Abstract
As human population and resource demands continue to grow, biodiversity conservation has never been more critical. About one-quarter of all mammals are in danger of extinction, and more than half of all mammal populations are in decline. A major priority for conservation science is to understand the ecological traits that predict extinction risk and the interactions among those predictors that make certain species more vulnerable than others. Here, using a new database of nearly 4,500 mammal species, we use decision-tree models to quantify the multiple interacting factors associated with extinction risk. We show that the correlates of extinction risk vary widely across mammals and that there are unique pathways to extinction for species with different lifestyles and combinations of traits. We find that risk is relative and that all kinds of mammals, across all body sizes, can be at risk depending on their specific ecologies. Our results increase the understanding of extinction processes, generate simple rules of thumb that identify species at greatest risk, and highlight the potential of decision-tree analyses to inform conservation efforts.
Keywords: conservation, biodiversity, body size, IUCN Red List, decision tree
Certain ecological traits, such as small geographic range, low population density, slow life history, and large body size are known to correlate strongly with extinction risk in mammals, and the importance of these traits can vary among different clades of mammals (1–5). Large body size, in particular, is a well-known predictor of both past and present human-related extinctions (4, 6, 7). Although the identification of these correlates of extinction has been an important first step in guiding conservation priorities, it is critical to understand how multiple ecological factors interact to predict risk across species that differ by orders of magnitude in body size, area of geographic range, abundance, life history, niche characteristics, and other traits. For example, it is not enough to know that species with small geographic ranges tend to be at greater risk; rather, we need to know how range size interacts with other ecological traits to make certain species with small ranges more vulnerable than others. By understanding how multiple key ecological predictors interact, we are able to identify the species at greatest risk and also to understand what makes them vulnerable. Additionally, to help avert the losses of populations and species of mammals (8–10), there is a real need for conservation scientists to provide results that are directly relevant and are easily interpretable for conservation practice. In this paper, we draw on a large dataset and methodological approach to build on current knowledge of extinction risk in mammals. Using a decision-tree modeling framework we (i) identify interactions among multiple ecological traits that lead to different pathways to extinction across mammals and (ii) use our model to codify simple rules of thumb that can be used to guide conservation.
Decision-Tree Modeling Approach.
Although decision trees have been used previously in ecology, their application to conservation biology has been limited (11–15). The decision-tree approach is a powerful alternative to traditional linear models and has documented advantages for extinction risk analyses, especially when the goal is predictive accuracy (14–16). Decision-tree models are designed to identify nonlinear, context-dependent associations among multiple correlated predictor variables (11, 14–17). They require fewer assumptions than correlational methods and do not assume a specific distribution of predictor variables. Further, they do not assume data independence, avoiding potential concerns about pseudoreplication and alleviating the need for explicit phylogenetic control (16, 18). In decision trees, the same predictor variables may reappear repeatedly in the model as necessary, a fundamental difference from the single-predictor variables of linear models. Consequently, the explanatory power of a predictor variable is not conflated by how many species share a particular trait value because of shared evolutionary history. Instead, the model quantifies the association of predictors and response variables on a species-by-species basis. Because decision trees predict outcomes of interest (i.e., extinction or survival) based on the nested internal structure of the predictor variables, they may provide a more accurate predictive framework for extinction risk than traditional parametric approaches. Whereas previous studies of mammalian extinction risk have addressed the interactions of variables in linear models (1, 4, 19), decision-tree analyses offer a major advance, because they reveal both how the interactions between predictors lead to the outcomes of interest and also how these interactions differ among subsets of the data (14). In contrast, linear models can indicate only whether a particular interaction is statistically significant over the entire dataset. This added insight into the context-dependence of interactions is a major advantage of decision-tree models. Finally, decision-tree analyses produce graphical outputs that quantify and summarize the interactions in a visual, easily interpretable format (14, 17). Because decision-tree models can be sensitive to small changes in the underlying data, we also used a random forest, a modeling technique that combines the predictions of many independent decision-tree models into a robust composite model (20).
Results and Discussion
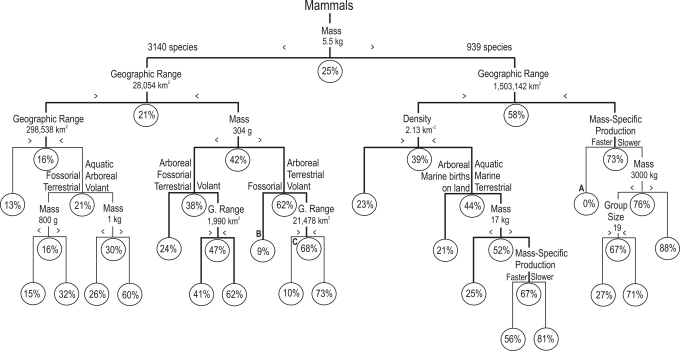
The random forest model classified mammal threat status with 82% accuracy [percent correctly classified (PCC), Cohen's kappa = 0.44, P < 0.001; supporting information (SI) Table S1], based solely on ecological traits. It identified 8 major predictors of extinction risk: small geographic range, low population density, small group size, slow production rate, large home range, large body size, habitat mode, and activity period (Fig. 1). These predictors were intercorrelated (Table S2), and most have been related to extinction risk in previous studies of mammals and other taxa (1–6, 21–23). However, the decision tree goes beyond previous work by showing quantitatively how these key predictors interact to create specific pathways to extinction (Fig. 2 and Fig. S1). Classification accuracy of the decision-tree model (Fig. 2) was similar to the random forest (PCC 81%, Cohen's kappa = 0.40, P < 0.001; Table S1). The first major split in the tree defines pathways separating large (> 5.5 kg) species and small (< 5.5 kg) species, a split similar to the 3-kg body size threshold identified by Cardillo et al. (4). Within each of these branches, the next variable is geographic range, followed by other ecological traits. Note that body size enters the model at several places throughout the tree, and at all these nodes species with relatively larger body sizes have higher risk. This finding suggests that size-selective extinction depends primarily on how large a species is relative to other species that share similar ecological traits rather than on its absolute body size. Similarly, at all nodes based on geographic range, species with relatively smaller ranges have a higher probability of extinction. A similar dichotomous pattern appears among different lifestyles of small mammals. For example, fossorial species consistently have lower risk, whereas volant species have higher risk, likely reflecting differences in survivorship related to these lifestyles (24, 25). In the statistically significant lower branches, reproduction rate is identified as a key factor for large mammals: species with slower reproductive rates are at higher risk than otherwise similar species with faster life histories. These patterns temper the roles of body size and geographic range as interpreted in previous studies, showing that species with a wide range of trait values have non-zero risk. For example, depending on their ecologies, small species can have risks equal to or greater than those of large species.
Fig. 1.
Relative importance, in rank order, of ecological predictors of mammalian extinction risk. Importance was measured by the drop in classification accuracy after predictor removal in a random forest of 500 trees. Differences in importance between predictors were quantified with pair-wise, 2-tailed z-tests, α = 0.05. The top 8 predictors (geographic range–activity period) were statistically indistinguishable, except that activity period was significantly less than geographic range; all 8 were significantly more important than landmass, trophic group, and sociality, and all other variables were significantly more important than sociality.
Fig. 2.
Decision tree showing extinction risk based on ecological traits (body mass, geographic range size, mass-specific production rate, population density, group size, home range, activity period, type of landmass, habitat mode, sociality, trophic group). Branches in the smaller, optimal tree (see Fig. S1) are shown in bold, but all depicted branches are statistically meaningful (χ2 test, P ≤ 0.001). The probability of being threatened is indicated at each node; species with higher risk are at the right of each branch point, and those with lower risk are at the left. Labeled nodes (A–C) are referenced in main text.
The decision tree provides the critical values of traits and quantifies how they interact to affect risk across the broad spectrum of mammalian ecologies. For example, not all large-bodied species with small geographic ranges have a high probability of extinction. Indeed, our model predicts that species larger than 5.5 kg with geographic ranges less than ≈1.5 million km2 have a negligible risk if they have fast reproductive rates for their body size (see node A in Fig. 2); it is the interaction between small geographic ranges and slow reproductive rates, rather than any of these traits in isolation, that creates a pathway to high extinction risk in large mammals. Similarly, within small-bodied species, fossorial species weighing between 304 g and 5.5 kg with geographic ranges less than 28,000 km2 have only a 9% risk of extinction (node B), whereas arboreal, terrestrial, or volant species of similar body and range size have a 68% risk (node C). In this case, it is the interaction of lifestyle with body size and range size that determines risk in these small mammals.
Many of the key predictors of extinction are highly correlated with body size (Fig. 3, Table S2). Indeed, extinction risk increases rapidly with body size (Fig. 4A). Figs. 3 and 4B show that species above 5.5 kg, about the size of a raccoon (Procyon lotor), have a disproportionately high risk. This pattern is consistent with the well-known impacts on large animals of human hunting and fishing for meat and secondary products, such as ivory and horn, during both the Pleistocene and present-day extinctions (3, 4, 6, 7). However, the decision-tree model demonstrates wide variability in extinction risk among large mammals (> 5.5 kg), ranging from 0% to 81%, depending on interactions of large body size with other ecological traits (Fig. 2).
Fig. 3.
Bivariate plots of the top 5 continuous ecological predictors as a function of body mass. Within each plot (A–E), the 4 categories of extinction risk cluster consistently. Note that home range (B), population density (C), and mass-specific production rate (E) correlate strongly with body size. The upper cloud of points in E represents placental mammals; the lower cluster represents marsupials.
Fig. 4.
(A) Body size and extinction risk. Proportion of species predicted to be threatened in each body size class (0.25 log g). (B) Mammalian body mass distribution showing the 4 risk categories based on the random forest model.
Our model provides insights into extinction risk in small mammals. The insights related to body size are particularly valuable, because, although small size generally is thought to be associated with lower extinction risk (4), about 85% of extant mammal species are smaller than 5.5 kg, about 40% of all mammal species predicted by our model to be at risk are below this size (Fig. 4B). Our finding that the interaction of multiple ecological traits causes many small species to be at high risk is consistent with the growing recognition that risk in the current extinction crisis does not scale simply with body size (6, 12, 23). Human impacts today include not only hunting, but also extermination programs, climate change, spread of diseases and exotic species, deforestation, habitat fragmentation, urbanization, desertification, and the conversion of landscapes to agriculture (26–28), and these factors affect biodiversity through multiple pathways at multiple scales.
Finally, our decision-tree model is an important conservation tool, providing simple rules of thumb and a map of pathways for predicting risk (Fig. 2). This type of general classification model for extinction risk may be particularly useful for rapid assessment and prioritization of poorly known species. We found that 28 International Union for the Conservation of Nature (IUCN) data-deficient species had high predicted risk (Table S3). Further, our model highlights the structure of interactions between lifestyles, ecological traits, and ranges of trait values associated with high risk in mammals. Traditional approaches to extinction risk analysis generally have been unable to quantify context-dependent interactions among multiple traits. Complex nonlinear interactions among correlated traits produce multiple, different pathways to extinction. Our results underscore the importance of natural history, ecology, and systematics in conservation biology and also the contribution of new quantitative tools, such as decision trees, to identify species at risk of extinction, improving our ability to guide policies and management for preserving biodiversity.
Materials and Methods
For 4,420 mammal species (out of a total of more than 5,400 mammals) we compiled a database of quantitative and categorical ecological traits, including body mass, geographic range size, speed of life history [calculated as the mass-specific production rate (24, 25)], population density, group size, home range, activity period (diurnal, nocturnal, or both), type of landmass (continent, island, or both), habitat mode (aquatic, arboreal, fossorial, marine, marine with births on land, terrestrial, or volant), sociality, and trophic category (carnivore, omnivore, herbivore). Our analyses excluded cetaceans, IUCN data-deficient species, and those listed on the basis of small geographic range (IUCN criterion B), (see SI Methods for details). We used a dichotomous response variable to represent extinction risk: species classified as vulnerable or higher by the IUCN were considered “threatened,” and least concerned or near threatened species were considered “nonthreatened” (29, 30). We used the rpart package in R (31) to build a classification-tree model that graphically depicts quantitative relationships between predictor variables and extinction risk. The tree was pruned to its optimal size, minimizing both classification error and tree complexity, and an extended tree including statistically significant lower branches (see SI Methods) was examined also (Fig. S2). A random forest model of 500 classification trees was used to estimate the relative importance of predictor variables and to make predictions about threat status (32). Model accuracy for the tree and random forest was quantified using standard metrics (Table S1). We then used our model to predict risk for data-deficient species and those with current range estimates. Lists of species predicted to be at high risk are provided in Tables S3–S5.
Supplementary Material
Acknowledgments.
We thank A. Hope for help with the database and M. Lee, J. McIntyre, and F. Smith, and two anonymous reviewers for comments. We are grateful for the invaluable databases that contributed to this work. This research was supported by National Science Foundation Grant OISE-0653296 to A.D.D., a Smithsonian Postdoctoral Fellowship and National Science Foundation Grant DBI-0805669 to A.G.B., and Grants DGAPA of the Universidad Nacional Autónoma de México and CONABIO to G.C.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901956106/DCSupplemental.
References
- 1.Cardillo M, et al. The predictability of extinction: Biological and external correlates of decline in mammals. Proc R Soc London Ser B. 2008;275:1441–1448. doi: 10.1098/rspb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones KE, Purvis A, Gittleman JL. Biological correlates of extinction risk in bats. Am Nat. 2003;161(4):601–613. doi: 10.1086/368289. [DOI] [PubMed] [Google Scholar]
- 3.Cardillo M. Biological determinants of extinction risk: Why are smaller species less vulnerable? Anim Conservation. 2003;6:63–69. [Google Scholar]
- 4.Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie NL, et al. Analysis of factors implicated in the recent decline of Australia's mammal fauna. Journal of Biogeography. 2007;34:597–611. [Google Scholar]
- 6.Olden JD, Hogan ZS, Zanden MJV. Small fish, big fish, red fish, blue fish: Size-based extinction risk of the world's freshwater and marine fishes. Global Ecology and Biogeography. 2007;16:694–701. [Google Scholar]
- 7.Lyons SK, Smith FA, Brown JH. Of mice, mastodons and men: Human-mediated extinctions on four continents. Evolutionary Ecology Research. 2004;6:339–358. [Google Scholar]
- 8.Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Science. 2002;296:904–907. doi: 10.1126/science.1069349. [DOI] [PubMed] [Google Scholar]
- 9.Ceballos G, Ehrlich PR, Soberon J, Salazar I, Fay JP. Global mammal conservation: What must we manage? Science. 2005;309:603–607. doi: 10.1126/science.1114015. [DOI] [PubMed] [Google Scholar]
- 10.Schipper J, et al. The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 11.Bell JF. Tree-based methods. In: Fielding AH, editor. Machine Learning Methods for Ecological Applications. Kluwer, Dordrecht: Springer; 1999. pp. 89–106. [Google Scholar]
- 12.Boyer AG. Extinction patterns in the avifauna of the Hawaiian islands. Diversity & Distribution. 2008;14:509–517. [Google Scholar]
- 13.Roff DA, Roff RJ. Of rats and Maoris: A novel method for the analysis of patterns of extinction in the New Zealand avifauna before European contact. Evolutionary Ecology Research. 2003;5:759–779. [Google Scholar]
- 14.Olden JD, Lawler JJ, Poff NL. Machine learning methods without tears: A primer for ecologists. Quarterly Review of Biology. 2008;83(2):171–193. doi: 10.1086/587826. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MS, et al. A comparison of predictive methods in extinction risk studies: Contrasts and decision trees. Biodiversity and Conservation. 2006;15:1977–1991. [Google Scholar]
- 16.Jones MJ, Fielding A, Sullivan M. Analysing extinction risk in parrots using decision trees. Biodiversity and Conservation. 2006;15:1993–2007. [Google Scholar]
- 17.De'ath G, Fabricius KE. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- 18.Westoby M, Leishman M, Lord J. Further remarks on phylogenetic correction. Journal of Ecology. 1995;83:727–729. [Google Scholar]
- 19.Cardillo M, Mace GM, Gittleman JL, Purvis A. Latent extinction risk and the future battlegrounds of mammal extinction. Proc Natl Acad Sci USA. 2006;103:4157–4161. doi: 10.1073/pnas.0510541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutler DR, et al. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- 21.Sodhi NS, et al. Measuring the meltdown: Drivers of global amphibian extinction and decline. PloS ONE. 2008;3(2):e1636. doi: 10.1371/journal.pone.0001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owens IPF, Bennett PM. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. Proc Natl Acad Sci USA. 2000;97(22):12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia VB, Lucifora LO, Myers RA. The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc R Soc London Ser B. 2008;275:83–89. doi: 10.1098/rspb.2007.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibly RM, Brown JH. Mammal reproductive strategies driven by offspring mortality-size relationships. Am Nat. 2009;173:E185–E199. doi: 10.1086/598680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibly RM, Brown JH. Effects of body size and lifestyle on evolution of mammal life histories. Proc Natl Acad Sci USA. 2007;104(45):17707–17712. doi: 10.1073/pnas.0707725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United Nations Environment Programme. Nairobi: United Nations Environment Programme; 2002. GEO-3: Global environment outlook; pp. 120–149. [Google Scholar]
- 27.Dickman C, Woodford Ganf R. A Fragile Balance: The Extraordinary Story of Australian Marsupials. Chicago: University of Chicago Press; 2007. [Google Scholar]
- 28.Foley JA, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 29.International Union for the Conservation of Nature. IUCN Red List of Threatened Species: Categories & criteria. Gland, Switzerland: IUCN/SSC Red List Programme; 2001. (version 3.1) [Google Scholar]
- 30.International Union for the Conservation of Nature. 2008 IUCN Red List of Threatened Species. Gland, Switzerland: IUCN/SSC Red List Programme; 2008. [Google Scholar]
- 31.Therneau TM, Atkinson EJ. Rochester: Mayo Clinic; 2002. An introduction to recursive partitioning using the RPART routines. [Google Scholar]
- 32.Liaw A, Wiener M. The randomForest package. R News. 2002;2/3:18–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.