Abstract
A new type of neutral donor–acceptor [2]-catenane, containing both complementary units in the same ring was synthesized from a dynamic combinatorial library in water. The yield of the water soluble [2]-catenane is enhanced by increasing either building-block concentrations or ionic strength, or by the addition of an electron-rich template. NMR spectroscopy demonstrates that the template is intercalated between the 2 electron-deficient naphthalenediimide units of the catenane.
Keywords: dynamic combinatorial chemistry, molecular recognition
We report here the spontaneous assembly of a donor–acceptor (D–A) [2]-catenane from a dynamic combinatorial library (DCL) in water. Unusually, this is a D–A catenane that contains the electron-deficient and electron-rich aromatic moieties in the same ring. Owing to their complex topology and the resulting synthetic challenge, mechanically interlocked molecules such as catenanes have captivated chemists for a long time (1). With advances in the efficient templated synthesis of these interlocked structures, applications of these interesting molecules have been found in molecular electronic devices, such as switches, motors, color displays, and molecular memory (2–5).
Conventional catenane synthesis relies on the use of noncovalent interactions to preorganize precursors in a suitable configuration that favors the formation of an interlocked structure, employing an irreversible, kinetically controlled chemical reaction as the final catenating step (for recent examples, see 6–9). The recent rise of dynamic covalent chemistry (10) using reversible chemical reactions under thermodynamic control has led to an increasing number of catenane syntheses that are either designed to lead to a particular structure (for recent examples, see 9, 11–19) or result from unpredictable dynamic combinatorial selection (20, 21). The advantage of either of these dynamic strategies is the possibility of recycling un-interlocked components, hence increasing the yield of the desired structure.
Interactions between electron-rich aromatics, such as dialkoxynaphthalene (DN) and tetrathiafulvalene (TTF), and electron deficient aromatics, like naphthalenediimide (NDI) and paraquat, have been extensively used in the preparation of catenanes (9, 22, 23). The vast majority of these catenane constructions rely on kinetically controlled reactions. Some examples of thermodynamically controlled syntheses of these structures include the neutral [2]-catenanes featuring zinc-pyridine coordination (24) and alkene metathesis as the ring-closing reactions (25). More recently, Stoddart and coworkers reported the iodide-catalyzed self-assembly of paraquat-based cationic D–A [2]- (16) and [3]-catenanes (14) from separate π-donor and π-acceptor rings using thermodynamically controlled nucleophilic substitution. Most of the examples of D–A catenane syntheses depend on a preformed, π-rich crown ether ring containing electron-donor units, and the subsequent formation of new electron-deficient rings followed by catenation. Hence, the resulting catenanes contain only a π-donor or π-acceptor in each of the individual rings.
Recently, we reported an aqueous disulfide DCL derived from the π-accepting NDI that uses an electronically complementary DN template to amplify a tetramer up to an 80% yield (26). Because interactions between the π-deficient NDI and π-rich DN have been successful in our previous syntheses of neutral D–A [2]-catenanes, it was expected that similar interlocked structures can be constructed if the electronically complementary aromatic subunits are incorporated into disulfide DCLs. This would allow the formation of macrocycles from both components through reversible disulfide exchanges (Scheme 1) (27). Here, we present the confirmation of our initial premise in the form of a new type of D–A [2]-catenane, obtained from an aqueous disulfide DCL containing initially only acyclic components. In this catenane, both donor and acceptor subunits are present in the same ring. We also prove that exerting stimuli on the equilibrating system, such as changing solvent ionic strength and template addition, can influence the yield of the [2]-catenane, and we demonstrate intercalation of the electron-rich template between the electron-deficient NDI units of the catenane.
Scheme 1.
Generation of donor-acceptor disulfide DCL with π-acceptor 1 and π-donor 2 in water.
Results and Discussion
Dithiol-building block 1, derived from a π-accepting NDI, was prepared as previously described (26). The cysteine-functionalized, π-donating counterpart 2 was synthesized in 4 straightforward steps from 1,5-dihydroxynaphthalene (see SI). Incorporation of the amino acid function in the building blocks provides both water solubility and a thiol group as a handle for reversible reactions.
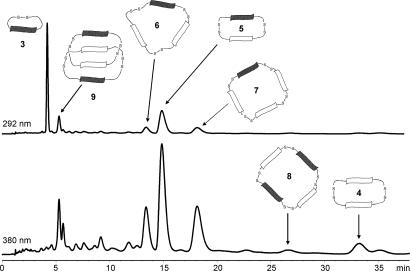
A DCL was set up by air oxidation of a 5 mM equimolar solution of 1 and 2 in water at pH 8. The library was equilibrated in a close-capped vial for 5 days and analyzed by reverse-phase HPLC and LC-MS. At equilibrium, the species containing only one kind of building block are the cyclic monomer 3 from the donor subunit 2 and the cyclic homodimer 4 from the acceptor subunit 1. Several macrocycles that incorporate both the donor and acceptor subunits are also present, including the heterodimer 5, the heterotrimer 6, and heterotetramers 7, 8, and 9 (Fig. 1).
Fig. 1.
HPLC analysis of a 5 mM DCL of 1 and 2. UV traces were recorded at 292 nm (upper trace) and 380 nm (lower trace) where the DN and NDI cores respectively absorb.
Macrocycle 7 contains 1 DN and 3 NDI subunits whereas the 2 heterotetramers 9 and 8 (retention time ≈5 and 27 min, respectively) have the same composition, containing 2 of each of the donor and acceptor building blocks. Tetramers with other building-block compositions were not observed. To help distinguish tetramers 8 and 9 and elucidate their cyclic structures, they were further analyzed by MS/MS (28–30). Molecular ions of these 2 tetramers have different fragmentation behavior: tetramer 8 shows fragments from trimeric species (m/z = 1,039.8) and dimeric species (m/z = 930.7, 566.9); whereas tetramer 9 has fragments from dimeric species only (Fig. 2). Fragments larger than the dimer were also observed for tetramer 7 (m/z = 1,038.6, 1,358.7, 1,510.5). Unlike in the case of 7, there were no homodimeric fragments observed in the MS/MS of 8 and 9. Because the direct fragmentation of a tetramer to dimer is characteristic of an interlocked structure, these observations suggest that the heterotetramer 9 is a [2]-catenane consisting of 2 interlocked heterodimeric donor-acceptor rings [–1–2–], while heterotetramer 8 is a cyclic tetramer with the cyclic structure [–1–2–1–2–].
Fig. 2.
Electrospray MS/MS spectra of the molecular ion of the tetrameric [2]-catenane 9 (A); the cyclic tetramer 8 (B); and the cyclic tetramer 7 (C).
The [2]-catenane 9 was isolated from a preparative scale DCL and characterized by 1H NMR and UV–Vis spectroscopies. The 1H spectrum of the [2]-catenane 9 in CD3OD (300 K, 500 MHz) consists of broad but assignable signals (see SI). In contrast, the 1H spectrum of the heterodimer 5 obtained under the same conditions shows sharp and well-defined peaks. Two coupled doublets were observed for the NDI unit of 9, but only one singlet for the corresponding protons of 5. Upfield shifts of 0.53–0.73 and 0.22–0.50 ppm were, respectively, observed for the NDI and DN aromatic protons of 9 compared with those of 5. These observations suggest that the aromatic cores in 9 are in closer proximity than in 5, as one would expect from the interlocked nature of the former compound.
The 1H spectrum recorded in D2O of 9 (300 K, 500 MHz) consists of sharp peaks with clear splitting patterns observed for the aromatic signals. Upfield shifts of 0.61–0.77 ppm were observed for the DN core protons when compared with the spectrum of 9 in CD3OD. These observations suggest that the catenane adopts an even more compact conformation in the more polar solvent. Similar behavior was also observed in the 1H spectrum of 5 (D2O, 300 K, 500 MHz): upfield shifts of 0.11 ppm and 0.14–0.39 ppm were observed for the NDI and DN aromatic protons, respectively, indicating the same kind of closer proximity between the donor and acceptor units in D2O versus CD3OD. In both solvents, the spectra are easily assignable: the NDI doublets of 9 suggest the presence of a well-defined symmetrical conformation, narrowing down the possible conformations for 9 to only I and III (Fig. 3). The larger upfield shift of the NDI protons in 9 when compared with 5, and the UV–Vis and templating data (see below), allow us to propose the D2 symmetric I as the major conformer of 9 in aqueous solution. This is consistent with the expectation of it being the most thermodynamically stable conformation, because the number of favorable interactions between the donor and acceptor is maximized while the repulsive interactions between electron-rich aromatic cores are minimized (31).
Fig. 3.
Three possible conformers of 9.
The UV–Vis spectrum of 9 is dominated by broad absorption bands at 367 and 383 nm (Fig. 4), corresponding to the NDI chromophores, and an even broader band <350 nm, likely due to a combination of the 2 chromophores. Red shifts of 6–8 nm and broadening were observed for the NDI absorption maxima, supporting a conformation of type I, with the NDI chromophores in close proximity to each other. The spectrum of uncatenated 5 is strikingly different, displaying only absorbances characteristic for the individual chromophores.
Fig. 4.
UV-Vis absorption profile of the [2]-catenane 9 (orange) and the heterodimer 5 (blue) in D2O.
Imposing Stimuli on the DCL.
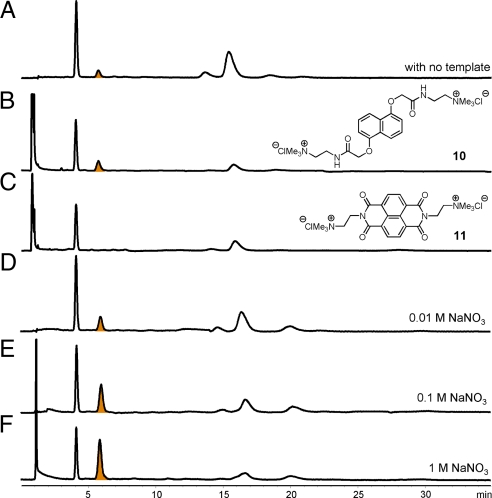
The adaptive ability to respond to external changes due to reversible chemical linkages between building blocks is the main feature of a DCL (27). Addition of a template to a DCL is perhaps the most common way to perturb product distribution of a DCL. Considering that the current building blocks are a π-donor and π-acceptor, the addition of another π-donating or π-accepting molecule as template to the DCL may stabilize some of the library members and induce a change in the library distribution. Because the library members in the DCL are anionic due to the carboxylic functionalities, the use of a cationic guest should induce stronger responses than a neutral or anionic one. Therefore, the π-rich guest 10, and the π-deficient guest 11 were tested as templates for the DCL (Fig. 5).
Fig. 5.
HPLC analysis of a 5 mM DCL mixture in the absence of template (A); the presence of 2.5 mM 10 (B); 2.5 mM 11 (C); 0.01 M NaNO3 (D); 0.1 M NaNO3 (E); and 1 M NaNO3 (F). UV traces shown were recorded at 292 nm.
Addition of the electron-rich template 10 to the DCL amplifies the [2]-catenane 9 at the expense of all other macrocycles. The amplification factor is approximately 1.5 when the library is conducted in water at pH 8 with 5 mM building block and 2.5 mM template concentration. However, addition of the electron-deficient template 11 under the same condition leads to the disappearance of 9, and the redistribution of the library material (Fig. 5). These results also support the conclusion that conformation I is most probable in water for 9, because it has the right donor–acceptor–acceptor–donor sequence for intercalating the electron rich 10, but not 11, between the 2 NDI cores.
Apart from introducing a template molecule, changing the concentration of the DCL solution can also alter the library distribution. Toward this end, DCLs of equimolar mixtures of 1 and 2 at different total concentrations were prepared. As expected, at higher concentrations, higher oligomers are favored over lower oligomers so the proportion of the monomeric 3 decreases while that of the tetrameric 9 increases. At total building-block concentrations of 2 mM, there is hardly any 9 detected while the amount of this compound increases from 5 mM to 10 mM. Solubility limitations prevented the exploration of higher concentrations.
Interactions between the hydrophobic surfaces of NDI and DN should be stronger in a solvent of higher ionic strength, and more hydrophobic surface should be buried in the compact [2]-catenane 9 than in the donor-acceptor dimer 5, providing another way to manipulate the DCL equilibrium position (32, 33). A new set of DCLs was prepared at 5 mM in the presence of NaNO3 (Fig. 5). Indeed the salt has a significant impact on the amount of 9 in the DCL, with the largest amplification of approximately 6-fold observed at 1 M NaNO3 (similar results have been obtained using NaCl, KNO3, Na2SO4, and K2SO4, see SI). Amplification of 9 is largely at the expense of 5, which was reduced by approximately 4 times while the proportion of monomer 3 also decreases, by a factor of approximately 1.5.
Binding of 10 to the [2]-catenane 9.
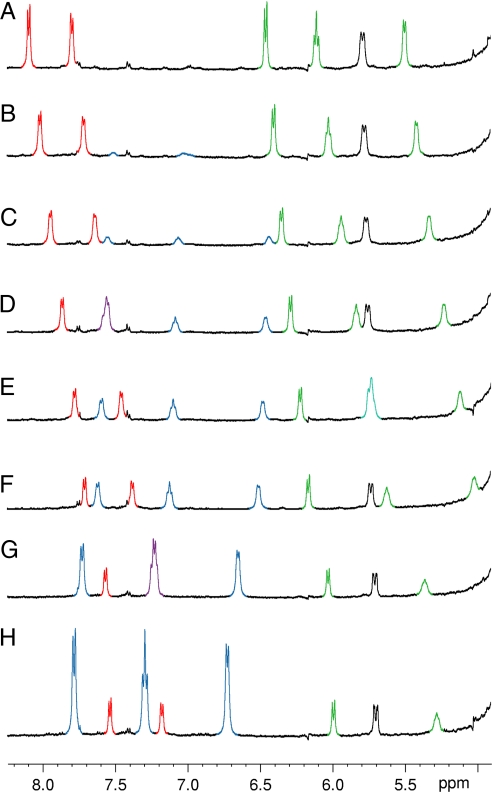
Upon titrating 10 (up to 3 equiv.) to a sample of 9, significant upfield shifts were observed for all of the aromatic protons of the catenane: 0.60 and 0.62 ppm shifts were found for the 2 NDI signals, while 0.47, 0.83, and >0.63 ppm shifts were observed for the DN protons (Fig. 6). Downfield shifts of approximately 0.3 ppm of the aromatic proton signals from the guest were also observed with increasing number of equivalents of 10 as the proportion of the bound guest decreases; this again is consistent with intercalation of the guest between aromatic rings of the host. An association constant of 7,700 ± 1,300 M–1 was estimated by monitoring the NDI resonances. Less precise association constants, but with the same order of magnitude, can be calculated by monitoring the DN signals of 9. The estimated binding strength between 9 and 10 is consistent with the modest amplification observed for 9 at 5 mM.
Fig. 6.
Partial 1H NMR spectra (D2O, 300 K, 500 MHz) of the [2]-catenane 9 in the presence of 0 (A); 0.2 (B); 0.4 (C); 0.6 (D); 0.8 (E); 1.0 (F); 2.0 (G); and 3.0 equiv. of 10 (H). Signals from NDI and DN core of 9 and the DN core of 10 are highlighted with red, green, and blue, respectively.
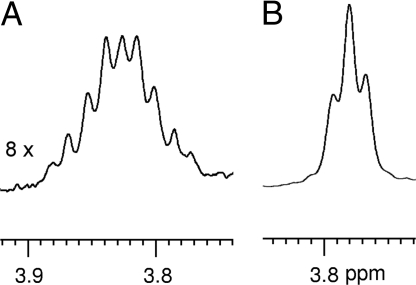
On the binding of 10 to the host 9, the protons of one of the methylene groups become diastereotopic (Fig. 7), indicative of the chiral environment (34). Evidence of the geometrical relationship between 9 and 10 comes from nOe experiments carried out on a sample of 10 and 9 in 3:1 molar ratio. Irradiation of the NDI protons shows close contacts with both the DN protons on the catenane and of the guest (Fig. 8). In contrast, irradiation of the DN protons of either 9 or 10 shows cross magnetization due to nOe only with the NDI protons of 9. These findings not only confirm the binding of 10 to 9, but also confirm the presence of conformation I in the complexed catenane as the aromatic plane of 10 is only in close proximity with the NDI moieties of 9 (molecular modeling at semiempirical levels supports this model, see SI). In contrast, no nOe was observed between protons of the complementary aromatic units in uncomplexed 9, suggesting the presence of a larger cavity due to the flexible linkages of the compound. Addition of 11 to an aqueous sample of 9 induces shifts in aromatic signals of both the guest and the catenane, but no nOe was observed between the aromatic protons of 9 and 11, indicating a different binding mode to that of 10 to the catenane.
Fig. 7.
Resonance of one of the methylene groups of the side chain of 10 when complexed by (A) 1.25 and (B) 0.33 equiv. of 9.
Fig. 8.
1D NOE spectra (D2O, 300 K, 500 MHz, mixing time = 1.2 s) of complexed 9 in the presence of 3 equiv. of 10 (top 3) and the reference spectrum (bottom). Irradiation (marked with an arrow) of DN signal of 9 (A); DN signal of 10 (B); and NDI signal of 9 (C). Signals from NDI and DN core of 9 and the NDI core of 10 are shaded with red, green, and blue in the reference spectrum, respectively.
Conclusions
In summary, we have identified an unusual donor-acceptor [2]-catenane from an aqueous DCL. The use of only acyclic components allows the first construction of a [2]-catenane that contains both donor and acceptor units in the same ring. The yield of the [2]-catenane depends on the library conditions: changing the concentration or ionic strength, the addition of an external template increases the yield of the interlocked compound. It is also found that a cationic, electron-rich molecule is intercalated between 2 electron-deficient moieties of the [2]-catenane, creating a supramolecular assembly featuring 5 alternating π-donor and π-acceptor units.
By appropriate modification of the cationic template, it is expected that supramolecular assemblies with more complex topology could be efficiently constructed using this dynamic combinatorial approach.
Materials and Methods
Chemicals were purchased from commercial suppliers and used as received. Water and MeOH for LC-MS were purchased from Romil or Rathburn. HPLC/LC-MS was performed on HP 1050 or Agilent 1100 LC/MSD trap XCT systems coupled to a diode array detector and the data processed using ChemStation software. Mass spectra (negative mode) were acquired in ultra scan mode using drying temperature of 325 °C, nebulizer pressure of 55 psi, drying gas flow of 10 L/min, capillary voltage of 4,000 V, an ICC target of 200,000 ions, and target mass of 1,000. Analytical separations were achieved by injecting 5 μL (for 5 mM DCL, scaled accordingly for DCL at different concentrations) of DCL solution onto a Symmetry C8 reverse phase column (150 × 4.6 cm, 3 μm particle size) with an isocratic elution of 58% MeOH in water (with 0.1% formic acid) at room temperature and a flow rate of 1 mL/min. Preparative separation was performed on a SymmetryPrep C18 column (300 × 7.8 mm, 7 μm particle size). Elution was performed using the same solvent system at 30 °C at a flow rate of 3 mL/min. UV–Vis spectra were recorded using Cary 400 UV Spectrometer at room temperature. 1H and 13C NMR spectra were recorded on Bruker DPX-400 or Advance 500 TCI Cryo Spectrometers and internally referenced to solvent residue.
A typical analytical DCL was prepared on a 1-ml scale by dissolving an equimolar mixture of 1 and 2 in 10 mM aqueous NaOH, followed by titration with 100 mM aqueous NaOH to pH 8, to the desired concentration. Where appropriate, alkali metal salts, guests 10 or 11, were added in solid form. The DCL was stirred in a close-capped vial at room temperature until being analyzed. Preparative DCL was prepared in the same manner on a 10-ml scale. For further experimental details, see SI.
Supplementary Material
Acknowledgments.
We thank the Croucher Foundation, Pembroke College, and the Engineering and Physical Sciences Research Council for financial support, and Dr. Ana Belenguer for maintaining the chromatography laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809934106/DCSupplemental.
References
- 1.Sauvage J–P, Dietrich–Buchecker C. Molecular catenanes, Rotaxanes and Knots: A Journey Through the World of Molecular Topology. Weinheim, Germany: Wiley; 1999. [Google Scholar]
- 2.Stoddart JF, Colquhoun HM. Big and little Meccano. Tetrahedron. 2008;64:8231–8263. [Google Scholar]
- 3.Kay ER, Leigh DA. Beyond switches: Rotaxane- and catenane-based synthetic molecular motors. Pure Appl Chem. 2008;80:17–29. [Google Scholar]
- 4.Ikeda T, Stoddart JF. Electrochromic materials using mechanically interlocked molecules. Sci Technol Adv Mater. 2008;9 doi: 10.1088/1468-6996/9/1/014104. 014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich–Buchecker C, Jimenez–Molero MC, Sartor V, Sauvage J–P. Rotaxanes and catenanes as prototypes of molecular machines and motors. Pure Appl Chem. 2003;75:1383–1393. [Google Scholar]
- 6.Megiatto JD, Jr., Schuster DI. General method for synthesis of functionalized macrocycles and catenanes utilizing “Click” chemistry. J Am Chem Soc. 2008;130:12872–12873. doi: 10.1021/ja8050519. [DOI] [PubMed] [Google Scholar]
- 7.Coskun A, Saha S, Aprahamian I, Stoddart JF. A reverse donor-acceptor bistable [2]catenane. Org Lett. 2008;10:3187–3190. doi: 10.1021/ol800931z. [DOI] [PubMed] [Google Scholar]
- 8.Aprahamian I, et al. Clicked interlocked molecules. Bull Chem Soc Jpn. 2007;80:1856–1869. [Google Scholar]
- 9.Dichtel WR, et al. Kinetic and thermodynamic approaches for the efficient formation of mechanical bonds. Acc Chem Res. 2008;41:1750–1761. doi: 10.1021/ar800067h. [DOI] [PubMed] [Google Scholar]
- 10.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Dynamic covalent chemistry. Angew Chem Int Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Goldup SM, Leigh DA, Lusby PJ, McBurney RT, Slawin AMZ. Gold(I)-template catenane and rotaxane synthesis. Angew Chem Int Ed. 2008;47:6999–7003. doi: 10.1002/anie.200801904. [DOI] [PubMed] [Google Scholar]
- 12.Westcott A, Fisher J, Harding LP, Rizkallah P, Hardie MJ. Self-assembly of a 3-D triply interlocked chiral [2]catenane. J Am Chem Soc. 2008;130:2950–2951. doi: 10.1021/ja8002149. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Bruneau A, He J, Abliz Z. Palladium(II)-directed self-assembly of dynamic donor-acceptor [2]catenanes. Org Lett. 2008;10:765–768. doi: 10.1021/ol702830p. [DOI] [PubMed] [Google Scholar]
- 14.Ratel K, Miljanić OŠ, Stoddard JF. Iodide-catalysed self-assembly of donor–acceptor [3]catenanes. Chem Commun. 2008:1853–1855. doi: 10.1039/b716245f. [DOI] [PubMed] [Google Scholar]
- 15.Miljanić OŠ, Stoddard JF. Dynamic donor–acceptor [2]catenanes. Proc Natl Acad Sci USA. 2007;104:12966–12970. doi: 10.1073/pnas.0704136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanco V, Chas M, Abella D, Peinador C, Quintela JM. Molecular catenation via metal-directed self-assembly and π-donor/π-acceptor interactions: Efficient one-pot synthesis, characterization, and crystal structures of [3]catenanes based on Pd or Pt dinuclear metallocycles. J Am Chem Soc. 2007;129:13978–13986. doi: 10.1021/ja074721a. [DOI] [PubMed] [Google Scholar]
- 17.Chas M, Blanco V, Peinador C, Quintela JM. Synthesis of [3]catenanes nased on metal-directed self-assembly and π-donor/π-acceptor interactions. Org Lett. 2007;9:675–678. doi: 10.1021/ol062824c. [DOI] [PubMed] [Google Scholar]
- 18.Blight BA, Wisner JA, Jennings MC. Reversible formation of a [2]catenane through first- and second-sphere coordination. Angew Chem Int Ed. 2007;46:2835–2838. doi: 10.1002/anie.200604724. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, et al. Dynamic [2]catenanes based on a hydrogen bonding-mediated Bis-Zinc prophyrin foldamer tweezer: A case study. J Org Chem. 2007;72:2897–2905. doi: 10.1021/jo062523g. [DOI] [PubMed] [Google Scholar]
- 20.West KR, et al. Dynamic combinatorial discovery of a [2]-catenane and its guest-induced conversion into a molecular square host. J Am Chem Soc. 2008;130:10834–10835. doi: 10.1021/ja801508q. [DOI] [PubMed] [Google Scholar]
- 21.Lam RTS, et al. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science. 2005;308:667–669. doi: 10.1126/science.1109999. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths KE, Stoddart JF. Template-directed synthesis of donor/acceptor [2]catenanes and [2]rotaxanes. Pure Appl Chem. 2008;80:485–506. [Google Scholar]
- 23.Raehm L, Hamilton DG, Sanders JKM. From kinetic to thermodynamic assembly of catenanes: Error checking, supramolecular protection and oligocatenanes. Synlett. 2002;11:1743–1761. [Google Scholar]
- 24.Try AC, Harding MM, Hamilton DG, Sanders JKM. Reversible five-component assembly of a [2]catenane from a chiral metallomacrocycle and a dinaphtho-crown ether. Chem Commun. 1998:723–724. [Google Scholar]
- 25.Hamilton DG, Feeder N, Teat SJ, Sanders JKM. Reversible synthesis of π-associated [2]catenanes by ring-closing metathesis: Towards dynamic combinatorial libraries of catenanes. New J Chem. 1998;22:1019–1021. [Google Scholar]
- 26.Au–Yeung HY, Pengo P, Pantoş GD, Otto S, Sanders JKM. Templated amplification of a naphthalenediimide-based receptor from a donor-acceptor dynamic combinatorial library in water. Chem Commun. 2009:419–421. doi: 10.1039/b816979a. [DOI] [PubMed] [Google Scholar]
- 27.Corbett PT, et al. Dynamic combinatorial chemistry. Chem Rev. 2006;106:3652–3711. doi: 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen S–A, Gates PJ, Cousins GRL, Sanders JKM. Electrospray ionisation Fourier-transform ion cyclotron resonance mass spectrometry of dynamic combinatorial libraries. Rapid Communs Mass Spectrom. 2000;14:44–48. doi: 10.1002/(SICI)1097-0231(20000115)14:1<44::AID-RCM832>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, West KR, Bondy CR, Sanders JKM. Dynamic combinatorial libraries of hydrazone-linked pseudo-peptides: Dependence of diversity on building block structure and chirality. Org Biomol Chem. 2007;5:778–786. doi: 10.1039/b617217b. [DOI] [PubMed] [Google Scholar]
- 30.Bäuerle P, et al. Oligothiophene-based catenanes: Synthesis and electronic properties of a novel conjugated topological structure. Angew Chem Int Ed. 2007;46:363–368. doi: 10.1002/anie.200602652. [DOI] [PubMed] [Google Scholar]
- 31.Hunter CA, Sanders JKM. The nature of π-π interactions. J Am Chem Soc. 1990;112:5525–5534. [Google Scholar]
- 32.Fujita M, Ibukuro F, Hagihara H, Ogura K. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature. 1994;367:720–723. [Google Scholar]
- 33.Fujita M, Ibukuro F, Yamaguchi K, Ogura K. A molecular lock. J Am Chem Soc. 1995;117:4175–4176. [Google Scholar]
- 34.Cousins GRL, Furlan RLE, Ng Y–F, Redman JE, Sanders JKM. Identification and isolation of a receptor for N-methyl alkylammonium salts: Molecular amplification in a pseudo-peptide dynamic combinatorial library. Angew Chem Int Ed. 2001;40:423–428. doi: 10.1002/1521-3773(20010119)40:2<423::AID-ANIE423>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.