Abstract
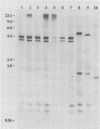
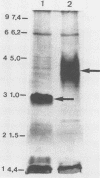
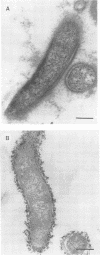
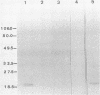
The study described here was carried out to further characterize reference strains of Serpulina (Treponema) hyodysenteriae representing serotypes 8 and 9. Results obtained from restriction fragment length polymorphism analysis, enteropathogenicity testing, and endotoxin profiles confirmed their identifications. Electron microscopy indicated that both strains were covered with a thin layer of capsule-like material. Immunoblot analysis indicated that an antigen in the 19-kDa region of proteinase K-digested whole cells reacted only with homologous antiserum. The serotype-specific antigens were sensitive to periodate oxidation but resistant to proteinase K digestion and migrated in the same region as purified lipopolysaccharides. Immunoblotting with proteinase K-digested whole cells appeared as useful as immunodiffusion with extracted lipopolysaccharide for the serological classification of S. hyodysenteriae. Immunogold labeling of whole cells and purified periplasmic flagella showed strong cross-reactions between S. hyodysenteriae and Serpulina innocens. Outer membrane preparations of strains representing serotypes 8 and 9 contained four major proteins which reacted with antisera against both species, and one major protein with a molecular mass of 46 kDa which reacted only with antisera against S. hyodysenteriae, irrespective of the serotype. Our findings suggest that periplasmic flagella and some outer membrane proteins are antigens common to both S. hyodysenteriae and S. innocens, whereas a 46-kDa outer membrane protein may be a species-specific antigen of S. hyodysenteriae. Finally, we propose immunoblotting as an alternative method to immunodiffusion for the serotyping of S. hyodysenteriae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M., Jacques M. Evaluation of the An-Ident system and an indole spot test for the rapid differentiation of porcine treponemes. J Clin Microbiol. 1991 Aug;29(8):1727–1729. doi: 10.1128/jcm.29.8.1727-1729.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield S. N., Fernie D. S., Penn C., Dougan G. Identification of the major antigens of Treponema hyodysenteriae and comparison with those of Treponema innocens. Infect Immun. 1988 May;56(5):1070–1075. doi: 10.1128/iai.56.5.1070-1075.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. M., Wannemuehler M. J. Comparison of the biological responses induced by lipopolysaccharide and endotoxin of Treponema hyodysenteriae and Treponema innocens. Infect Immun. 1989 Mar;57(3):717–723. doi: 10.1128/iai.57.3.717-723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer J. M., Wannemuehler M. J. Pathogenesis of Treponema hyodysenteriae: induction of interleukin-1 and tumor necrosis factor by a treponemal butanol/water extract (endotoxin). Microb Pathog. 1989 Oct;7(4):279–288. doi: 10.1016/0882-4010(89)90046-6. [DOI] [PubMed] [Google Scholar]
- Halter M. R., Joens L. A. Lipooligosaccharides from Treponema hyodysenteriae and Treponema innocens. Infect Immun. 1988 Dec;56(12):3152–3156. doi: 10.1128/iai.56.12.3152-3156.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B., Buddle J. R. Proposed revisions to the serological typing system for Treponema hyodysenteriae. Epidemiol Infect. 1989 Feb;102(1):75–84. doi: 10.1017/s0950268800029708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Hovind-Hougen K., Høgh P., Birch-Andersen A. Morphological heterogeneity among spirochetes isolated from cases of swine dysentery. Zentralbl Bakteriol. 1990 Oct;274(1):1–15. doi: 10.1016/s0934-8840(11)80970-9. [DOI] [PubMed] [Google Scholar]
- Hudson M. J., Alexander T. J., Lysons R. J. Diagnosis of swine dysentery: spirochaetes which may be confused with Treponema hyodysenteriae. Vet Rec. 1976 Dec 18;99(25-26):498–500. doi: 10.1136/vr.99.25-26.498. [DOI] [PubMed] [Google Scholar]
- Hunter D., Saunders C. N. Diagnosis of swine dysentery using an absorbed fluorescent antiserum. Vet Rec. 1977 Oct 8;101(15):303–304. doi: 10.1136/vr.101.15.303. [DOI] [PubMed] [Google Scholar]
- Jacques M., Gottschalk M., Foiry B., Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990 Jun;172(6):2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen N. S., Casey T. A., Stanton T. B. Detection and identification of Treponema hyodysenteriae by using oligodeoxynucleotide probes complementary to 16S rRNA. J Clin Microbiol. 1990 Dec;28(12):2717–2721. doi: 10.1128/jcm.28.12.2717-2721.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Harris D. L., Kinyon J. M., Kaeberle M. L. Microtitration agglutination for detection of Treponema hyodysenteriae antibody. J Clin Microbiol. 1978 Sep;8(3):293–298. doi: 10.1128/jcm.8.3.293-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Marquez R. B. Molecular characterization of proteins from porcine spirochetes. Infect Immun. 1986 Dec;54(3):893–896. doi: 10.1128/iai.54.3.893-896.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joens L. A., Whipp S. C., Glock R. D., Neussen M. E. Serotype-specific protection against Treponema hyodysenteriae infection in ligated colonic loops of pigs recovered from swine dysentery. Infect Immun. 1983 Jan;39(1):460–462. doi: 10.1128/iai.39.1.460-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. A., Sellwood R., Lemcke R. M., Burrows M. R., Lysons R. J. Analysis of the axial filaments of Treponema hyodysenteriae by SDS-PAGE and immunoblotting. J Gen Microbiol. 1989 Jun;135(6):1625–1632. doi: 10.1099/00221287-135-6-1625. [DOI] [PubMed] [Google Scholar]
- Knoop F. C. Experimental infection of rabbit ligated ileal loops with Treponema hyodysenteriae. Infect Immun. 1979 Dec;26(3):1196–1201. doi: 10.1128/iai.26.3.1196-1201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Z. S., Bélanger M., Jacques M. Serotyping of Canadian isolates of Treponema hyodysenteriae and description of two new serotypes. J Clin Microbiol. 1991 Dec;29(12):2794–2797. doi: 10.1128/jcm.29.12.2794-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysons R. J., Kent K. A., Bland A. P., Sellwood R., Robinson W. F., Frost A. J. A cytotoxic haemolysin from Treponema hyodysenteriae--a probable virulence determinant in swine dysentery. J Med Microbiol. 1991 Feb;34(2):97–102. doi: 10.1099/00222615-34-2-97. [DOI] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. P., Toivio-Kinnucan M., Wu G., Wilt G. R. Ultrastructural and electrophoretic analysis of Treponema hyodysenteriae axial filaments. Am J Vet Res. 1988 Jun;49(6):786–789. [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A., Glock R. D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983 Aug;131(2):997–999. [PubMed] [Google Scholar]
- Penn C. W., Cockayne A., Bailey M. J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985 Sep;131(9):2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Sellwood R., Kent K. A., Burrows M. R., Lysons R. J., Bland A. P. Antibodies to a common outer envelope antigen of Treponema hyodysenteriae with antibacterial activity. J Gen Microbiol. 1989 Aug;135(8):2249–2257. doi: 10.1099/00221287-135-8-2249. [DOI] [PubMed] [Google Scholar]
- Smith S. C., Roddick F., Ling S., Gerraty N. L., Coloe P. J. Biochemical and immunochemical characterisation of strains of Treponema hyodysenteriae. Vet Microbiol. 1990 Jul;24(1):29–41. doi: 10.1016/0378-1135(90)90048-z. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Stanton T. B. Proposal to change the genus designation Serpula to Serpulina gen. nov. containing the species Serpulina hyodysenteriae comb. nov. and Serpulina innocens comb. nov. Int J Syst Bacteriol. 1992 Jan;42(1):189–190. doi: 10.1099/00207713-42-1-189. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wannemuehler M. J., Hubbard R. D., Greer J. M. Characterization of the major outer membrane antigens of Treponema hyodysenteriae. Infect Immun. 1988 Dec;56(12):3032–3039. doi: 10.1128/iai.56.12.3032-3039.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]