Abstract
Adaptive behaviors are guided by motivation and memory. Motivational states specify goals, and memory can inform motivated behavior by providing detailed records of past experiences when goals were obtained. These 2 fundamental processes interact to guide animals to biologically relevant targets, but the neuronal mechanisms that integrate them remain unknown. To investigate these mechanisms, we recorded unit activity from the same population of hippocampal neurons as rats performed identical tasks while either food or water deprived. We compared the influence of motivational state (hunger and thirst), memory demand, and spatial behavior in 2 tasks: hippocampus-dependent contextual memory retrieval and hippocampus-independent random foraging. We found that: (i) hippocampal coding was most strongly influenced by motivational state during contextual memory retrieval, when motivational cues were required to select among remembered, goal-directed actions in the same places; (ii) the same neuronal populations were relatively unaffected by motivational state during random foraging, when hunger and thirst were incidental to behavior, and signals derived from deprivation states thus informed, but did not determine, hippocampal coding; and (iii) “prospective coding” in the contextual retrieval task was not influenced by allocentric spatial trajectory, but rather by the animal's deprivation state and the associated, non-spatial target, suggesting that hippocampal coding includes a wide range of predictive associations. The results show that beyond coding spatiotemporal context, hippocampal representations encode the relationships between internal states, the external environment, and action to provide a mechanism by which motivation and memory are coordinated to guide behavior.
Keywords: hippocampus, memory, neuronal coding
Episodic memory provides a record of past experience and is structured by spatial, temporal, and personal contexts (1), multiple frames of reference that organize the features of events and provide global categories for information storage and retrieval. Motivational states arising from interoceptive cues provide an internal context that modulates the relative significance, meaning, or organization of events in memory (2, 3). Thus, motivational states such as hunger and thirst define internal, contextual cues that can specify behavioral goals and inform memory retrieval.
The hippocampus is required for episodic memory in humans (4, 5) and episodic-like memory in other animals (6). Hippocampal neurons typically fire in place fields, local regions of an environment that selectively elicit significant activity (7). Place field properties suggest that the hippocampus helps code the animal's location within spatial contexts (7–11). Place fields are modulated by the behavioral, cognitive, and mnemonic demands of spatial tasks, suggesting that hippocampal codes support memory guided spatial navigation (12–17). In non-spatial memory tasks, hippocampal activity is also modulated by salient perceptual and cognitive task features independent of location, implying that the neurons may provide a global memory signal (17–22). Indeed, hippocampal neurons respond to organizing features of experience other than spatial context by distinguishing events that occur in the same place at different times (23) or in association with different emotional significance (24, 35).
The hippocampus may support episodic memory by encoding organized associations among the multimodal elements that characterize an experience. Such coding exemplifies a content-addressable memory system that allows any subset of event features to help reconstruct memories that contain those features (25–27). From this view, each feature provides a potential retrieval cue for events that contain it. A particular fragrance, for example, can evoke specific and detailed associative memories. The neuronal mechanisms that support such memory reconstruction have not been tested outside of the spatial domain in rats, and the mechanisms by which internal motivational signals may elicit memory retrieval to guide goal-directed behaviors remain largely unknown. We now report that different motivational states elicit distinct hippocampal codes in identical places. The codes distinguished competing goal-directed actions, reflected both internal and external stimuli, and signaled non-spatial targets. The results demonstrate a neuronal mechanism by which different motivational states can influence memory processes and help select among adaptive behaviors based on past experience.
Results
To test the interactions between motivational state and memory demand, rats were trained in a non-spatial, hippocampus-dependent, contextual memory retrieval (CR) task. Rats deprived of food or water on alternate days were trained to approach 1 of 3 visually distinct goal boxes to obtain reward in a trident maze (Fig. 1). For each rat, 1 goal box was paired consistently with food, another with water, and a third was never rewarded (e.g., food in the black box, water in the white box, nothing in the lit box). The presence of reward in a given goal box was contingent on the rat's current deprivation state so that only the goal box paired with the appropriate item contained reward (food or water) on a given trial. To prevent rats from adopting allocentric or egocentric spatial response strategies, the goal boxes were moved pseudorandomly among 3 possible locations at the start of each trial. Thus, the animals learned to differentially approach and avoid equally reinforced goal objects in an identical external environment depending on their current deprivation state. The task required the rats to use motivational states as discriminative cues to retrieve learned associations between specific rewards and non-spatial targets. Both radiofrequency fornix and excitotoxic hippocampal lesions severely impaired memory performance in this task, but left intact non-specific stimulus-reward associative learning as well as internal and external cue discrimination (3).
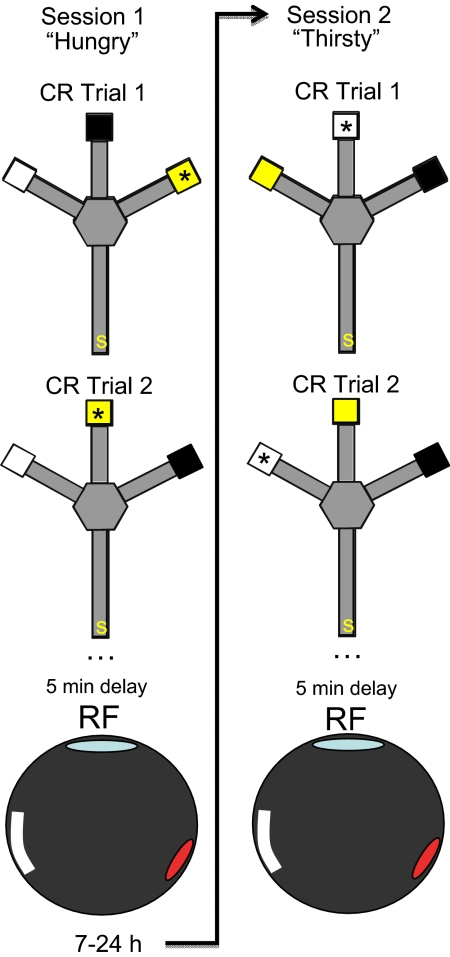
Fig. 1.
Apparatus and design. In the CR task each rat was placed on the start arm (s) of a trident maze and was required to approach a specific, visually distinct goal box (*) to obtain the reward relevant to its current deprivation state. In this figure, a “hungry” rat could find food in session 1 by approaching the yellow box. Because the boxes were moved among the 3 goal arms after each trial, the spatial location of the box was irrelevant to performance. Unit activity was recorded as the rat performed the CR task for 25–35 trials (≈50 min). Five to 10 min after the CR session, an open box was placed on the Trident maze, and the same population of neurons was recorded as the same “hungry” rat foraged for randomly scattered chocolate sprinkles (RF: 8 min). The rat was returned to the vivarium (7–24 h) and the alternate deprivation state was induced (arrow). Session 2 followed the same procedure, but the now “thirsty” rat could find water by approaching the white goal box in the CR task, and find randomly scattered chocolate sprinkles in the RF task. Thus, the only difference between RF sessions was deprivation state. A complete experimental session had 4 recording sessions (2 states and tasks).
To assess hippocampal coding crucial for memory performance, the activities of 2 to 17 well-isolated principal neurons from the CA1 layer (100–370 μV) were recorded simultaneously as 6 rats performed the non-spatial CR task, yielding a total of 9 experimental sessions. Histology and EEG (ripple, sharp waves, and theta rhythm) (28, 29) verified that the electrodes entered the CA1 cell layer and the recorded complex-spike (CS) units were CA1 neurons. A complete experimental session included recording during contextual memory retrieval followed immediately by recording during random foraging for each deprivation condition (Fig. 1). The random foraging (RF) session assessed the extent to which hippocampal activity was altered by motivational states that were irrelevant to contingent memory retrieval. Rats were strongly motivated throughout the CR and RF sessions, as demonstrated by the immediate approach and consumption of the deprived substance when it was made available after recording sessions.
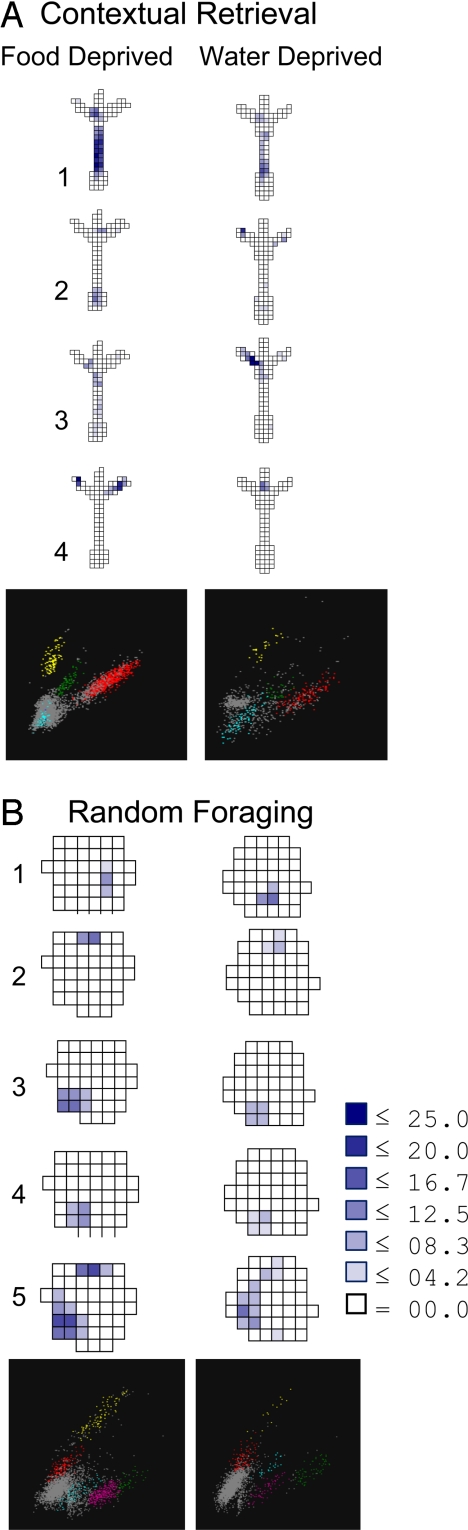
Stable unit activity was recorded across deprivation conditions. To ensure that activity from the same population of neurons was compared across deprivation conditions, only probes with stable recordings throughout an experimental session were included in the analyses (Fig. 2). Units were isolated independently in recordings obtained from CR and RF sessions, and relative cluster boundaries and measures of waveform similarity were used to identify neurons active in both deprivation conditions and tasks. The average waveforms of each isolated unit on a tetrode were compared across session pairs (e.g., CR food versus CR water deprivation) using Pearson's r and the Mahalanobis distance of waveform parameters. Waveforms identified as belonging to the same neuron were most highly correlated across deprivation conditions compared with other waveforms (CR, r ≥ 0.95 and RF, r ≥ 0.95), and this measure of waveform stability was equivalent in the 2 tasks (CR vs. RF, r values converted to Fisher Z scores and evaluated with a t test, t = 0.3, P = 0.7).
Fig. 2.
Firing rate maps and cluster projections recorded from the same tetrode for interleaved CR and RF test sessions. Recordings were stable across deprivation conditions in both the CR (A) and RF (B) tasks. Rate maps compare the spatial firing patterns of the same CA1 neurons matched across deprivation conditions in the CR (4 neurons) and RF (5 neurons) tasks. The legend shows firing rate in each pixel (spikes/s). (A) Most units fired in different patterns across deprivation conditions in the CR task. Some cells fired in the same locations but at different rates (cells 1 and 3), others fired in different locations (cells 2 and 4), and others fired exclusively in one condition (see Fig. 4.). (B) Most neurons fired in stable patterns across deprivation conditions in the RF task (cells 3–5), while others fired in different locations (cell 1) or in the same location at different rates (cell 2). Only 1 neuron recorded during RF fired exclusively in 1 condition. The cluster plots show the waveform peaks on 2 of the tetrode wires (max x, y = 254 μV).
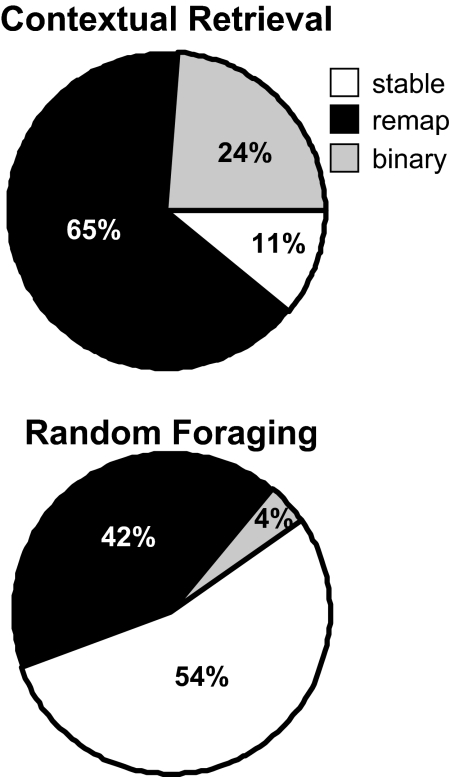
Hippocampal unit activity strongly distinguished the motivation-based memory retrieval conditions despite identical spatial behaviors in a constant spatial environment. The activities of 63 CS neurons with distinct, stable waveforms and well-defined place fields on the maze were compared across deprivation conditions in the CR task. Direction, running speed, and visits were compared across all session pairs using paired t-tests, and data obtained from pairs that differed significantly on any of these behavioral measures were eliminated from subsequent analyses, yielding a total of 46 cells and 67 subfields. Eleven of these 46 (24%) neurons had binary responses (on vs. off) between deprivation conditions, and were active and had a well-defined firing field in only 1 of the task conditions. The remaining 35/46 (76%) neurons fired in both hunger and thirst conditions. Thirty (65%) remapped across deprivation conditions and fired either at different rates, in different locations or both as revealed by t-tests and spatial correlations (Pearson's r), while only 5 (11%) neurons had stable firing fields (Fig. 3). The same pattern occurred in subsets of simultaneously recorded groups of cells (Fig. S1 and Table S1).
Fig. 3.
Place fields distinguished deprivation states and task demands. Firing patterns were significantly more distinct across deprivation states in the CR than the RF task [χ2 (1) = 15.48, P < 0.0001].
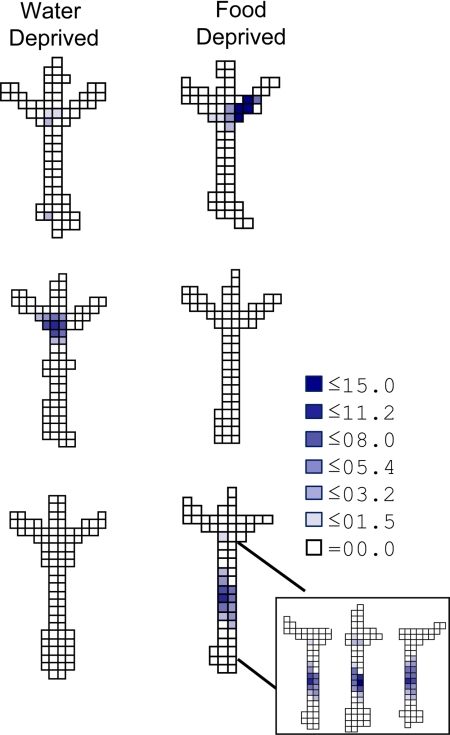
Hippocampal neurons encoded multiple relationships among the salient features of the CR task. To respond correctly, a rat had to discriminate deprivation states, associate the current state with a specific visual cue that was conditionally associated with reward, and direct movement through space toward that goal. Although the start arm, choice point, and goal arms occupied fixed positions, the goal boxes moved among the arms from trial to trial, so that the behavioral significance of arm location varied with the position of the goal boxes. To determine how hippocampal neurons coded these key task components, we compared unit activity in the start arm, the choice point, and the 3 goal arms across CR conditions. CS activity clearly distinguished the conditions in each of these maze regions (Figs. 2 and 4). Most task-sensitive fields (22/54 fields, 41%) coded conjunctions of goal box and location, firing near the end of a specific goal arm only as the rat approached 1 particular goal box. Many other cells fired differently in the choice point (15/54 fields, 28%; Figs. 2 and 4). The remaining task sensitive cells fired differentially in the start arm (17/54 fields, 31%), showing “prospective” coding that distinguished the non-spatial target.
Fig. 4.
Disjunctive coding in the CR task. Firing rate maps for the 3 neurons recorded in the CR task are shown across deprivation conditions. Cells 2 and 3 fired exclusively in 1 deprivation condition, showing binary responses. Cell 1 (200 μV max) coded the conjunction of goal and location, firing only when the “food” goal box occupied the right maze arm. Cell 2 (198 μV) fired in the choice point only during approach to the “water” goal box, while cell 3 (178 μV max) fired in the start arm only during approach to the “food” goal box. (Inset) Firing rate maps obtained for cell 3 after parsing the data file into right-, middle-, and left-going trajectories. Cells that fired discriminately in the start arm in different deprivation conditions did not fire selectively depending on the trajectory taken by the rat. The legend shows firing rate (spikes/s).
Hippocampal coding in the start arm varied with deprivation state and the associated goal object, but not spatial trajectories. In spatial tasks, hippocampal neurons fire in distinct, reliable sequences that correspond to the future position (“prospective coding”) of the animal, even as the rat traverses identical spatial paths (12, 13, 16, 17, 30). To assess the relative influence of allocentric spatial trajectory and non-spatial goal-directed sequences on hippocampal coding, we parsed data files into left-, middle, and right-going trajectories and compared the activity of neurons with start-arm fields in food and water deprivation sessions. In contrast to previously reported spatial trajectory coding, no cells (0/23) distinguished the allocentric spatial trajectories taken by the rat, whether or not the firing fields distinguished the 2 deprivation conditions. Rather, cells with fields on the start arm either revealed discriminative coding with respect to the defining features of the goal (17/23, 74%; Fig. 4 and Fig. S2) or signaled location independent of motivational state and trajectory (6/23, 26%; Fig. S2). Thus, rather than coding a sequence of spatial locations, the differential firing in the start arm across deprivation conditions was related to the animal's motivational state and approach to a specific non-spatial target, suggesting that the hippocampus codes goal-directed memory sequences in general and not only spatial trajectories.
Hippocampal CS activity was only weakly influenced by deprivation state during RF compared with CR (Fig. 3). CS activity in 26 neurons was compared across deprivation conditions in the RF task excluding session pairs that differed in the animal's running speed or position (eliminating 2 cells). Most cells, (13/24, 54%) had stable firing fields across deprivation conditions, as revealed by spatial correlations and t-tests. Ten (42%) neurons remapped and only 1 (4%) cell fired discordantly as rats foraged while either hungry or thirsty. The distribution of stable, remapped, and discordant fields showed significantly higher place fields stability across deprivations conditions in the RF than in the CR tasks [χ2 (1) = 15.48, P < 0.0001; Fig. 3]. Subsets of simultaneously recorded groups of neurons revealed similar firing patterns across deprivation conditions (Fig. S1). These results verify that CS activity is sensitive to motivational state (internal context), and is consistent with prior studies describing non-spatial contextual coding by hippocampal neurons (23, 24). When motivational state was not required for selective memory retrieval, however, it had relatively minor influence on hippocampal coding.
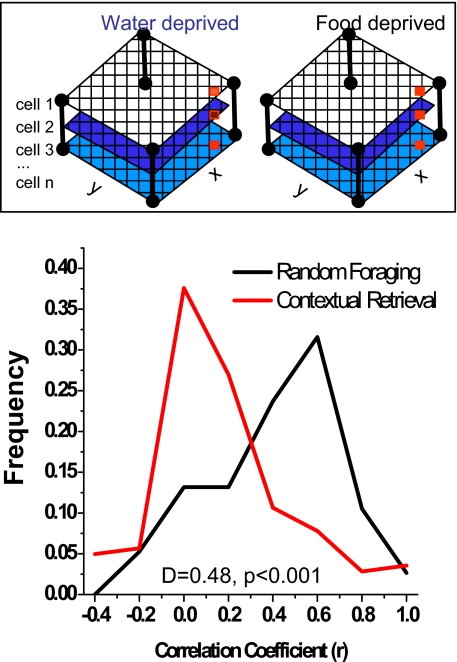
Hippocampal population coding more strongly discriminated memory demands than deprivation states. Population vector correlations (31) further quantified hippocampal coding on the entire dataset across deprivation conditions in the 2 tasks. A population vector was computed for each task and deprivation condition from the mean firing rate of each cell in each grid unit across all ensembles and rats (see Experimental Procedures). Two arrays of population vectors, one recorded during food and the other during water deprivation sessions, were compared using Pearson's r, and the data from CR and RF tasks were analyzed separately. As suggested by the categorical single unit data described above, quantitative differences in the population codes distinguished deprivation conditions markedly in the CR but not in the RF task. The population vectors were largely uncorrelated across deprivation conditions in the CR task (r mode = 0, skewed toward zero; KS test, P < 0.01), but were similar in the random foraging task (r mode = 0.6). The distributions of population vector correlations differed significantly between the 2 tasks (CR versus RF: KS test; D = 0.48, P < 0.001; Fig. 5), showing that the hippocampal population code distinguished between different memory retrieval conditions more markedly than the different deprivation states per se.
Fig. 5.
Population vector correlations across deprivation conditions. Firing rate vectors represented the activity of all cells (1 … n) in each grid location (x, y) and are depicted by the columns of red squares (Inset). Population vectors from each grid location compared food and water deprivation conditions using Pearson's r; the curves show the frequency distributions of these. Higher r values (x axis) show greater similarity in the ensemble code between deprivation conditions. The vectors distinguished deprivation conditions in the CR memory task (r mode = 0, skewed toward zero; KS test, P < 0.01), but were similar in the RF task where no memory discrimination was required (r mode = 0.6; CR versus RF: KS test, D = 0.48, P < 0.001).
Discussion
When motivational states signaled memory discriminations, they were accompanied by distinct CA1 representations. During contextual memory retrieval, most single units either fired at different rates, in different maze locations, or were exclusively active either when the animal was food deprived and approaching a food-associated goal, or water deprived and approaching a water-associated goal. By contrast, the same neuronal populations had largely stable firing patterns during random foraging, when no memory discrimination was required and deprivation state was incidental to ongoing behavior. The coding observed in the CR task converge with the effect of hippocampal lesions in this task, which impair memory processing—selecting among competing goal directed behaviors—but not discriminating motivational states, goal boxes, or stimulus-reward associations (3). Thus, hippocampal networks respond to motivational states as they do to external stimuli: information dynamically influences hippocampal coding, depending upon its relative importance for memory discrimination. These features of hippocampal coding suggest a natural mechanism by which internal states and environmental features can be associated in memory and used selectively to guide goal-directed, adaptive behavior.
Hippocampal neurons code the key features required for motivation-based contextual memory retrieval: the associative reconstruction of specific relationships among the internal and external features that distinguished different trials. Task performance required the rat to retrieve from memory the relationships between specific goals (food or water) and their associated non-spatial targets, and use this information to guide different responses to the same external stimuli. The hippocampal coding patterns we report here correspond well to these task features. Some cells responded to conjunctions of features, firing as the rat approached a particular goal box when it occupied a specific location. Many neurons fired differentially on the common start arm revealing “prospective” coding (12), but rather than coding pending trajectories, the prospective signal depended on whether the animal was hungry and approaching the food-associated goal or thirsty and approaching the water-associated one. The observed firing patterns thus dovetail with the computational requirements for contextual memory retrieval.
Motivational state per se had a small but measurable influence on hippocampal coding. In the random foraging task, when deprivation state was irrelevant to memory discrimination, most cells had stable fields with correlations well within the range of prior reports comparing repeated measures of unit activity in the same task in a constant environment (8, 10, 32–36). Other neurons, however, fired with different rates or in different locations between deprivation conditions. These deprivation-sensitive cells shifted the population vector correlations and demonstrated that motivation-related information is included in the hippocampal representation. Indeed, the present results are similar quantitatively to those reported in a random foraging task following changes from one familiar box shape or color to another in a constant room (31). Thus deprivation state per se may be a perceptual variable, albeit an internal one, that distinguishes different episodes within the same spatial context or reference frame (37–39). Alternatively, deprivation-sensitive place fields may have been established by training in the CR task. The RF box occupied the same location and room as the maze used in CR, and all of the rats were trained extensively to use motivational state as a discriminative memory stimulus before random foraging sessions. This training may have increased the influence of deprivation state on hippocampal coding during subsequent random foraging. Future experiments are needed to determine the extent to which internal state coding is endogenous or a consequence of learning (cf. 16).
Prospective coding signaled motivation-defined goals independent of their location. Hippocampal neurons are sensitive to the history of ongoing behavior, reflecting the past and anticipating future actions (12, 13, 16, 17, 30, 40). Thus, place fields can be modulated by future spatial goals or past locations even as perceptual, behavioral, and motivational variables are constant. Differential firing in the same location has been interpreted as an example of spatial sequence coding (12, 13, 16, 17), and as “journey” coding that reflect the beginning and end of goal-directed episodes (12, 26). Our prior work suggested that prospective coding in a spatial task signaled immediately pending locations (12). The present results suggest that such differential hippocampal coding is not limited to signaling spatiotemporal sequences but is related more generally to behavioral goals. If hippocampal coding discriminates only spatial sequences, then prospective activity in the start arm should vary with the allocentric location of the target box. If such coding discriminates goal-directed sequences more generally, then prospective activity should vary with the defining features of the goal. In the present experiment, the goals were defined by the animal's motivational state and associated boxes that moved from trial to trial, making their non-spatial properties crucial and their location irrelevant. Under these conditions, the deprivation-defined goal strongly altered unit activity, but the allocentric location of the goal box never did, so that differential unit activity in the start arm was indistinguishable during left, right, and middle-going spatial trajectories. Thus, the sequence coding reported in other studies may not reflect spatial trajectories per se, but rather a goal-directed cognitive path through an associative memory space in which allocentric location is incidental.
Experimental Procedures
Subjects.
Six male Long Evans rats (Charles River Laboratories) weighed between 300–350 g at the onset of training, were maintained at 85% of their free-feeding body weight and housed individually on a 12-h light/dark cycle. All experiments and surgical procedures were conducted in accordance with the guidelines set forth by the Mount Sinai Institutional Animal Care and Use Committee and the National Institute of Health.
Apparatus.
A wooden trident maze (Fig. 1), painted gray and elevated 63 cm from the floor, was located in the center of a dimly lit room, with black curtains surrounding 2/3 of the maze to minimize visual access to room cues. Three goal arms (49 cm long, 6.4 cm wide, with edges 2.5 cm high) separated by 45° angles were located directly across a hexagonal central platform (17.8 cm across) from a start arm (85 cm long, 6.4 cm wide, with edges 2.5 cm high). Three interchangeable wooden goal boxes (30 cm high, 15 cm wide and 13.5 cm deep), distinguished by color and illumination, were mounted on the ends of each of the goal arms. A wooden rectangular waiting platform (41 cm long, 30 cm wide, elevated 94 cm from the floor) was located next to the maze.
A square black Plexiglas box (71 cm/side) was elevated 15 cm from the floor, placed in the same room and location as the trident maze, and used for random foraging recording sessions. The floor of the box was covered with black construction paper to reduce light reflection. A black plastic cylinder (38 cm high, 70 cm diameter) with various visual cues taped to its surface was inserted into the box for recording sessions.
Behavioral Training.
Habituation, training, and pre-surgery probe tests were carried out as described previously (3) except that wooden blocks were used in place of guillotine doors to limit access to goal arms. Rats were food and water deprived on alternate days and trained to approach one goal box for food reward when hungry, a second goal box for water reward when thirsty and to avoid a third goal box that was never rewarded. Box-reward pairings were consistent for each rat throughout training and counterbalanced between rats. The presence of reward was contingent on the rat's deprivation state; only the box designated to contain the reward appropriate to the animal's current deprivation state held reward on any given trial, the other 2 goal boxes were empty. The goal boxes were moved pseudorandomly between the 3 possible goal locations after each trial to prevent rats from adopting egocentric or allocentric spatial strategies. Rats were trained for 6 trials/day with 10-min inter-trial intervals until they reached and maintained an 80% correct choice criterion level of performance.
Surgery.
Rats were implanted with recording electrodes after they maintained the 80% correct choice performance for 6 consecutive days (3 days/deprivation state). Rats were anesthetized with isoflurane (Forane, Baxter) and a 14-drive recording assembly (Neuro-hyperdrive; Kopf Instruments) was mounted on the skull over the left dorsal hippocampus (AP −3.8 mm, ML −2.2 mm from Bregma) with dental cement. Twelve of 14 independently movable probes were tetrodes made from 4 twisted wires (Ni-Cr wire, Rediohm-800, 12.7 μm, Kanthal); 2 probes were reference electrodes. After surgery, probes were lowered 1 mm into the brain and rats were injected with Banamine (flunixin meglumine; 1.5 mg/kg, i.m.). All rats were given 8 days recovery following surgery. After the recovery period electrodes were advanced 40–80 μm/day while rats were retrained in the CR task and familiarized with the RF environment.
Recording Equipment.
The electrode assembly was connected to a headstage (Kopf 54, Neuralynx) with source follower amplifiers; 10 color LEDs on the headstage were detected by an overhead color LCD camera and used to track the animal's position and heading (640 × 480 pixel resolution, 60-Hz sampling rate). Electrode signals were amplified 1–6 K, filtered between 0.6 and 6 kHz, digitized at 32 kHz, and stored together with tracking information by computer (Cheetah 64 Data Acquisition System, Neuralynx).
Testing Procedures.
To help ensure stable recordings, the electrodes were not moved for >24 h before recording sessions. Before testing rats were deprived of food for 20 h or water for 16 h and had free access to the non-deprived substance. Recording sessions in the CR task lasted approximately 50 min and included 25–35 trials. At the start of each trial, the rat was moved from the waiting platform to the start arm of the maze. Entering the correct box led to a cup containing the reward appropriate to the rat's current deprivation state (0.2 g powdered rat chow or 4 drops of water). The rat was allowed to consume the reward and was then returned to the waiting platform. If the rat entered an incorrect goal box, it was allowed to inspect the box for 5–10 s and was then returned to the waiting platform. The rat remained on the waiting platform for approximately 1 min between trials while goal boxes were moved pseudorandomly among the 3 goal arms to ensure that each box occupied each location for the same number of trials. After the last CR trial the rat was returned either to its home cage (2 rats) or to the waiting platform where it remained while the trident maze was switched with the open field (4 rats). The CR task was followed immediately by an 8-min RF session. Recording began when the rat was placed in the open field in a constant location and orientation, and continued for 8 min while the rat explored the box to obtain chocolate sprinkles tossed by the experimenter at random intervals and locations. After a recording session the rat was returned to the vivarium and given immediate access to the deprived item; the non-deprived item was removed. Eight to 24 h later, a second recording session assessed unit activity in the alternate deprivation state. For longer intersession intervals, rats were given free access to both food and water for 2 h before the removal of the next deprived item.
Unit Isolation and Screening.
Single units with greater than or equal to 2:1 signal-to-noise ratios and peaks >100 μV were discriminated offline using digitized waveform parameters (e.g., peak amplitudes, spike widths, etc.). A computer program displayed waveforms and represented parameter values as points in a multidimensional space, with each dimension defined by one parameter. Points were assigned to distinct and homogenous clusters (units) using a non-linear elliptical cluster-cutting algorithm (12). The clusters were adjusted and the waveforms were inspected to ensure that the units were discriminated appropriately. The discrimination was further quantified by Mahalanobis distance between clusters in a normalized parameter space. Any pair of clusters <2 standard deviations apart was flagged, and only units with minimal overlap with neighboring clusters and noise were included in subsequent analyses. Units recorded in each session were discriminated independently of other sessions. Only probes with stable recordings throughout an experimental session were included in the final dataset.
Recording stability across consecutive food and water deprivation sessions was assessed by comparing waveforms and cluster distributions qualitatively and quantitatively. Units identified in consecutive food and water deprivation recording sessions were compared visually and matched based on waveform similarity, and cluster location, size, and boundaries. Waveform stability across sessions was quantified by extracting peak and trough amplitudes (32 measures total) from the averaged waveform recorded on each of the 4 wires of a given tetrode that were compared by Pearson's r. The same procedure was used for CR and RF sessions.
Field Analysis.
To define place fields, the camera pixels that defined the maze area was divided into grid units to ensure reliable and adequate sampling. For all recordings firing rates were calculated only if the rat was moving faster than 2 cm/s and obtained by dividing the total number of spikes by the total amount of time spent in each grid unit. Only cells with a mean firing rate greater than or equal to 1 spike/s and only those grid units occupied for greater than or equal to 300 ms and visited at least 4 times in each session of a pair (i.e., food and water deprivation) were analyzed for place field activity. Spatial behavior was quantified using paired t tests to assess whether speed, direction, or visit arrays differed in session pairs. A significant difference (P < 0.05) on any of the behavioral parameters excluded the associated unit data from further analyses. A place field was defined as an area of greater than or equal to 2 adjacent grid units with mean firing rate >1 spike/s and greater than or equal to 3 spikes/subfield visit. Analyses excluded activity in the goal box, and only cells with greater than or equal to 1 place field in at least one of the complimentary recording sessions were compared between sessions. Differences in place field activity were quantified using Student's t tests to assess firing rate means and Pearson's r to assess firing rate spatial distributions. The CR maze area was divided into a 40 × 40 array of 5.6-cm2 grid units. This relatively fine array was used to analyze activity across the width of the maze arms, but the granularity of the spatial grid did not affect the results (SI Text). The spatial distribution of unit activity was analyzed for place fields in each maze arm and the choice point, and firing rates along the length and width of a maze arm were compared across corresponding grid units between session pairs. Activity was assessed separately in specific maze regions and in the entire maze. The RF arena was divided into a 30 × 30 array of 8.7-cm2 grid units, and spatial behavior was quantified across an entire recording session.
Population Vector Analysis.
Firing rate maps were constructed from each cell's mean firing rate in each visited grid unit. Separate 3D arrays (grid location x, y, celln) were defined for each deprivation condition and task, and rate vectors for each grid unit were extracted from each array. Pearson's r compared the rate vectors across deprivation states for each grid unit that contained neuronal activity in at least 1 of the 2 deprivation states. To quantify population coding stability in the 2 tasks, the distribution of the r values from all grid units were assessed with Kolmogorov-Smirnov (KS) goodness of fit tests.
Histology.
Rats were perfused with PBS followed by 4% paraformaldehyde. Brains were cryoprotected in 10% sucrose in 0.1 M PB for 1 day, 20% sucrose in 0.1 M PB for a second day, then blocked and sectioned at 40 μm on a cryostat and stained with formol-thionin (Fig. S3).
Supplementary Material
Acknowledgments.
We thank Janina Ferbinteanu, Prasad Shirvalkar, Erin Rich, Jake Young, Amir Bahar and Justin Riceberg for helpful comments; Maojuan Zhuang for technical work, and Anna Balk for programming. This work was supported by National Institutes of Health Grants MH65658 and MH073689 and the Mount Sinai School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903259106/DCSupplemental.
References
- 1.Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 6.Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- 7.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 8.Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 10.Jeffery KJ, Anderson MI. Dissociation of the geometric and contextual influences on place cells. Hippocampus. 2003;13:868–872. doi: 10.1002/hipo.10162. [DOI] [PubMed] [Google Scholar]
- 11.O'Keefe J, Conway DH. Hippocampal place units in the freely moving rat: Why they fire where they fire. Exp Brain Res. 1978;31:573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- 12.Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- 13.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 14.Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;27:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markus EJ, et al. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DM, Mizumori SJY. Learning-related development of context-specific neuronal responses to places and events: The hippocampal role in context processing. J Neurosci. 2006;26:3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 18.Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: Evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- 19.Weiss C, Kronforst-Collins MA, Disterhoft JF. Activity of hippocampal pyramidal neurons during trace eyeblink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Wible CG, et al. Mnemonic correlates of unit activity in the hippocampus. Brain Res. 1986;399:97–110. doi: 10.1016/0006-8993(86)90604-9. [DOI] [PubMed] [Google Scholar]
- 21.Wiener SI, Paul CA, Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. J Neurosci. 1989;9:2737–2763. doi: 10.1523/JNEUROSCI.09-08-02737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young BJ, Fox GD, Eichenbaum H. Correlates of hippocampal complex-spike cell activity in rats performing a nonspatial radial maze task. J Neurosci. 1994;14:6553–6563. doi: 10.1523/JNEUROSCI.14-11-06553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Putting fear in its place: remapping of hippocampal place cells during fear conditioning. J Neurosci. 2004;24:7015–7023. doi: 10.1523/JNEUROSCI.5492-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen NJ, Poldrack RA, Eichenbaum H. Memory for items and memory for relations in the procedural/declarative memory framework. Memory. 1997;5:131–178. doi: 10.1080/741941149. [DOI] [PubMed] [Google Scholar]
- 26.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 27.Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 29.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 30.Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leutgeb S, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. J Neurosci. 2003;23:8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeffery KJ, Gilbert A, Burton S, Strudwick A. Preserved performance in a hippocampal-dependent spatial task despite complete place cell remapping. Hippocampus. 2003;13:175–189. doi: 10.1002/hipo.10047. [DOI] [PubMed] [Google Scholar]
- 34.Lenck-Santini PP, Rivard B, Muller RU, Poucet B. Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus. 2005;15:356–369. doi: 10.1002/hipo.20060. [DOI] [PubMed] [Google Scholar]
- 35.Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro ML, et al. Intrahippocampal grafts of fetal basal forebrain tissue alter place fields in the hippocampus of rats with fimbria-fornix lesions. Neuroscience. 1989;32:1–18. doi: 10.1016/0306-4522(89)90103-6. [DOI] [PubMed] [Google Scholar]
- 37.Gothard KM, Hoffman KL, Battaglia FP, McNaughton BL. Dentate gyrus and ca1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J Neurosci. 2001;21:7284–7292. doi: 10.1523/JNEUROSCI.21-18-07284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leutgeb S, Leutgeb JK, Moser MB, Moser EI. Place cells, spatial maps, and the population code for memory. Curr Opin Neurobiol. 2005;15:738–746. doi: 10.1016/j.conb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Hampson RE, Deadwyler SA. Ensemble codes involving hippocampal neurons are at risk during delayed performance tests. Proc Natl Acad Sci USA. 1996;93:13487–13493. doi: 10.1073/pnas.93.24.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.