/Tbet or CD8/Tbet DKO recipients treated with CTLA4Ig+MR1 (n = 5 for CD4/Tbet DKO group, and n = 6 for CD8/Tbet DKO and control Tbet KO groups). Survival data presented as Kaplan-Meier plot. (B) Immunofluorescence staining and confocal microscopy of heart allografts harvested 2 weeks after transplantation for IL-17 expression by CD4 and CD8 T cells (400× magnification). CD4 and CD8 T cells infiltrate in the CD8/Tbet DKO and CD4/Tbet DKO, respectively, and only CD8 T cells produce IL-17 (Fig. 3B Upper). Note absence of CD4 T cell infiltration in CD8/Tbet DKO recipients but grafts from CD4/Tbet DKO recipients show reduced but persistent IL-17 producing CD8 T cell infiltration (Fig. 3B Lower). (C) Bar graph illustrating Th1, Th2 and T17 proinflammatory cytokine production by the splenocytes of CD4/Tbet and CD8/Tbet DKO recipients 14 days after transplantation of heart grafts, treated with (Upper) or without CTLA4Ig+MR1 (Lower). Presented are mean ± SD; representative of at least 3 independent experiments.
Fig. 4.
In vivo IL-17 neutralization inhibits rejection and facilitates allograft survival with combined co-stimulation blockade in Tbet KO recipients. (A) Fully mismatched cardiac allograft survival in Tbet KO recipients treated with CTLA4Ig+MR1 and anti-IL-17 mAb (n = 4) or control IgG (n = 6). Survival data presented as a Kaplan-Meier plot. (B) Bar graph illustrating Th1, Th2, and T17 proinflammatory cytokine production by the splenocytes of Tbet KO recipients 14 and 21 days after transplantation of heart graft. Presented are mean ± SD; representative of at least 3 independent experiments.
Tim-1 Blockade Overcomes Allograft Tolerance Resistance in Tbet KO Recipients.
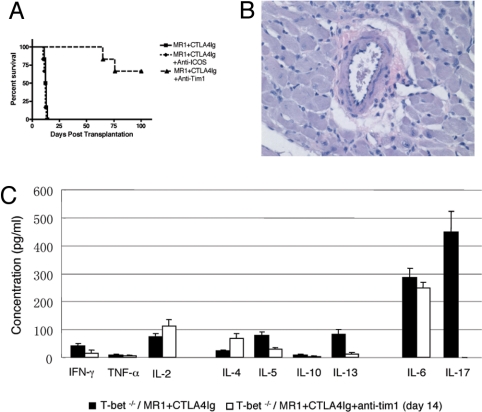
Ligation of Tim-1 with its ligand Tim-4 in vitro induces T cell activation and proliferation (23). High-avidity binding of Tim-1 with an agonistic anti-Tim-1 antibody (3B3) induces production of proinflammatory cytokines IFN-γ and IL-17 (14). Blocking this interaction with a low-avidity blocking anti-Tim-1 antibody (RMT1–10) inhibits the generation of pathogenic T17 cells in vitro (14). Therefore, we combined RMT1–10 treatment with CTLA4Ig+MR1 in Tbet KO recipients, to see whether this facilitated long-term allograft survival in our T17-biased model of allograft tolerance resistance. Indeed, the addition of Tim-1:Tim-4 blockade resulted in striking restoration of the ability of CTLA4Ig+MR1 to induce long-term cardiac allograft survival in Tbet KO recipients with MST of 74.3 ± 7.2 vs. 12.3 ± 1.21, P = 0.0037 (Fig. 5A) with no evidence of acute or chronic rejection on histological examination of the heart allografts surviving >100 days after transplantation (Fig. 5B). Interestingly, targeting Tim-1 completely abrogated IL-17 production (449.0 ± 73.7 pg/mL with CTLA4Ig+MR1 vs. 0.56 ± 0.87 pg/mL with CTLA4Ig+MR1+anti-Tim-1; P < 0.001) and, notably in contrast to IL-17 neutralization, significantly inhibited IL-5, IL-13, and IL-6 production (Fig. 5C). On the other hand, even though ICOS signaling has been reported to be important for CD4 T17 differentiation (18), addition of ICOS blockade had no effect on IL-17 production (449.0 ± 73.7 with CTLA4Ig+MR1 vs. 512.2 ± 98.5 pg/mL with CTLA4Ig+MR1+anti-ICOS; P = 0.237) and was unable to reverse the resistance to allograft tolerance by CTLA4Ig+MR1 in Tbet KO recipients (Fig. 5A), indicating that Tim-1 and not ICOS signaling is important in generating an aggressive CD8 T17 alloimmune response and mediate resistance to tolerance inducation in Tbet KO recipients.
Fig. 5.
Tim-1, but not ICOS, blockade restores the ability of combined costimulation blockade to induce transplantation tolerance in Tbet KO recipients. (A) Fully mismatched cardiac allograft survival in Tbet KO recipients treated with CTLA4Ig+MR1 and anti-Tim-1 mAb or anti-ICOS mAb (n = 6 for all 3 groups). Survival data presented as a Kaplan-Meier plot. (B) Representative photomicrograph of an H&E-stained section of cardiac allograft, > 100 days after transplantation, in Tbet KO recipients treated with anti-Tim-1 mAb. Note the absence of inflammatory infiltrates and signs of acute or chronic rejection in the Tbet KO recipients treated with anti-Tim-1 mAb. (C) Bar graph illustrating Th1, Th2, and T17 proinflammatory cytokine production by the splenocytes of Tbet KO recipients 14 days after transplantation heart graft. Presented are mean ± SD; representative of at least 3 independent experiments.
Discussion
Tbet plays a crucial role in Th1 development, in part, via the induction of IFN-γ. In the absence of Tbet, CD4 T cells fail to differentiate into the Th1 lineage and default to a Th2 fate (24). Tbet KO mice fail to generate functional Th1 responses and are protected from a number of T cell mediated autoimmune diseases but show evidence of heightened allergic (Th2) responses (24). The ability to generate CD8 effector cells is also impaired in Tbet KO mice, and these mice respond poorly to infection with lymphocytic choriomeningitis (25). In contrast, we have recently demonstrated that Tbet KO mice, despite exhibiting a Th1-deficient environment characterized by profound deficiency of IFN-γ and increased Th2 cytokines, mount an aggressive alloimmune response characterized by increased proinflammatory cytokine IL-6, IL-12p40, and IL-17 production, and massive infiltration of the allograft with PMN cells (12). Further, in a model of EAM, heightened disease severity in Tbet KO mice was suggested to be due to increased CD4 T cell-mediated IL-17 production in the heart (26). Lohr et al. in another model of systemic autoimmune disease reported worsening of disease in Tbet KO mice, suggesting a reciprocal relationship between Th1 responses and IL-17 production indicating that IL-17 is an important cytokine in tissue inflammation (27). T17 cells induce non-hematopoietic cells, including epithelial cells, to produce chemokines that recruit neutrophils to the site of infection (8). Our observation of accelerated rejection in Tbet KO recipients (Fig. 1) characterized by PMN and IL-17-producing T cell infiltration are in keeping with these previous reports of Tbet as a negative regulator of T17-mediated immune responses.
CD4 T17 cells have been the focus of most studies on T17 immunity; however, recently CD8 T cells have also been noted to produce IL-17 and mediate inflammation (25). In a viral infection model, mice with T cells lacking both Tbet and Eomes develop a CD8 T cell-dependent, progressive inflammatory and wasting syndrome characterized by multiorgan infiltration of neutrophils (25). More recently, Burrell et al. (13) by depleting CD4 or CD8 T cells with mAbs demonstrated that CD8 T cells are the primary cells producing IL-17 and mediate CD154-CD40 costimulation blockade-resistant allograft rejection in Tbet KO recipients. We confirmed these observations by studying allograft survival in CD4/Tbet DKO or CD8/Tbet DKO and established that CD8 T17 cells are the principle cells producing IL-17 (Fig. 3C) and mediate resistance to tolerance induction even with combined T cell–co-stimulation (CD28-CD80/86 and CD154-CD40) blockade (Figs. 2 and 3). Taken together with our previous observations (12), Tbet appears to regulate both CD4- and CD8 T17-aggressive alloimmune responses in different settings; while CD4 T17 mediate allograft rejection and vasculopathy, CD8 T17 cells are critical for mediating resistance to immunosuppression and induction of tolerance.
Treatment with IL-17 antibodies after the onset of experimental collagen-induced arthritis decreased joint damage and histologic destruction of cartilage and bone. IL-17 KO mice exhibit delayed onset and reduced maximum-severity scores in experimental autoimmune encephalomyelitis (7). In keeping with this, brief in vivo neutralization of IL-17 with mAb facilitated prolongation of graft survival with combined costimulation blockade in Tbet KO recipients (Fig. 4A). As expected, this was associated with abrogation of IL-17 and other proinflammatory cytokine production at day 14 but eventual rejection of the grafts was associated with return to higher levels at day 21 (Fig. 4B).
From the foregoing, it is clear that T17 immunity is now firmly implicated in alloimmunity and various autoimmune diseases, particularly in the absence of a Th1 environment (7, 27, 28). Co-stimulation blockade of either CD28-CD80/86 or CD154-CD40 pathways in transplantation is associated with dampened Th1 and intact/enhanced Th2 immunity (29, 30). Interestingly, current immunosuppressive medications including glucocorticoids, CsA, and SRL inhibit both IFNγ and IL-17 (31, 32). However, the extent of inhibition of IL-17 needed to eliminate T17 responses is unknown, and there are conflicting data regarding IL-17 inhibition with tacrolimus (31, 33), and both CsA and SRL had little impact on allograft survival in Tbet KO recipients. Taken together with our observations of combined co-stimulation blockade-resistant rejection in Tbet KO recipients mediated by T17 immunity, these data indicate that in clinical transplantation where Th1 immunity is effectively suppressed, T17 immune responses may emerge and contribute to resistance to transplantation tolerance induction with current treatment protocols. Further, T17 cells are predominantly of the memory phenotype (34, 35), and memory CD8 T cells are critical in mediating resistance to co-stimulation blockade-induced transplantation tolerance (5, 6). These co-stimulation blockade-resistant CD8 memory T cells could be CD8 T17 cells. Indeed, in Tbet KO recipients there was reduced but persistent production of proinflammatory cytokine IL-17, particularly by CD8 T cells as seen in CD4/Tbet DKO recipients, despite combined co-stimulation blockade (Figs. 2B and 3C), indicating that CD8 T17 cells in particular are resistant to CTLA4Ig+MR1, and other pathways and mechanisms are operative in maintaining T17 immune responses. Two such pathways are the ICOS-ICOSL pathway and the costimulatory Tim-1:Tim-4 pathway (14–16, 18). Naïve CD4 T cells up-regulate Tim-1 expression early after activation, and Tim-1 cell-surface expression is maintained through differentiation into the Th1 or Th2 phenotype, with higher expression on Th2 cells (36). Tim-4, a molecule expressed by DCs, is a ligand of Tim-1 (23). Cross-linking of Tim-1 on the surface of T cells in vitro by Tim-4Ig enhanced T cell proliferation, and production of Th1 and Th2 cytokines and in vivo administration of Tim-4Ig during an ongoing immune response created similar effects (23). Tim-1:Tim-4 signaling also greatly enhances proinflammatory T17 responses (15). Using an antagonistic Tim-1-specific mAb (RMT1–10), we recently reported the role of Tim-1 in alloimmunity (16). A short course of RMT1–10 monotherapy prolonged survival of fully MHC-mismatched mouse cardiac allografts. This prolongation was associated with inhibition of alloreactive Th1 responses and preservation of Th2 responses. Interestingly Tim-1-specific antibody treatment was more effective in Th1-type cytokine-deficient Stat4 KO recipients compared with Th2-type cytokine-deficient Stat6 KO recipients. Therefore, we added Tim-1 blockade (RMT1–10) to CTLA4Ig+MR1 in Tbet KO recipients. Interestingly targeting Tim-1, unlike ICOS blockade, dramatically restored the ability of CTLA4Ig+MR1 to prevent acute and chronic rejection and promote transplantation tolerance (Fig. 5 A and B). This was associated with complete abrogation of proinflammatory cytokine IL-17 production with the addition of Tim-1 blockade but not ICOS blockade (Fig. 5 C and Results), indicating the effect is specifically due to targeting the Tim-1 pathway. Our in vivo data are in keeping with previous in vitro studies linking Tim-1 signaling with Th2/T17 immunity and indicates that Tim-1:Tim-4 pathway plays a critical role in regulating alloreactive T17 responses. Further, Tim-1:Tim-4 signaling, in addition to greatly enhancing proinflammatory Th1 and T17 responses, limits the generation and functional capacity of Tregs (15). However until recently, Tim-1 signaling was thought to be primarily important in CD4 T cell co-stimulation, but a recent study noted that CD8 T cell proliferation was also enhanced by agonistic anti-Tim-1 mAb (15). Taken together with our data, it appears that Tim-1 signaling is critical for mounting an aggressive CD8 T17 alloimmune response, explaining the dramatically beneficial effect of adding Tim-1 blockade to CTLA4Ig+MR1 where CD8 T cells are known to mediate co-stimulation blockade-resistant rejection (2–6). Whether the CD8 T cell-mediated co-stimulation blockade-resistant rejection is due to CD8 T17 cells and whether the beneficial effect of targeting Tim-1 is due to a direct effect of the blocking anti-Tim-1 mAb (RMT1–10) on CD8 T17 cells or due to upstream effects at the time of T cell priming remains to be investigated. What is known is that Tim-1 deprograms Tregs, manifested by decrease in Foxp3, GITR, CTLA4, and IL-10 gene expression and markedly impairs function of Tregs indicated by profound decrease in the ability of Tregs to inhibit proliferation of CD4 T effectors. Whether Tim-1 blockade preserved Treg function in Tbet KO recipients in our studies is under investigation. Interestingly, IL-6 has opposing effects on the generation of Tregs and T17 cells. Moreover, Tregs can be reprogrammed to develop into IL-17-producing effectors (37). However IL-6, which is believed to be upstream of IL-17 in the differentiation of T17 cells, remains elevated with no deleterious effects on graft survival suggesting that the beneficial effects of Tim-1 blockade are independent of IL-6. Further, IL-2 levels were increased in CD8/Tbet DKO recipients enjoying long-term allograft survival with combined costimulation blockade (Fig. 3C Lower), suggesting that an immunoregulatory cytokine milieu may be established by this combination therapy to promote regulation by enhancing Treg survival and function (22). It is worth noting that even though ICOS signaling is reported to be important for CD4 T17 differentiation (18), ICOS blockade, unlike Tim-1 blockade, was unable to control CD8 T17 responses and restore the ability of CTLA4Ig+MR1 to induce transplantation tolerance in Tbet KO recipients, indicating the Tim-1 blockade uniquely targets the aggressive CD8 T17 cells and promotes tolerance. Taken together, T17 alloimmunity appears to be clinically important where Th1/Th2 immunity is effectively suppressed (with current immunosuppressive protocols) allowing CD8 T17 cells to emerge and mediate rejection. In addition, although there are conflicting data on the role of IL-17 in carcinogenesis (38), a recent report suggests that TGF-β in the tumor microenvironment can subvert CD8 T cells into making IL-17, which then promotes tumor growth through direct pro-survival effects on the tumor cells (39), suggesting that targeting CD8 T17 cells may be beneficial for both prevention of rejection and tumor growth.
In summary, alloimmune responses involve a complex interplay between pathogenic/inflammatory immune mechanisms that promote rejection and regulatory/anti-inflammatory immune mechanisms that facilitate tolerance toward the transplant. Tipping this balance either way will ultimately determine the fate of the transplanted organ. From the data presented here, it is clear that CD8 T17 cells are the major mediators of resistance to allograft tolerance. We have shown that neutralization of IL-17 facilitates prolongation of allograft survival with CTLA4Ig+MR1. Further, Tim-1, but not ICOS, blockade overcomes the CD8 T17-mediated resistance to allograft tolerance. In conclusion IL-17-producing CD8 T17 cells are important in allograft tolerance resistance and targeting TIM-1 provides an approach to overcome resistance to tolerance in clinical transplantation.
Materials and Methods
Mice.
BALB/c (H-2d), B6 (H-2b), Tbet, CD4, CD8, and Ig KO mice, all on the B6 background, were purchased from Jackson Laboratories. Tbet KO mice lacking CD4, CD8, or B cells were generated by crossbreeding of Tbet KO mice with CD4, CD8, or Ig KO mice, respectively. Genotyping was used to confirm homozygous deletion of the Tbet, CD4, CD8, or Ig genes. Animals were maintained in accordance with institutional guidelines.
Heart Transplantation.
BALB/c mice were used as donors and B6, Tbet KO, CD4/Tbet, CD8/Tbet, or Tbet/Ig DKO as recipients. Vascularized heart grafts were transplanted using microsurgical techniques as described (16). Graft function was assessed by daily palpation of the abdomen. Rejection was defined as complete cessation of cardiac contractility as determined by direct visualization. Graft survival is shown as MST in days.
Skin Transplantation.
Full thickness trunk (1 cm2) harvested from BALB/c donors were transplanted onto the flank of B6 Tbet KO recipients, sutured with 6.0 silk, and secured with dry gauze and a bandage for 7 days. Skin graft survival was monitored daily thereafter, and rejection was defined as complete graft necrosis.
Treatment Protocols.
Anti-CD154L mAb (MR1) and CTLA4Ig, 0.5 mg each, were given IP on day 0, and 0.25 mg on days 2, 4, and 6 post-transplantation. CD4 and CD8 depletion was achieved by IP injections of 0.1 mg mAb GK1.5 and 2.43 (both from BioExpress) respectively on days −6, −3, and −1. Neutralizing IL-17 mAb (MAB421; R&D Systems) was administered IP at a dose of 0.1 mg per dose daily on day 0 to 3 followed by every other day until day 13 post-transplantation. Anti-Tim-1 (RMT1–10) mAb (14, 16) or anti-ICOS mAb was administered IP at a dose of 0.5 mg on day 0, and 0.25 mg on days 2, 4, 6, 8, and 10.
Histology.
The harvested grafts were sectioned transversely, frozen in OCT compound (Ames Co.) and stored at −80 °C, and/or fixed in 10% buffered formalin for morphological examination. Four-micrometer thick sections of heart were stained with hematoxylin and eosin or elastin stains. Frozen sections were used for immunofluorescence staining using goat anti-mouse IL-17 (R&D Systems), rat anti-Mouse CD4 and CD8 (both from BioExpress) as primary antibodies. Secondary detection was performed using Cy2-conjugated donkey anti-rat IgG and Cy3-conjugated donkey anti-goat IgG (Jackson Immunoresearch Laboratories). Images were captured using Nikon C1 Plus Confocal Laser Scanning microscope.
Cytokine Analysis by LUMINEX Assay.
Splenocytes harvested at 7, 14, or 21 days after transplantation from recipients of heart allografts were re-stimulated by irradiated donor spleen cells. The cell-free supernatants were removed after 48 h and analyzed by multiplexed cytokine bead-based immunoassay using a 21-plex mouse cytokine detection kit (Upstate) according to the manufacturer's instructions as described previously (12). All samples were tested in triplicate wells.
Statistics.
For graft survival analysis, Kaplan-Meier graphs were constructed and log-rank comparison of the groups was used to calculate P values. For cytokine levels by LUMINEX assay, data are presented as mean ± SD and comparisons between the values were performed using the 2-tailed Student's t test. For all statistical analyses, the level of significance was set at a probability of P < 0.05. All experiments were repeated at least 3–5 times.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health (NIH) Grants: RO1 AI-51559, R01AI-37691, R01 AI-70820, and P01 AI-41521 (to M.H.S.) and CA112663 and NS038037 (to L.H.G.). X.Y. was supported in part by American Society of Transplantation (AST) Basic Science Faculty Development Grant Award. M.J.A. is supported in part by the AST-Wyeth Basic Science Faculty Development Grant Award and NIH Grant K08 AI080836–01.
Footnotes
Conflict of interest statement: L.H.G. is on the Board of Directors and holds equity in Bristol Myers Squibb Pharmaceutical Company.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812538106/DCSupplemental.
References
- 1.Ansari MJ, Sayegh MH. The arduous road to achieving an immunosuppression-free state in kidney transplant recipients. Nature Clinical Practice. 2007;3:464–465. doi: 10.1038/ncpneph0568. [DOI] [PubMed] [Google Scholar]
- 2.Newell KA, et al. Cutting edge: Blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–2362. [PubMed] [Google Scholar]
- 3.Jones ND, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165:1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 4.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: Therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 7.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 9.Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 10.Piccotti JR, Chan SY, VanBuskirk AM, Eichwald EJ, Bishop DK. Are Th2 helper T lymphocytes beneficial, deleterious, or irrelevant in promoting allograft survival? Transplantation. 1997;63:619–624. doi: 10.1097/00007890-199703150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Goriely S, Goldman M. The interleukin-12 family: New players in transplantation immunity? Am J Transplant. 2007;7:278–284. doi: 10.1111/j.1600-6143.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- 12.Yuan X, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao S, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degauque N, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118:735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno T, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118:742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 18.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin HC, Benbernou N, Fekkar H, Esnault S, Guenounou M. Regulation of IL-17, IFN-gamma and IL-10 in human CD8(+) T cells by cyclic AMP-dependent signal transduction pathway. Cytokine. 1998;10:841–850. doi: 10.1006/cyto.1998.0375. [DOI] [PubMed] [Google Scholar]
- 20.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 22.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008;13:1440–1446. doi: 10.2741/2773. [DOI] [PubMed] [Google Scholar]
- 23.Meyers JH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 24.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 25.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangachari M, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 29.Sayegh MH, et al. CD28–B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan MJ, et al. Requirement for donor and recipient CD40 expression in cardiac allograft rejection: Induction of Th1 responses and influence of donor-derived dendritic cells. J Immunol. 2004;172:6626–6633. doi: 10.4049/jimmunol.172.11.6626. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, et al. Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood. Cytokine. 2009;45:141–147. doi: 10.1016/j.cyto.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Yang K, et al. Inhibitory effect of rapamycin and dexamethasone on production of IL-17 and IFN-gamma in Vogt-Koyanagi-Harada patients. Br J Ophthalmol. 2009;93:249–253. doi: 10.1136/bjo.2008.142489. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa Y, Yoshikawa H, Iwasa K, Yamada M. linical efficacy and cytokine network-modulating effects of tacrolimus in myasthenia gravis. J neuroimmunol. 2008;195:108–115. doi: 10.1016/j.jneuroim.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Zhang J, Yang B, Wu C. Cyclosporin A inhibits the production of IL-17 by memory Th17 cells from healthy individuals and patients with rheumatoid arthritis. Cytokine. 2008;42:345–352. doi: 10.1016/j.cyto.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Umetsu SE, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 37.Radhakrishnan S, et al. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137–3147. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Numasaki M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 39.Nam JS, et al. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.