Abstract
Hypoxia, through the hypoxia-inducible transcription factors HIF-1α and HIF-2α (HIFs), induces angiogenesis by up-regulating a common set of angiogenic cytokines. Unlike HIF-1α, which regulates a unique set of genes, most genes regulated by HIF-2α overlap with those induced by HIF-1α. Thus, the unique contribution of HIF-2α remains largely obscure. By using adenoviral mutant HIF-1α and adenoviral mutant HIF-2α constructs, where the HIFs are transcriptionally active under normoxic conditions, we show that HIF-2α but not HIF-1α regulates adenosine A2A receptor in primary cultures of human lung endothelial cells. Further, siRNA knockdown of HIF-2α completely inhibits hypoxic induction of A2A receptor. Promoter studies show a 2.5-fold induction of luciferase activity with HIF-2α cotransfection. Analysis of the A2A receptor gene promoter revealed a hypoxia-responsive element in the region between −704 and −595 upstream of the transcription start site. By using a ChIP assay, we demonstrate that HIF-2α binding to this region is specific. In addition, we demonstrate that A2A receptor has angiogenic potential, as assessed by increases in cell proliferation, cell migration, and tube formation. Additional data show increased expression of A2A receptor in human lung tumor cancer samples relative to adjacent normal lung tissue. These data also demonstrate that A2A receptor is regulated by hypoxia and HIF-2α in human lung endothelial cells but not in mouse-derived endothelial cells.
Keywords: angiogenesis, lung cancer, hypoxia-inducible factors
Angiogenesis is a highly complex process that plays a central role in the lung, both in normal development and during the evolution of disease states, such as cancer. Hypoxia appears to be a critical factor in both situations, although the signaling events involved are incompletely understood. Hypoxia causes up-regulation of genes largely through stabilization of hypoxia-inducible transcription factors (HIFs). Hypoxia-inducible transcription factors HIF-1α and HIF-2α recognize the same consensus DNA-binding element, and one or both of these can up-regulate a number of genes involved in cell growth, proliferation, glucose metabolism, and angiogenesis (1). These include vascular endothelial growth factor (VEGF) and its receptors, including VEGFR-1 (also called flt-1) and VEGFR-2 (also called KDR or flk-1); glucose transporter 1; and carbonic anhydrase XII. A number of genes, including hexokinase-2, glucose phosphate isomerase, phosphofructokinase, aldolase A, aldolase C, GAPDH, and carbonic anhydrase IX, among others, are uniquely regulated by HIF-1α in almost all cell types. In contrast, HIF-2α regulation of a few unique genes is limited largely to specific cell lines. In most cell types, genes regulated by HIF-2α overlap with those of HIF-1α (2). Thus, the role of HIF-2α appears to be unclear. However, HIF-2α has been linked to poor prognosis in a number of cancer types, but the mechanisms by which it promotes tumor growth and vascularization remain largely obscure (3–9). Recently, interest in establishing a role for HIF-2α independent of HIF-1α has led to identification of genes that are specifically regulated by this transcription factor. For example, HIF-2α regulates expression of the transcription factor Oct-4 in mice (10). In the MCF-7 breast cancer cell line, preferential targets of HIF-2α include insulin-like growth factor-binding protein 3 (IGFBP3), SRY-related HMG-box gene 9 (SOX9), CBP/p300-interacting transactivator, with Glu/ASP-rich C-terminal domain, 2 (CITED2), and a few more marginally regulated genes (11). Erythropoietin (Epo) is regulated largely by HIF-2α in Kelly cells and Hep3B cells (12), in cultured astrocytes from mice (13), and also in the liver of mice (14). In the liver of a knockout model, HIF-2α was also shown to regulate expression of superoxide dismutase 1 (SOD1), SOD2, glutathione peroxidase (GPx), catalase, and frataxin (15, 16). Thus, HIF-2α appears to regulate different genes in different cell types. Although HIF-1α was originally discovered as a regulator of Epo, these recent new studies have indicated that, paradoxically, HIF-2α may be responsible for Epo regulation in some tissues. In addition to causing a release of VEGF that acts on its receptors in endothelial cells, hypoxia also can cause a release of adenosine (17, 18). Adenosine stimulates both proliferation of endothelial cells (19, 20) and expression of VEGF (21). The biological effects of adenosine are mediated through the different adenosine receptor subtypes, A1, A2A, A2B, and A3, which signal mainly through coupling with G proteins. The A1 and A3 adenosine receptors inhibit adenylyl cyclase and are inhibitory G protein (Gi)-coupled, whereas the A2A and A2B receptors are stimulatory G protein (Gs)-coupled. Both the high-affinity A2A and the low-affinity A2B receptors activate adenylyl cyclase, resulting in increases in intracellular cAMP. Among the adenosine receptors, both adenosine A2A and the related adenosine A2B receptors have unique properties. The activated adenosine A2A receptor can protect against tissue injury in heart (22), kidney (23), skin (24), spinal cord (25), vascular smooth muscle (26), and lung (27). Adenosine A2A receptor also can exhibit antiinflammatory properties (28). Endothelial cells can express either A2A receptor or A2B receptor or both (21, 29). In human dermal microvascular endothelial cells, activation of A2B but not A2A receptor promotes angiogenesis (21). By contrast, activation of A2A but not A2B receptor promotes angiogenesis in human umbilical vein endothelial cells and human lung microvascular endothelial cells (HLMVECs) (30). Therefore differential expression and function of adenosine receptor subtypes could contribute to functional heterogeneity in different types of endothelia.
Tumor growth is critically dependent on its complex microenvironment. The coexistence of antitumor immune cells and an immunosuppressive hypoxic tumor microenvironment has increasingly been recognized (31, 32). Interestingly, part of this immunosuppression has been attributed to the presence of adenosine, A2A receptor and HIF-1α (33, 34). The hypoxic tumor microenvironment supports increased stabilization of HIF-1α and/or HIF-2α. Several studies have shown that HIF-2α is associated with increased vascular density in tumors (5, 6). The present study establishes the role of adenosine A2A receptor in promoting angiogenesis under conditions that stabilize HIFs. The results indicate that adenosine A2A receptor, and not the related adenosine A2B receptor, is a unique angiogenic target regulated by hypoxia via HIF-2α, but not HIF-1α.
Results
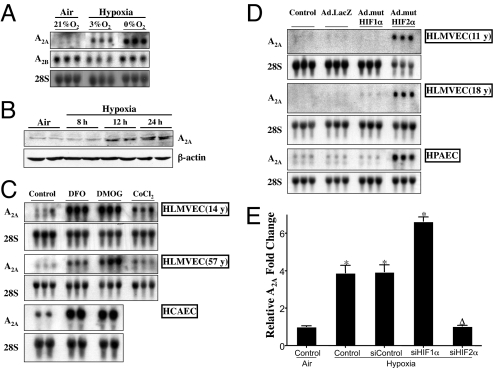
Multiple pathways regulated by hypoxia and HIFs promote angiogenesis. One such pathway involves adenosine receptors. Among adenosine receptors, both A2A and A2B receptors can be regulated by hypoxia. To assess the role of adenosine receptors in hypoxia, primary HLMVECs were exposed to air or hypoxia. Fig. 1A shows that steady-state mRNA levels of adenosine A2A receptor, and not the related adenosine A2B receptor, increased when HLMVECs were exposed to hypoxia. Furthermore, there was also an increase in A2A receptor protein starting at 8 h, when HLMVECs were exposed to hypoxia (Fig. 1B).
Fig. 1.
Effect of hypoxia, HIF stabilizers, and adenoviral HIF-1α and HIF-2α on adenosine A2A receptor steady-state mRNA levels. (A) Primary HLMVECs were exposed to air (21% O2), fetal/physiologic hypoxia (3% O2), or severe hypoxia (0% O2) for 24 h. Total RNA was isolated, and 15 μg were loaded per well. Blots were probed with cDNA for human A2A receptor, A2B receptor, and 28S rRNA, in that order. (B) Primary HLMVECs were exposed to hypoxia for different time periods. Whole-cell lysates were made, and a total of 100 μg was resolved on a 4–15% gel. Blots were probed with adenosine A2A antibody followed by β-actin antibody after stripping the blot. (C) Primary HLMVECs and primary HCAECs from 2 different donors were treated with 200 μM DFO, 1 mM DMOG, or 200 μM CoCl2 for 24 h. Blots were probed with cDNA from human A2A, followed by stripping and then probing with 28S rRNA. (D) Primary HLMVECs and HPAECs were transduced with 15 pfu per cell of either Ad.mutHIF-1α, Ad.mutHIF-2α, or Ad.LacZ. Twenty-four hours after transduction, total RNA was isolated, and 15 μg was loaded per well. Blots were probed with cDNA for human A2A receptor, followed by 28S. The age of the cell donor is indicated in years (y). The order of presentation of the groups shown in A, C, and D has been rearranged from the same original Northern blot. (E) Primary HLMVECs were transfected with siRNA targeted against HIF-1α, HIF-2α, or the nontargeting control. Twenty-four hours after transfection, cells were exposed to severe hypoxia (0% O2) or air (21% O2) for an additional 24 h. After exposure, total RNA was isolated and real-time RT-PCR performed by using Taqman primers and probes for adenosine A2A receptor. ∗, Statistical difference from the control expression in air. Δ, Statistical difference from the Control, siControl, and siHIF-1α samples in hypoxia.
Because hypoxic regulation of a large number of genes is mediated by HIF-1α and HIF-2α, we evaluated the effect of HIF-stabilizing agents desferoxamine (DFO) and dimethyloxalylglycine (DMOG) at concentrations that we have demonstrated previously can stabilize both HIF-1α and HIF-2α (35). Fig. 1C demonstrates that HIF stabilization increased adenosine A2A receptor steady-state mRNA levels. This HIF-mediated regulation of A2A mRNA level was not restricted to one donor or one endothelial cell type, like the HLMVEC, but was also consistently present in a number of donors and endothelial cells from other sources, like the coronary artery. To further dissect the role of individual HIFs in regulating adenosine A2A receptor in primary human endothelial cells, we constructed adenoviral vectors encoding mutant HIF-1α (mutHIF-1α) or mutant HIF-2α (mutHIF-2α). These mutant HIFs are both stable and transcriptionally active under normoxic conditions. Both mutHIF-1α and mutHIF-2α transcriptionally up-regulated VEGF, but only HIF-1α up-regulated hexokinase-2 (SI Methods and Fig. S1). Interestingly, only HIF-2α increased adenosine A2A mRNA in primary endothelial cells derived from lung (HLMVECs and HPAECs; Fig. 1D). This HIF-2α-specific regulation of A2A receptor was reproducible in at least 3 different donors of HLMVECs, of which 2 are shown in Fig. 1D. In addition to microvascular endothelial cells, endothelial cells from the macrovessel also showed similar regulation. The contribution of HIF-1α in up-regulating adenosine A2A receptor mRNA was insignificant (Fig. 1D). To further elucidate the physiological role of HIF-2α in regulating A2A receptor, HLMVECs were transfected with siRNA against HIF-1α, HIF-2α, or the nontargeting control siRNA and were exposed to hypoxia. Again, hypoxia increased A2A receptor expression, and HIF-2α knockdown reversed this change. As expected, the nontargeting controls did not change expression of the receptor under hypoxic conditions, and both HIF-1α and HIF-2α knockdowns decreased VEGF expression (SI Methods and Fig. S2). Interestingly, HIF-1α knockdown increased A2A receptor (Fig. 1E). In all cases, siRNA transfection efficiencies reached ≥95%, as assessed by using siGLO Green (Dharmacon) as an indicator. These findings in primary human-derived endothelial cells were in contrast to those using mouse-derived endothelial cells, SVEC and MB114, where hypoxia or HIF stabilization did not alter the expression of A2A receptor (SI Methods and Fig. S3).
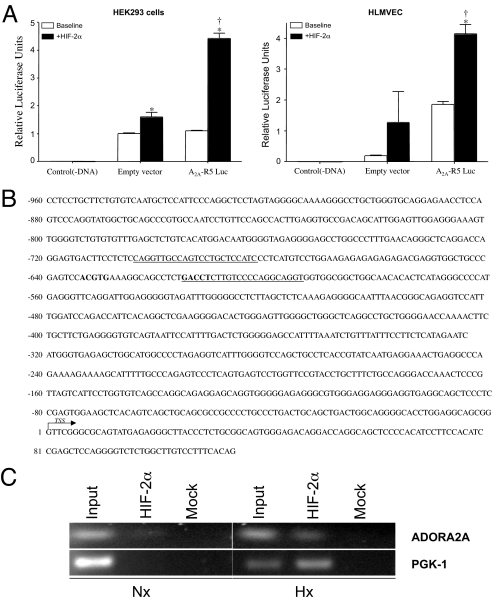
To further evaluate the transcriptional regulation of A2A receptor by HIF-2α, we analyzed the promoter region of this receptor. Earlier bioinformatics analysis of the human A2A receptor gene suggested the presence of multiple promoters (36). Putative promoters upstream of the A2A receptor gene were previously determined as described (37, 38). Among the putative promoters cloned in the luciferase reporter construct, R5 showed consistent induction when cotransfected with the HIF-2α constructs. Both 293 cells and HLMVECs showed a similar increase in luciferase activity when cotransfected with a mutated, constitutively active HIF-2α construct (Fig. 2A). To further examine in vivo association of the endogenously active HIF-2α with hypoxia-responsive elements within the A2A receptor promoter, ChIP assays were performed. Immunoprecipitation of the chromatin complexes formed when HLMVECs were exposed to hypoxia showed significant enrichment of the A2A promoter fragment with the specific HIF-2α antibody compared with the normoxic control or the mock antibody control (Fig. 2C). Similar enrichment of PGK-1 was also observed in HLMVECs under identical conditions and was used as a positive control (Fig. 2C). In Fig. 2B, the primers used in amplifying the hypoxia response element in the R5 promoter are underlined, and the hypoxia response elements are shown in bold.
Fig. 2.
Identification of HIF-2α response element-binding site in the adenosine A2A receptor promoter. (A) Luciferase assay showing A2A receptor promoter activity in HLMVECs and HEK293 cells. ∗, Statistical difference from baseline controls. †, Statistical difference from empty vector activity. (B) Sequence of the A2A receptor promoter showing response elements in bold and primers used for ChIP PCR amplification as underlined. (C) HLMVECs were exposed to air (Nx:21% O2) or hypoxia (Hx:1% O2) for 6 h, after which cells were fixed, chromatin immunoprecipitated with anti-HIF-2α antibody or the control nonspecific antibody, and DNA isolated from the bound complexes. DNA from ChIP was PCR amplified by using specific primers corresponding to the adenosine A2A receptor response element present in the R5 promoter. As a positive control, the PGK-1 hypoxia-inducible factor response element was also PCR-amplified.
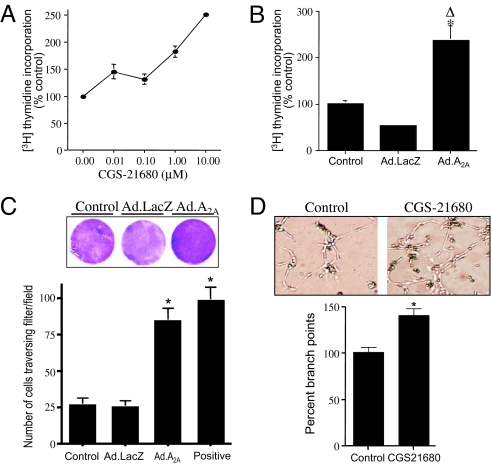
Angiogenesis is a multistep process involving migration, invasion, and cell proliferation, resulting in tube formation and branching. Adenosine A2A receptor can be angiogenic in some cell types (30, 39) but have no effect in other cell types (40). Fig. 3A shows that activation of adenosine A2A receptor increased cellular proliferation in a dose-dependent manner. Because hypoxia and HIF-2α increased A2A receptor expression, we determined whether A2A receptor by itself could alter cellular function. We therefore overexpressed A2A receptor by using an adenoviral vector and measured cellular proliferation, as assessed by [3H]thymidine incorporation. Cellular proliferation increased significantly in the presence of overexpressed A2A receptor compared with control nontransduced cells or the adenovirus LacZ (Ad.LacZ)-transduced cells (Fig. 3B). Because HIF-2α promotes migration of endothelial cells (41), we determined whether adenosine A2A receptor also could increase endothelial cell migration. The increase in migration of HLMVECs across a fibronectin-coated membrane in response to increased A2A receptor expression is shown in Fig. 3C. There was increased migration of cells transduced with Ad.A2A compared with both the Ad.LacZ control and the nontransduced control. Finally, endothelial cells in which adenosine A2A receptor was activated exhibited increased cell sprouting resulting in formation of branches relative to control cells (Fig. 3D). These data demonstrate that adenosine A2A receptor is an angiogenesis-promoting molecule in primary HLMVECs.
Fig. 3.
Adenosine A2A receptor activation promotes cellular proliferation, migration, and angiogenic activity in primary HLMVECs. (A) Serum-starved cells were treated with varying concentrations of the adenosine A2A receptor agonist CGS-21680 for 24 h in the presence of [3H]thymidine at 1 μCi/mL. (B) Cells were transduced with 10 pfu per cell Ad.LacZ or Ad.A2A receptor. Cells were then serum starved for 24 h and incubated with [3H]thymidine at 1 μCi/mL for an additional 24 h. After [3H]thymidine incorporation, cells were washed and incorporated radioactivity determined in cell lysates. ∗, Statistically significant difference from the nontransduced control cells. Δ, Statistical difference from the Lac-Z transduced controls. (C) Nontransduced, Ad.A2A receptor-transduced, or Ad.LacZ-transduced primary HLMVECs (100,000 per well in a 24-well plate) were plated on fibronectin-coated inserts in serum-free medium. Positive represents nontransduced cells incubated in complete EBM-2 medium containing growth factors and serum. After incubation for 24 h, the lower sides of the inserts were stained with crystal violet and scanned for photography (Magnification: 1×). Photographs were taken of randomly selected fields, and the number of cells per field was quantitated. ∗, Statistically significant difference from the nontransduced and Ad.LacZ-transduced control cells. (D) Primary HLMVECs (100,000 per well in a 24-well plate) were plated on Matrigel (BD Biosciences) in the presence or absence of the A2A receptor agonist, CGS-21680 (1 μM). After incubation for 4 h, fields were randomly selected and photographed (Magnification: 10×). Representative picture shows angiogenic tube formation. Branch points were calculated from 11 random fields from each group and plotted. ∗, Statistically significant difference from control cells.
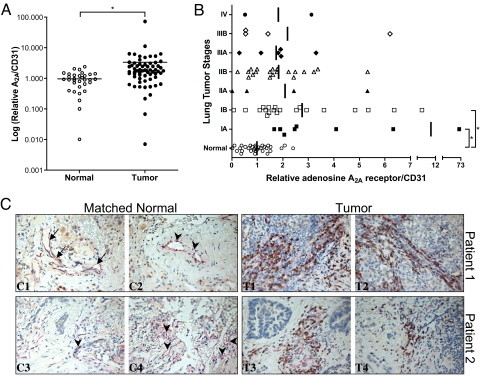
To further explore the potential biological significance of our findings, we analyzed the expression of A2A receptor in 64 lung cancer samples at different stages and compared it to the expression of 32 normal lung tissue samples. Twenty-four of the tumor samples had matched normal lung tissue from the same patient. Fig. 4 A and B showed that there was increased expression of A2A receptor in many cancer samples compared with normal lung tissue. The expression of A2A receptor was normalized to the expression of CD31, a predominantly endothelial marker. Additional comparison of the different tumor stages to the controls showed significant increases only in the early stages, IA and IB. To verify that A2A mRNA expression was associated with protein expression in the tumors, samples that showed increased mRNA expression of A2A receptor were further probed by immunohistochemistry. Fig. 4C demonstrates increased immunostaining of A2A receptor in lung tumors relative to normal tissue from the same patients. These regions also coimmunostained positively for CD31, representing contributions likely from endothelial cells and/or macrophages, further indicating that these were vascular regions.
Fig. 4.
Adenosine A2A receptor expression is increased in lung tumor samples at different stages. (A) Human lung cancer expression panels (I and IV) containing reverse-transcribed cDNA from the different tumor stages were probed with real-time Taqman primers and probes for adenosine A2A receptor and CD31. The A2A expression data were normalized to CD31 expression. The different tumor stages were grouped together and plotted after log transformation. ∗, Statistical difference from the normal lung tissue using a 2-sample t test. (B) Normalized A2A expression data were plotted against the different stages of tumors. ANOVA was used to compare the groups, followed by pairwise comparisons of normal tissue versus each of the other groups using Dunnett's procedure. ∗, Statistically different from the normal lung tissue. (C) CC1–CC4 show significant positive CD31 (PECAM-1; pinkish-red) immunostaining in the bronchovascular bundles (CC1–CC3) and parenchyma (CC3 and CC4) of normal (atelectatic) lung tissue obtained at surgery. Staining of venous and/or lymphatic vessel endothelium (CC1, arrow) and arterial vessel endothelium (CC2 and CC3, arrowheads) was variable but notably positive, as was the capillary endothelium (CC3 and CC4). Immunostaining for adenosine receptor A2A (brown) was largely absent from these tissues. By contrast, lung cancer specimens from both of these patients (CT1, CT2, CT3, and CT4) stained markedly positive, especially in the tumor stroma. In these regions, CD31-positive-staining cells, likely endothelial cells and/or tumor macrophages, coimmunostained positively for adenosine receptor A2A or, in some cases, A2A-positive cells were immediately adjacent to those staining positively for CD31 (Magnification: 40×).
Discussion
HIF-2α is a transcription factor that, unlike the related HIF-1α, is abundant in the vasculature, as indicated by its other name, endothelial PAS domain protein 1 (EPAS1) (42). Vascular growth and development are important in both the developing lung and in growing malignant tumors. With this in mind, we sought to investigate unique HIF-2α-regulated genes important for growth and proliferation of the vasculature. To this end, we used primary microvascular endothelial cells, which are essential for alveolar formation (43, 44) and are also a principal target for antiangiogenic therapy in cancers (45).
Hypoxia, HIF-stabilizing agents, and HIF-2α all regulate adenosine A2A receptor at the mRNA level. Unlike previous studies of HIF-2α-mediated induction of specific genes in cell lines and mouse models, our studies point to a more global role for this transcription factor in primary human lung microvessels and macrovessels. This regulation of the adenosine A2A receptor that we observed was not restricted to a single donor, but was reproduced in more than 3 donors. Interestingly, HIF-2α also increased adenosine A2A receptor mRNA in human pulmonary artery endothelial cells. Although siRNA knockdown of HIF-2α under hypoxia demonstrated a decrease in A2A receptor expression, studies also showed a paradoxical increase in receptor expression when the related HIF-1α was knocked down. It appears that both HIFs bind to the A2A receptor HRE. However, only HIF-1α binding to the regulatory element appears to form an inhibitory complex resulting in inhibition of transcription. A similar regulation exists for HK2, a known HIF-1α-specific gene, where HIF-2α rather than HIF-1α, perhaps πε△ηαπσ forms an inhibitory complex under conditions of hypoxia (SI Methods and Fig. S2). These results further corroborate earlier findings that selective activation of target gene by HIF-1α or HIF-2α does not depend on its DNA-binding ability (46, 47). Unlike the HIF-2α regulation of adenosine A2A receptor in lung-derived endothelial cells, Colgan and coworkers (48) have demonstrated an HIF-1α-dependent regulation of adenosine A2B receptor in dermal microvascular endothelial cells. It is interesting that adenosine receptors are regulated so differently even in microvascular endothelial cells of different tissue origins. This suggests potentially important phenotypic differences in the growth regulation of the microvasculature in the pulmonary versus the systemic circulation, just as occur in microvessels versus macrovessels (49). The finding that mouse-derived endothelial cells do not up-regulate adenosine A2A receptor in response to hypoxia and HIF-stabilizing agents was rather surprising, given the consistent findings in the human cells. Although more recent studies in mice show an increased role for HIF-2α in tumor vascularization (50, 51), the mechanisms are likely to differ from those of humans. Despite the fact that the laboratory mouse model has been an invaluable tool in understanding numerous disease processes, it appears that this model system would not be useful for studies of this phenomenon as it occurs in human cells. Given the fact that human adenosine A2A receptor has multiple tissue-specific transcription start sites and promoters (36), it would not be surprising to find distinct regulation of this receptor in different cell types. Interestingly, among the putative promoters, only R5 showed consistent induction with HIF-2α cotransfection. The higher basal level of luciferase activity in endothelial cells is likely due to higher basal levels of HIF-2α in these cells. The specificity of this interaction is further substantiated by using a ChIP assay, in which we show that HIF-2α does indeed bind to the A2A receptor promoter in vivo. It remains to be seen whether this promoter is unique to lung endothelial cells or is also active in other cell types. Although PGK-1, used as a positive control in the ChIP experiment, is an HIF-1α-regulated unique gene, it is now well accepted that both HIF-1α and HIF-2α bind to the same hypoxia response elements (46, 47).
Angiogenesis promotes growth and proliferation of the microvasculature both in normal and disease states, the latter being potentially less well-regulated. Cell proliferation is an essential element in angiogenesis. Compared with endothelial cells from the macrovessels, microvascular endothelial cells have a greater proliferative potential (49). Our findings indicate that the adenosine A2A receptor promotes proliferation of lung microvascular endothelial cells under conditions of both agonist-mediated receptor activation and adenoviral-mediated overexpression. The proproliferative characteristic of this receptor is consistent with other reports that show increased endothelial cell proliferation upon its activation (52). This is in contrast to studies in other cell types, including PC12 (pheochromocytoma) and smooth muscle cells, where decreased proliferation upon activation of adenosine A2A receptor was observed (52, 53). In other studies with PC12 cells, there was a decrease in cell viability upon activation of the receptor in one study (54), whereas inhibition of apoptosis was observed in another (55). Taken together, these studies point to a cell- and tissue-specific regulation and function of the receptor. These studies, in addition to our own findings, show that either adenosine A2A receptor or the related adenosine A2B receptor can be regulated by hypoxia in specific cell types. Cells expressing either of these receptors may have differential sensitivities based, in part, on local adenosine concentrations. Specifically, (i) adenosine A2B receptor has a much lower-affinity Km for adenosine relative to the adenosine A2A receptor and would likely be activated in areas where adenosine concentrations are high, and (ii) higher adenosine concentrations can, while activating A2B receptor, simultaneously desensitize the high-affinity A2A receptor (56). Thus, adenosine A2A receptors would likely be more important and active at lower adenosine concentrations. Finally, endothelial cell migration and sprouting assays demonstrate that increased expression or activation of A2A receptor can contribute to the angiogenic processes in these microvascular cells.
The biological significance of A2A receptor in tumor growth has been elegantly demonstrated by Sitkovsky and coworkers (57). Their study shows that A2A receptor increases tumor growth through inhibition of antitumor T cells. Interestingly, these studies also showed decreased neovascularization in tumors from the A2A receptor knockout animals compared with the wild-type animals. Likewise, several studies have also shown that HIF-2α, and not the related HIF-1α, is an important prognostic marker of tumor grade, tumor aggressiveness, invasion, and metastasis in a number of cancers (3, 5, 6, 58, 59). However, the mechanism by which HIF-2α promotes such growth independently of HIF-1α in human cancers is still not clear. Our studies show significant increase in expression of A2A receptor in whole-lung tumors, as assessed through normalization of data to the vascular marker CD31. Interestingly, the expression of A2A receptor was significantly increased during the early, more differentiated stages of tumor growth (Fig. 4B), suggesting that vascular growth and proliferation are of great importance in establishing early tumor growth. This is in agreement with recent findings where vascular growth was attributed to tumorigenicity only during the early stages of tumor growth (60, 61). Furthermore, immunohistochemistry results from human lung tumors showed that A2A receptor expression was increased mainly in the tumor stroma, a region actively involved in new blood vessel recruitment to support tumor growth. Some of the A2A receptor expression could also be contributed by cell types other than the endothelial cells. Taken together, these earlier studies (62) along with our current findings underscore the importance of A2A receptor in human lung tumor growth and also suggest a possible mechanism by which HIF-2α can promote vascular growth in tumors. These studies warrant further investigations into the role of this receptor in tumor progression.
In summary, the present study identifies the adenosine A2A receptor as a new angiogenic target of HIF-2α. This receptor could play an important role in tumor angiogenesis during the evolution of cancer, and therefore could offer a potential target for therapy in this process.
Experimental Procedures
Materials.
Primary HLMVECs, human coronary artery endothelial cells (HCAECs), pulmonary artery endothelial cells (HPAECs), and endothelial cell growth medium were obtained from Lonza.
Generation of Adenoviruses.
The HIF-1α construct containing mutations at P564A and N803A that allow the protein to be stable and constitutively active under normoxic conditions was obtained from Murray Whitelaw (University of Adelaide, Adelaide, Australia) (63). An additional mutation was generated at P402A to prevent any ubiquitylation and subsequent degradation of the HIF-1α protein (64). The construct was then subcloned into an adenoviral shuttle vector (pShuttle-CMV) by using the restriction sites KpnI/XbaI. An adenovirus vector encoding the mutHIF-1α (Ad.mutHIF-1α) was generated by using standard procedures. Briefly, the plasmid was linearized by using PmeI and used to transform Escherichia coli strain BJ5183 carrying the plasmid AdEasy-1 (65) to generate the recombinant plasmids containing the entire vector chromosome. Recombinant vector DNA encoding the mutHIF-1α was released from the plasmid by digestion with PacI and used to transfect 293 cells to generate Ad.mutHIF-1α. The vector was plaque-purified, grown in large scale, and purified by using CsCl step- and isopycnic-gradient centrifugation. Ad.mutHIF-2α, encoding the mutant human HIF-2α construct (also from Murray Whitelaw) (63) containing mutations at P531A and N847A, was generated similarly.
For generation of an adenovirus vector encoding A2A receptor (Ad.A2A), human adenosine A2A receptor (a kind gift from Marlene A. Jacobson, Merck Research Laboratories, West Point, PA) cDNA was excised from pSVL plasmid by using XhoI and BamH1 (blunted) and was subcloned into the adenoviral shuttle vector pShuttle-CMV by using the restriction sites XhoI and EcoRV. Ad.A2A was generated following the protocols outlined above.
Adenoviral transductions of HLMVECs were carried out at a multiplicity of infection of 10 pfu per cell, as described previously (66). For transduced controls, Ad.LacZ (67) was used.
RNA Interference.
Knockdown of HIF-1α and HIF-2α in HLMVECs was carried out by using predesigned SmartPool siRNA purchased from Dharmacon. Initially, transfection efficiencies were optimized by using siGLO Green as an indicator. Transfections were carried out in 6-well plates by using 25 nM siRNA complexed to 3 μL of DharmaFect1 transfection reagent (Dharmacon) in a total volume of 2.0 mL, per the manufacturer's protocol. Twenty-four hours after transfection, cells were exposed to hypoxia (0% O2, 5% CO2, balance N2) for an additional 24 h, after which RNA was isolated and real-time RT-PCR performed by using Taqman primers and probes for adenosine A2A receptor, HK2, and VEGFA (synthesized by Sigma-Aldrich).
Promoter Luciferase Assay.
The A2A receptor promoter constructs, cloned in pSSG-luciferase reporter vectors (a variant of pGL4.11 vector from Promega), were obtained from SwitchGear Genomics. Details are provided in SI Methods.
ChIP.
ChIP assays were performed on HLMVECs by using standard protocol. Details are provided in SI Methods.
Tumor Tissue and Expression Profile.
Human lung cancer expression panels (I and IV) containing cDNA from normal tissue and the different tumor stages were procured from Origene Technologies. Tumor and normal tissue RNA were also obtained from the University of Colorado Cancer Center Core facility (Aurora, CO) under an institutional review board-exempt protocol. Tumor and normal tissue sections used in immunohistochemistry were obtained from the Core facility. RNA samples were reverse transcribed to cDNA. All cDNA samples were probed with Taqman primers and probes for adenosine A2A receptor, and the data were then normalized to CD31 expression.
Statistical Analysis.
All statistical analyses were performed with the JMP software (SAS Institute). Data are represented as mean ± SEM of n ≥ 3 and were compared by ANOVA followed by Tukey–Kramer test for multiple comparisons. A P value of <0.05 was considered significant. Data from the tumor tissues as a group were compared to the normal tissues and analyzed by using a 2-sample t test. Data from the individual tumor stages were analyzed by using ANOVA followed by Dunnett's procedure.
Supplementary Material
Acknowledgments.
The authors thank Jonathan Kurche of the Integrated Department of Immunology (National Jewish Health, Denver, CO) for help in creating an additional mutation in the HIF-1α construct, Kelly Schneider of the Department of Pediatrics (National Jewish Health) for help with cell culture, and Mathew Strand for help with statistical analysis of data. This study was supported by a Scientist Development Grant from the American Heart Asso ciation (to A.A.), grants from the Cancer League of Colorado (to A.A.), and National Institutes of Health Grants K12-KL2RR025779 (to S.A.), P50 HL084923 (to C.W.W.), U01 HL56263 (to C.W.W.), HL60569 (to S.P.C.), DK50189 (to S.P.C.), and HL084376 (to J.M.S.).
Footnotes
Conflict of interest statement: National Jewish Health, A.A. and C.W.W. hold a pending patent on use of adenosine A2A receptor as a marker of HIF-2α activation in various disease states.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901326106/DCSupplemental.
References
- 1.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91:803–806. doi: 10.1113/expphysiol.2006.033498. [DOI] [PubMed] [Google Scholar]
- 2.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. Hypoxia-inducible factor-2 alpha (HIF-2 alpha) induces angiogenesis in breast carcinomas. Appl Immunohistochem Mol Morphol. 2006;14:78–82. doi: 10.1097/01.pai.0000145182.98577.10. [DOI] [PubMed] [Google Scholar]
- 6.Holmquist-Mengelbier L, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Koga F, et al. Prognostic significance of endothelial Per-Arnt-sim domain protein 1/hypoxia-inducible factor-2alpha expression in a subset of tumor associated macrophages in invasive bladder cancer. J Urol. 2004;171:1080–1084. doi: 10.1097/01.ju.0000110541.62972.08. [DOI] [PubMed] [Google Scholar]
- 8.Lofstedt T, et al. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura H, et al. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: Correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- 10.Covello KL, et al. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–5647. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- 12.Warnecke C, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 13.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oktay Y, et al. Hypoxia-inducible factor 2alpha regulates expression of the mitochondrial aconitase chaperone protein frataxin. J Biol Chem. 2007;282:11750–11756. doi: 10.1074/jbc.M611133200. [DOI] [PubMed] [Google Scholar]
- 16.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 17.Daval JL, Nicolas F, Doriat JF. Adenosine physiology and pharmacology: How about A2 receptors? Pharmacol Ther. 1996;71:325–335. doi: 10.1016/s0163-7258(96)00094-0. [DOI] [PubMed] [Google Scholar]
- 18.Nees S, Gerbes AL, Willershausen-Zonnchen B, Gerlach E. Purine metabolism in cultured coronary endothelial cells. Adv Exp Med Biol. 1979;122B:25–30. doi: 10.1007/978-1-4684-8559-2_5. [DOI] [PubMed] [Google Scholar]
- 19.Ethier MF, Chander V, Dobson JG., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- 20.Meininger CJ, Granger HJ. Mechanisms leading to adenosine-stimulated proliferation of microvascular endothelial cells. Am J Physiol. 1990;258:H198–H206. doi: 10.1152/ajpheart.1990.258.1.H198. [DOI] [PubMed] [Google Scholar]
- 21.Feoktistov I, et al. Differential expression of adenosine receptors in human endothelial cells: Role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 22.Lasley RD, Jahania MS, Mentzer RM., Jr Beneficial effects of adenosine A(2a) agonist CGS-21680 in infarcted and stunned porcine myocardium. Am J Physiol Heart Circ Physiol. 2001;280:H1660–H1666. doi: 10.1152/ajpheart.2001.280.4.H1660. [DOI] [PubMed] [Google Scholar]
- 23.Okusa MD, et al. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol. 2000;279:F809–F818. doi: 10.1152/ajprenal.2000.279.5.F809. [DOI] [PubMed] [Google Scholar]
- 24.Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. Selective A(2A) adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am J Physiol Heart Circ Physiol. 2001;281:H67–H74. doi: 10.1152/ajpheart.2001.281.1.H67. [DOI] [PubMed] [Google Scholar]
- 25.Cassada DC, et al. An adenosine A2A agonist, ATL-146e, reduces paralysis and apoptosis during rabbit spinal cord reperfusion. J Vasc Surg. 2001;34:482–488. doi: 10.1067/mva.2001.117996. [DOI] [PubMed] [Google Scholar]
- 26.McPherson JA, et al. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 27.Ross SD, et al. Selective adenosine-A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant. 1999;18:994–1002. doi: 10.1016/s1053-2498(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 29.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H650–H656. doi: 10.1152/ajpheart.2000.279.2.H650. [DOI] [PubMed] [Google Scholar]
- 30.Desai A, et al. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 31.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: Molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 32.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adenosinergic cancer immunotherapy. Purinergic Signal. 2007;3:129–134. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26:273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 34.Sitkovsky MV. T regulatory cells: Hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Asikainen TM, et al. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, et al. Characterization of genomic organization of the adenosine A2A receptor gene by molecular and bioinformatics analyses. Brain Res. 2004;1000:156–173. doi: 10.1016/j.brainres.2003.11.072. [DOI] [PubMed] [Google Scholar]
- 37.Kim TH, et al. Direct isolation and identification of promoters in the human genome. Genome Res. 2005;15:830–839. doi: 10.1101/gr.3430605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinklein ND, Aldred SJ, Saldanha AJ, Myers RM. Identification and functional analysis of human transcriptional promoters. Genome Res. 2003;13:308–312. doi: 10.1101/gr.794803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feoktistov I, et al. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44:649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- 42.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 43.Hislop AA. Airway and blood vessel interaction during lung development. J Anat. 2002;201:325–334. doi: 10.1046/j.1469-7580.2002.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thebaud B, Abman SH. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 46.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau KW, Tian YM, Raval RR, Ratcliffe PJ, Pugh CW. Target gene selectivity of hypoxia-inducible factor-alpha in renal cancer cells is conveyed by post-DNA-binding mechanisms. Br J Cancer. 2007;96:1284–1292. doi: 10.1038/sj.bjc.6603675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 49.King J, et al. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Rankin EB, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita T, et al. Hypoxia-inducible transcription factor-2alpha in endothelial cells regulates tumor neovascularization through activation of ephrin A1. J Biol Chem. 2008;283:18926–18936. doi: 10.1074/jbc.M709133200. [DOI] [PubMed] [Google Scholar]
- 52.Martin PL. Adenosine agonists for the prevention of restenosis? IDrugs. 1999;2:44–51. [PubMed] [Google Scholar]
- 53.Sun CN, et al. Rescue of p53 blockage by the A(2A) adenosine receptor via a novel interacting protein, translin-associated protein X. Mol Pharmacol. 2006;70:454–466. doi: 10.1124/mol.105.021261. [DOI] [PubMed] [Google Scholar]
- 54.Trincavelli ML, et al. A(2A) adenosine receptor ligands and proinflammatory cytokines induce PC 12 cell death through apoptosis. Biochem Pharmacol. 2003;66:1953–1962. doi: 10.1016/j.bcp.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Huang NK. Adenosine A2A receptors regulate oxidative stress formation in rat pheochromocytoma PC12 cells during serum deprivation. Neurosci Lett. 2003;350:127–131. doi: 10.1016/s0304-3940(03)00860-7. [DOI] [PubMed] [Google Scholar]
- 56.Palmer TM, Stiles GL. Identification of an A2a adenosine receptor domain specifically responsible for mediating short-term desensitization. Biochemistry. 1997;36:832–838. doi: 10.1021/bi962290v. [DOI] [PubMed] [Google Scholar]
- 57.Ohta A, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bangoura G, et al. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3176–3182. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raval RR, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasarre P, et al. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 2009;69:1324–1333. doi: 10.1158/0008-5472.CAN-08-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chikazawa M, Inoue K, Fukata S, Karashima T, Shuin T. Expression of angiogenesis-related genes regulates different steps in the process of tumor growth and metastasis in human urothelial cell carcinoma of the urinary bladder. Pathobiology. 2008;75:335–345. doi: 10.1159/000164218. [DOI] [PubMed] [Google Scholar]
- 62.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 63.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 64.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He TC, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad A, Ahmad S, Chang LY, Schaack J, White CW. Endothelial Akt activation by hyperoxia: Role in cell survival. Free Radic Biol Med. 2006;40:1108–1118. doi: 10.1016/j.freeradbiomed.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 67.Schaack J, Langer S, Guo X. Efficient selection of recombinant adenoviruses by vectors that express beta-galactosidase. J Virol. 1995;69:3920–3923. doi: 10.1128/jvi.69.6.3920-3923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.