Abstract
A complement-independent bactericidal IgG1 against the OspB of Borrelia burgdorferi increased the permeability of the outer membrane through the creation of openings of 2.8 – 4.4 nm, resulting in its osmotic lysis. Cryo-electron microscopy and tomography demonstrated that exposure to the antibody causes the formation of outer membrane projections and large breaks which may precede the increase in permeability of the outer membrane. The bactericidal effect of this antibody is not transferable to Escherichia coli expressing rOspB on its outer membrane. Additionally, the porin P66, the only protein that coprecipitated with OspB, is dispensable for the bactericidal mechanism.
Keywords: osmotic lysis, spirochete, outer membrane, pore, Lyme disease
Borrelia organisms, the agents of Lyme disease (1, 2) and relapsing fever (3), are extracellular pathogens. Their extracellular life cycle makes them uniquely susceptible to antibodies (4, 5). Antibodies require the recruitment of complement for bacterial lysis through formation of the membrane attack complex. However, lytic complement is not required for efficient host defense against Borrelia infections (6–8). The binding of Factor H (9) and C4BP (10), regulators of the alternative and classical complement pathways, respectively, to the Borrelia surface accounts for complement inhibition. In contrast, antibodies are the main immune effectors against both diseases and are required for an efficient host response (4).
Indeed, there are antibodies against Borrelia that require the classical complement pathway to eliminate the spirochetes (4, 11). However, there are also numerous antibodies to Borrelia that exert bactericidal effects in a complement-independent manner (4, 6, 8, 12, 13). Two such monoclonal antibodies against relapsing fever organisms are H4825 (IgG2a) and CB515 (IgM), which are directed against variable major proteins (8, 13). Two monoclonal antibodies against B. burgdorferi are CB2 (IgG1) and H6831 (IgG2a), which are directed against outer surface protein B (OspB) (12, 13). Monovalent Fab fragments of the IgG monoclonal antibodies can also kill B. burgdorferi, suggesting that agglutination is not the bactericidal mechanism. Although some requirements for the bactericidal function are known, the mechanism of complement-independent antibodies has remained elusive. The antibodies are highly specific regardless of isotype. CB515 is specific for one serotype of relapsing fever Borrelia (14) whereas CB2 and H6831 are specific to one amino acid of OspB (Lys 253) (13, 15). Furthermore, the bactericidal function resides in the antibody-variable region, as shown through experiments using a single-chain variable fragment (scFv) of CB515 (14). The variable region alone can eliminate the entire serotype population to which it is specific. That the constant (effector) region is dispensable is unusual and underscores the importance of the variable region in conjunction with its antigen in creating an effect that is extraordinarily lethal. Outer membrane (OM) damage is apparent during exposure to bactericidal antibodies observed through the release of periplasmic flagella (8, 12, 13), although the precise nature of this damage remains unknown. Additionally, OspB of B. burgdorferi undergoes structural changes upon the binding of CB2 and H6831 (16, 17), underscoring the importance of the antigen, but the changes could not explain the bactericidal mechanism.
For the present study, the direct effect of the antibody on the OM of Borrelia, resulting in its osmotic lysis and subsequently lysis of the spirochete, was probed. The capacity for CB2 to function in a bactericidal manner in the presence of different osmoprotective sugars was measured as was the activity of CB2 against Escherichia coli expressing full-length, recombinant OspB (rOspB).
Results
Destruction of the Borrelia OM Occurs By Formation of Openings and Osmotic Lysis.
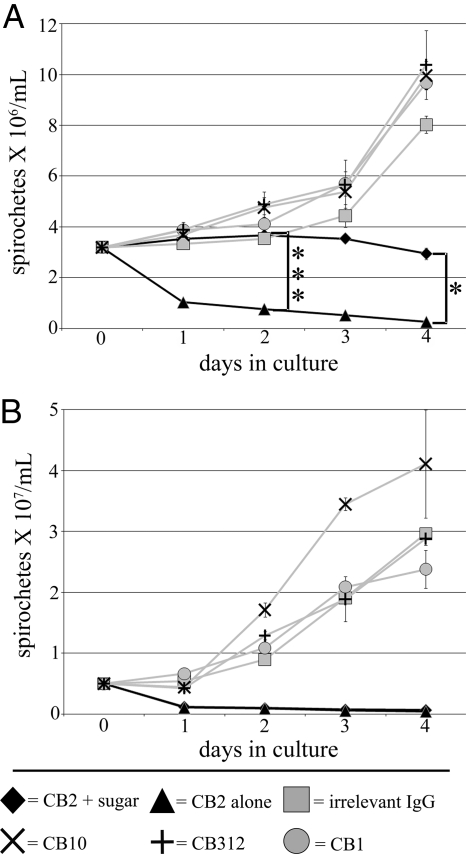
A characteristic of exposure to complement-independent bactericidal antibodies is the formation of blebs in the OM of Borrelia (8, 12–14). This consistent observation led to the idea that OM blebbing could result in the formation of openings or pores and cause osmotic lysis. To investigate this idea, we chose dextran T500 and sucrose (of 28 nm and 0.92 nm molecular diameter, respectively) for potential osmoprotection in a 4-day growth inhibition assay in the presence of CB2 (Fig. 1). Controls consisted of an irrelevant IgG and IgG antibodies to cytosolic DNAk (CB312), periplasmic flagella (CB1), and OspA (CB10) of B. burgdorferi in the presence of the specified sugars. OspA is cotranscribed with OspB and both are very similar in their primary structure and isoelectric points (18, 19). Cultures with control antibodies grew normally compared with cultures with no sugar or sugars only without antibodies (Fig. S1), whereas spirochetes with CB2 decreased in numbers and did not grow. Spirochetes cultured with CB2 and dextran T500 did not grow but did not decrease in numbers (Fig. 1A). In contrast, spirochetes cultured with CB2 and sucrose were not protected, decreasing to similar numbers as those cultured with CB2 alone (Fig. 1B). That dextran T500 inhibited lysis is an indication that B. burgdorferi were protected osmotically from injury to the OM by the action of CB2. Because spirochetes were killed by CB2 in the presence of sucrose but not dextran T500, it appears that the osmotic protection that prevents lysis is size-dependent, suggesting the presence of openings or pores of a defined size in the OM.
Fig. 1.
B. burgdorferi persists for 4 days during exposure to CB2 when protected osmotically. (A) Spirochetes exposed to CB2 with dextran T500 for 4 days did not decrease in numbers in contrast to CB2 alone. ANOVA, ***, P < 0.001, *, P < 0.05. (B) Spirochetes exposed to CB2 with sucrose decreased to numbers comparable to CB2 alone by 1 day of exposure. These results suggest that spirochetes survive due to an osmotically protected OM in the presence of dextran T500. ANOVA, P < 0.001. Control antibodies were added in the presence of the specified sugar for each experiment.
CB2-Induced Osmotic Lysis of the OM Is Due To the Formation of Membrane Openings of 2.8–4.4 nm in Diameter.
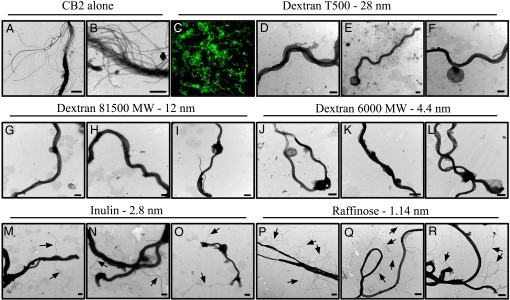
B. burgdorferi incubated with CB2 and dextran T500 for 15 min were analyzed by negative-stain transmission electron microscopy (TEM) (Fig. 2 D–F). Those spirochetes protected by dextran T500 had the characteristic membrane blebs, however, there was no disruption of the OM as evidenced by retained periplasmic flagella. Thus, the OM was not ruptured as a result of the osmotic protection provided by dextran T500. Dextran T500 does not affect or inhibit CB2 binding as determined by fluorescence microscopy (Fig. 2C), therefore, its protective effects are due to osmoprotection and not an inhibition of antibody binding.
Fig. 2.
CB2 causes the formation of openings of 2.8–4.4 nm in the B. burgdorferi OM. (A and B) Exposure to CB2 alone completely kills B. burgdorferi. (C) CB2 binds B. burgdorferi despite the presence of dextran T500 (100×). (D–L) The OM of B. burgdorferi is not ruptured when exposed to CB2 with dextran T500 (D–F), dextran 81500 MW (G–I), or dextran 6000 MW (J–L), due to osmotic protection. (P–R) The exposure of periplasmic flagella (arrows) in those spirochetes exposed to CB2 with inulin (M–O) or raffinose (P–R) indicates a ruptured OM. This suggests that CB2 induces the formation of openings in the OM of 2.8–4.4 nm leading to osmotic lysis. (Scale bars: 500 nm.)
The size of the putative openings in the OM that may be responsible for osmotic lysis was determined by using sugars of several molecular sizes. The sizes correspond to the molecular diameters of the sugars, therefore, a sugar of a molecular diameter larger than the openings will stay in the external medium and balance osmotic pressure protecting the OM from lysis. Conversely, a sugar of a molecular diameter smaller than that of the openings will diffuse through and afford no protection to the membrane (20). As shown earlier, sucrose (molecular diameter = 0.92 nm) did not provide osmotic protection whereas dextran T500 (molecular diameter = 28 nm) did, giving us a wide range of sizes, 0.92–28 nm, to consider for the putative openings. The sugars (and their molecular diameters) used were: Raffinose (1.14 nm), inulin (2.8 nm), dextran 6000 MW (4.4 nm), and dextran 81500 MW (12 nm). After a 15 min incubation with CB2 and the sugars, spirochetes were examined for OM rupture by negative-stain TEM (Fig. 2), as this is a critical time-point where spirochetes decrease in numbers sharply (14). Exposure of periplasmic flagella was the key indicator of OM rupture in this experiment. Dextran 81500 MW and dextran 6000 MW served as osmoprotectants to the Borrelia OM (Fig. 2 G–L) as they produced results identical to dextran T500 (Fig. 2 D–F). Periplasmic flagella exposure began to occur with inulin (Fig. 2 M–O), and was readily apparent with raffinose (Fig. 2 P–R). Thus, we determined that CB2 causes the formation of OM openings in the range of 2.8–4.4 nm in diameter, based on the molecular diameters of the sugars used, and that these openings result in the osmotic rupture of the OM.
To determine whether the pores were of a fixed size or could increase in size over time, we performed osmoprotection studies for 4 days. Raffinose and inulin afforded no protection against CB2 as in the prior experiment (Fig. S2 A and B). Spirochetes were partially protected by dextran 6000 MW because their numbers after 1 day of CB2 exposure were 2-times higher than those exposed to CB2 alone. A similar trend was observed when B. burgdorferi was cultured with CB2 and dextran 81500 MW, where spirochetes did not decrease in numbers until the second day (Fig. S2 C–E). Although spirochetes decreased by day 2, their numbers were still 6-times greater than those exposed to CB2 alone (Fig. S2E). Dextrans do not slow the effects of CB2, but rather osmotic protection is lost in these cases. This result is indicative of the presence of membrane openings larger than is suggested by the size of these sugars so that the openings in the OM increase in size with increasing incubation time with CB2, and the sugars lose the ability to osmoprotect.
CB2-Mediated OM Disruptions Occur Rapidly.
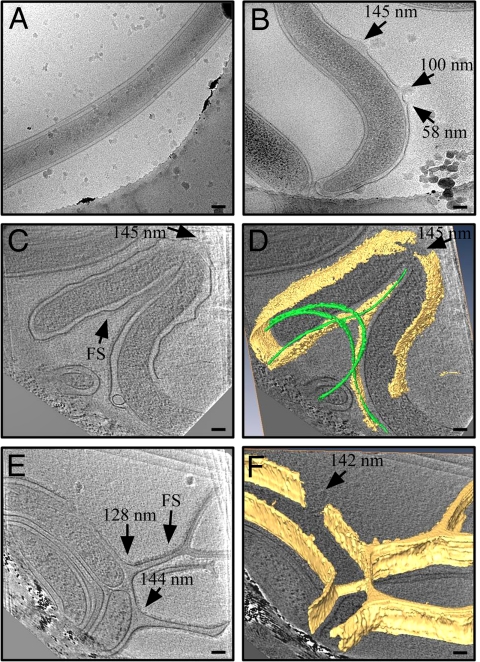
To observe openings formed in the OM at an early time-point we analyzed the effects of CB2 against B. burgdorferi by using cyro-electron microscopy and tomography (Fig. 3 and Fig. S3). This high-resolution technique allows for 3D visualization of surface structures in great detail without the use of fixatives or dehydration steps. Instead, specimens are instantly frozen, preserving structures in their native state. Because specimens are immediately frozen after experiments, this allowed us to observe the effects of CB2 at strict 2 and 10 min time-points. Micrographs from this experiment showed distention of the Borrelia OM after exposure to CB2 (Fig. S3 C, D, and F), which we have observed previously in negative-stain TEM as membrane blebs (8, 12, 14). We have previously observed disruption of the OM indirectly as determined by the exposure of periplasmic flagella (8, 12, 14), however, these images clearly show disruptions that appear as breaks in the membrane (Fig. 3 B–D and Fig. S3 C–F). When measured at their base these disruptions were of varying size, ranging from 58–145 nm. Tomograms from this experiment also showed the formation of thin, branch-like OM projections (Fig. 3 E and F), seen also in negative-stain TEM images (Fig. S4). The diameters of the projections (128 nm and 144 nm) correlate with the diameters of the OM breaks observed here, suggesting that the projections are the cause of the breaks. Due to the large size of these breaks and the nature of flash-freezing the spirochetes, it is possible that the breaks are transient after forming from the thin projections. In this manner CB2 creates the large breaks, which we believe are a precursor to the smaller openings that we have observed through osmoprotection (2.8–4.4 nm). After the large breaks are formed, lipid redistribution or sealing of the OM would account for the appearance of the smaller openings (21), which are critical for osmotic lysis of the spirochetes. An additional observation was the presence of a fringe/shadowing (FS) on areas of the outer membrane in CB2-exposed Borrelia but not in controls (Fig. 3C and Fig. S5), suggestive of a bound antibody to the spirochete surface. The FS was particularly localized around outer membrane projections (Fig. 3E). When measured in the tomograms, the particles that comprise the fringe are 16–25 nm, a range consistent with the size of intact IgG (Fig. S5F). Immunogold experiments showed that CB2 was bound to the outer membrane projections (Fig. S6), further proof that CB2 is directly involved in the creation of the outer membrane projections.
Fig. 3.
The early events of exposure to CB2 include the formation of OM projections and breaks. (A) Spirochetes exposed to an irrelevant IgG are intact and unaffected. (B) Cryo-electron microscopic images of spirochetes exposed to CB2 show OM disruptions which appear as “breaks” in the membrane (arrows, with diameters of the breaks indicated). (C–F) Cryo-electron tomography shows the 3-D structure of CB2-induced breaks as well as thin OM projections all around the organism. (C and E) Individual 3.6 nm thick slices from 2 tomographic volumes. (D and F) Surface rendered models of the OM (yellow) and flagella (green) in the corresponding tomograms. The diameters at the base of the OM projections correlate with those of the OM breaks, suggesting that the OM projections precede the breaks and break off to form them. CB2-treated cells displayed a fringe/shadow in several regions at the outer face of the OM (signified by arrows labeled FS). (Scale bars: 100 nm.)
The Bactericidal Effects of CB2 Are Specific to the Borrelia Organisms.
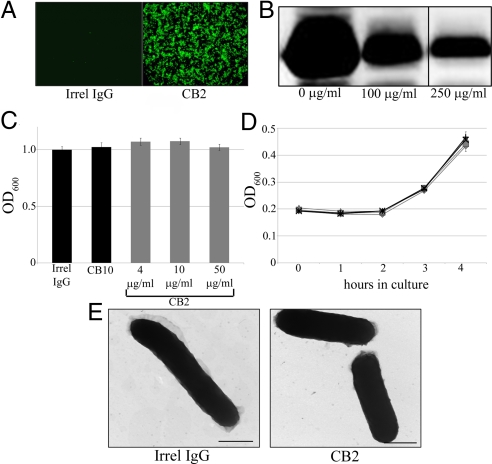
We have previously shown that the bactericidal action of complement-independent antibodies resides in the variable region (14). However, properties of the antigen and of the organisms themselves may be critical for the bactericidal effect as well. To investigate bactericidal specificity, we analyzed the effect of CB2 against recombinant E. coli expressing lipidated OspB. Upon induction with 1 mM IPTG for 3 h, E. coli expressed large quantities of rOspB. We first tested whether the bacteria could express this Borrelia antigen on their surfaces. Through immunofluorescence using live, unfixed E. coli, we have shown that rOspB is expressed on their OM, which also proves that CB2 is capable of binding to rOspB on E. coli (Fig. 4A). Proteinase K degradation assays showed cleavage of rOspB, further demonstrating its surface exposure on E. coli (Fig. 4B). When tested for activity, CB2 did not cause a decrease in E. coli numbers after 3 h (Fig. 4C). E. coli were completely capable of normal growth after CB2 exposure (Fig. 4D) and also exhibited no ultrastructural damage after CB2 exposure (Fig. 4E). Because CB2 had no effect against E. coli despite binding rOspB, these results collectively demonstrate that there are unique factors present in the OM of Borrelia that contribute to the effects of complement-independent bactericidal antibodies and that are not transferable to other organisms.
Fig. 4.
CB2 requires unique factors in Borrelia to create its bactericidal effects. (A) Fluorescence assay demonstrating the surface exposure of rOspB on E. coli and the ability of CB2 to recognize it. (B) Proteinase K (100 or 250 μg/mL) cleaves rOspB, further demonstrating its surface exposure. (C–E) CB2 has no bactericidal effect against recombinant E. coli (C), the E. coli have no defect in growth following CB2 exposure (D), (♦, 5 μg/mL CB2; ■, 15 μg/mL CB2; ▴, 30 μg/mL CB2; +, 30 μg/mL irrel IgG; X, 30 μg/mL CB10), and there is no evidence of damage to recombinant E. coli after CB2 exposure (E).
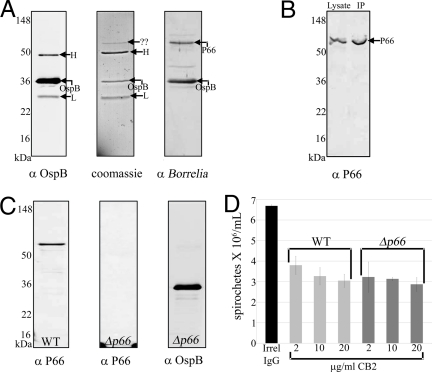
P66, a Porin That Coprecipitates with OspB, Is Not Critical for the Bactericidal Mechanism of CB2.
In searching for additional molecules in Borrelia required for the bactericidal action of CB2, several immunoprecipitations with CB2 demonstrated one protein band that consistently coprecipitated with OspB (Fig. 5A). This protein had a spirochetal origin because it was recognized by antisera to B. burgdorferi (Fig. 5A). Mass spectrometry analysis revealed the protein to be P66, a porin/adhesin in B. burgdorferi (22, 23), and this was confirmed through the use of antisera to P66 in immunoblot (Fig. 5B). To determine whether or not P66 is critical for the bactericidal mechanism, we analyzed the effect of CB2 against a strain of B. burgdorferi with a targeted mutation (Δp66) (Fig. 5C). There was no difference in the effect exerted by CB2 against Δp66 compared to wild-type B. burgdorferi, indicating that although P66 coprecipitates with OspB, it is not critical for the bactericidal action of CB2 (Fig. 5D).
Fig. 5.
The candidate accessory protein, P66, is not critical for the bactericidal activity of CB2. (A) Immunoblots and Coomassie-stained gel of OspB immunoprecipitation showing the coprecipitant later identified as P66. (B) Immunoblot showing recognition of P66 in B. burgdorferi lysate and in the OspB immunoprecpitate, confirming mass spectrometry results that identified P66 as the coprecipitant. (C) Immunoblots showing the absence of P66 from Δp66 and the presence of the version of OspB recognized by CB2. (D) Killing assay showing that CB2 has identical effects when used against WT or Δp66, suggesting that P66 is not critical for the bactericidal mechanism of CB2. Irrelevant IgG was added at 20 μg/mL.
Discussion
Typically, antibodies exert a bactericidal effect against microorganisms with the assistance of complement and formation of the membrane attack complex. Monoclonal antibodies and polyclonal IgM that work against Lyme disease and relapsing fever spirochetes are unique in that they are directly bactericidal in the absence of complement. Although some of the bactericidal requirements are known, the actual mechanism of complement-independent antibodies has not been worked out. We now show that complement-independent bactericidal antibodies to Borrelia exert their effects through formation of openings and subsequent osmotic lysis of the OM. In a previous study, we showed that the bactericidal effect resides in the variable region of the antibodies (14). In addition to this previous finding, we have demonstrated here that the bactericidal effect of the antibodies is not transferable to other bacteria because CB2 had no bactericidal effect against E. coli expressing lipidated rOspB. Thus, there appears to be unique factors present in Borrelia that are just as critical as the variable region of the antibody for completion of this mechanism. One such factor, the only OM protein candidate that coprecipitated with OspB, P66, was not required for the bactericidal activity of CB2.
Based on our osmoprotection experiments, CB2 creates openings in the OM of 2.8 – 4.4 nm in diameter. These may not be pores, per se, which would involve the formation of fixed-size protein channels, but rather, they may be openings in the OM that result in increased permeability as a result of CB2 binding and blebbing of the OM. Longer incubation times (2–4 days) with dextran 6000 MW and dextran 81500 MW resulted in a loss of protection. This result is indicative of OM openings that increase in size over time, which is likely the result of continual membrane blebbing or micellization caused by CB2. This is a fundamentally different mechanism from the pores that are formed during the assembly of the membrane attack complex after complement recruitment. A current model for the function of cationic antimicrobial peptides, called the “carpet” mechanism (where the peptides coat the membrane and insert causing micellization, leading to the formation of transient, toroidal pores resulting in increased permeability and resulting in osmotic lysis), is reminiscent of the increasingly larger pores created by CB2 (24). If lysis is prevented, blebbing will continue causing larger membrane openings and thus increased permeability over time to the point where the membrane disintegrates (24). CB2 and related antibodies may increase the permeability of the OM in a manner similar to the carpet mechanism, allowing for osmotic changes and lysis of this barrier. However, the variable region of CB2 shares no homology with known cationic antimicrobial peptides, as has been suggested in another case (25). The range of 2.8–4.4 nm appears to be the critical size for openings responsible for increased permeability and susceptibility to osmotic lysis, because this size was observed at a time in the reaction when the majority of spirochetes are killed. That spirochetes can persist in culture during constant CB2 exposure and recover to normal growth after exposure in the presence of dextran T500, but not when smaller sugars are used, is an additional indication of CB2-induced osmotic lysis of the OM due to openings.
Cryo-electron tomography showed the very early effects of CB2 on the spirochetes in a 2-min exposure and has demonstrated previously overlooked characteristics of the OM “frozen in time” due to the flash-freezing preparation of the organisms. The thin, branch-like membrane projections are one of these characteristics. The outer membrane projections are a consistent feature of damage caused by CB2, and Immunogold TEM has shown that CB2 is directly involved in their creation. Another cryo-TEM observation is the large OM breaks. That the diameters of the projections at their base are similar to those of the breaks suggests that the breaks may be the result of the projections breaking off of the bacterial surface. Additionally, the membrane breaks are pointed in an outward orientation (Fig. 3B and Fig. S3 D–F ), reminiscent of the projections. However, there is a discrepancy between the size of the openings deduced from the osmoprotection studies and the size of the breaks visualized in cryo-electron microscopy and tomography. From the osmoprotection studies, the size of the openings was deduced to be ≈2.8–4.4 nm, which can also increase in size during additional incubation with the antibody in the presence of osmoprotective sugars. The breaks in cryo-electron microscopy images are larger (58–145 nm) after a 2 min incubation with CB2. We have considered 2 possibilities to explain this discrepancy. The first possibility considers that the OM projections seen in cryo-electron tomography form very rapidly after exposure to CB2, break off, and leave temporary breaks of 58–45 nm. These large breaks are transient and energetically unfavorable for the membrane and may then be sealed by the remaining lipids in the OM (21). As this process continues and more membrane lipids are lost, the sealing will continue until the openings become of a smaller, semifixed, or equal size (2.8–4.4 nm) all around the membrane, thus resulting in an OM with increased permeability. This first possibility suggests steps of a mechanism that would include: (i) binding of CB2 to OspB; (ii) creation of thin membrane projections; (iii) breaking-off of the projections resulting in transient large breaks (58–145 nm); (iv) redistribution of lipids to seal the gaping breaks resulting in (v) increased permeability of the OM due to small, equal-sized openings around the membrane (2.8–4.4 nm) as surrounding membrane lipids become more scarce, and finally; (vi) osmotic lysis of the spirochete. The other possibility is that the osmoprotective sugars do not allow for the larger openings to occur. Only after lengthier antibody and sugar exposure (1–2 days) do the openings increase in size. It is important to note, however, that none of the sugars used in this study are surfactants. Previous observations with CB2 and a related antibody, H6831, demonstrated structural changes in OspB upon binding (16, 17), but neither were consistent with development of the outer membrane openings.
There was no bactericidal activity against E. coli expressing rOspB on their surface. Recombinant E. coli did not decrease in numbers, grew normally, and were not damaged after exposure to CB2. Because CB2 bound rOspB on these bacteria but did nothing else, it seems there must be unique accessory factors to this mechanism in the OM of Borrelia that are not found in E. coli. Whatever these factors may be, they are absolutely required for the effects of complement-independent bactericidal antibodies. P66, which coprecipitated with OspB, appeared to be the ideal candidate accessory protein because it is localized to the OM and it is a porin (23). It was conceivable that a porin could be required to effect osmotic changes by allowing the passage of ions, or could be in an open conformation after CB2 binding allowing for the influx of water and, thus, osmotic lysis. However, CB2 had bactericidal activity against Δp66, indicating that P66 is not critical for the bactericidal mechanism. It is possible that P66 coprecipitated with OspB because of natural colocalization, as is the case with P66 and OspA (26). In fact, CB10, an IgG directed against OspA, is not bactericidal in the absence of complement, and was used as a control for all of the studies with CB2. OspA and OspB are similar, highly cationic lipoproteins that are cotranscribed from a shared operon (18, 19). It now appears that the openings responsible for lysis of the OM are created by the binding of CB2, and may be the result of a critical role for OspB in the fluidity of the spirochetal OM.
This unique bactericidal effect is the result of a lethal association of the variable region of the antibody, its targeted antigen, and unique properties within the organism itself. Because susceptibility to the bactericidal effect is not transferable to other organisms and because there is no obvious accessory protein required for this mechanism, what makes the Borrelia OM so susceptible to the effects of complement-independent bactericidal antibodies? One feature that needs to be considered in the context of OM fluidity in Borrelia is the absence of LPS and the presence of cholesterol-containing glycolipids (27–29). Because the OM of Borrelia contains cholesterol, a characteristic that is not common for prokaryotes, there is the possibility that lipid raft domains could contribute to increased blebbing and membrane permeability following antibody binding. Whatever the case, it has become abundantly clear that the combination of the variable region, antigen, and the Borrelia organisms create a specific effect that is extraordinarily lethal and is triggered by increased OM permeability and osmotic lysis as the mechanism.
Materials and Methods
Additional methods are given in SI Text.
Osmoprotection Experiments.
B. burgdoferi strain B31 was harvested from mid-log phase culture and resuspended in serum-free BSK-H (Sigma) supplemented with raffinose (Fisher), inulin (Fisher), dextran 6000 MW (Fluka), dextran 81500 MW (Sigma), or dextran T500 (Amersham Pharmacia). CB2, CB1, CB10, CB312, or an irrelevant IgG (Sigma) were added to the spirochetes at 2 μg/mL and CB2 alone was also added to spirochetes as a positive control for 15 min, after which spirochetes were enumerated or prepared for negative-stain TEM analysis, which was performed as previously described (14). For experiments where B. burgdorferi was cultured in the presence of the antibodies and sugars for 4 days, the starting concentration was 3.2 × 106 or 5 × 106 spirochetes per mL for dextran T500 and sucrose ( JT Baker), respectively, and antibodies were added at 2 μg/mL. Spirochetes were grown at 33 °C in BSK-H containing heat-inactivated serum and enumerated under dark-field microscopy daily.
rOspB Surface Exposure.
To assess whether rOspB was expressed on the E. coli surface, 10 μg of CB2 or an irrelevant IgG (Sigma) was added to live, unfixed E. coli after expression and incubated at 4 °C for 1 h in 1X PBS (Gibco). The bacteria were washed, resuspended in PBS/goat anti-mouse IgG FITC conjugate (Sigma, 1:1000), and incubated at 4 °C for 1 h. E. coli were again washed, added to the wells of a Teflon-coated indirect immunofluorescence assay slide, dried, fixed in 100% methanol, and viewed in a Nikon Eclipse E400 microscope. For proteinase K degradation assays, the protease was added to live E. coli at 100 or 250 μg/mL and allowed to incubate for 1 h at room temperature. HALT protease inhibitor mixture (Pierce) was added to stop the reaction and the samples were electrophoresed, blotted, and probed with CB2.
rOspB E. coli Bactericidal Assays.
CB2, CB10, or an irrelevant IgG (Sigma) were added to E. coli (containing intact LPS) at 4, 10, or 50 μg/mL after expression, and incubated in 1X Hank's Balance Salt Solution (Gibco) for 3 h at 37 °C (which is the optimal growth temperature for E. coli, as opposed to 33 °C for Borrelia). Bactericidal effects were determined by OD600 measurements or negative-stain TEM analysis. The growth/recovery of E. coli was assessed by exposure to CB2 at 5, 15, or 30 μg/mL, or an irrelevant IgG or CB10 at 30 μg/mL, for 3 h in 1X HBSS. E. coli were diluted 1:10 in LB and incubated at 37 °C for 4 h. The OD600 was measured hourly.
Immunoprecipitations.
B. burgdorferi was exposed to 10 μg of CB2 for 1 h at 4 °C, resuspended in 1X radioimmunoprecipitation assay buffer (Pierce) with HALT protease inhibitor mixture (Pierce), and incubated for 1 h at 37 °C, after which insoluble materials were removed. Protein G agarose beads (Pierce) were added and allowed to incubate overnight at 4 °C. Agarose beads were washed 4 times and resuspended in 1X Laemmli buffer. Samples were electrophoresed and stained with 0.1% Coomassie or blotted and probed with CB2, antisera to B. burgdorferi, or antisera to P66.
Supplementary Material
Acknowledgments.
This work was supported by NIH grants R37-AI027044, R01-AR0404415, and the Northeast Biodefense Center grant U54-AI057158 – Lipkin. The Wadsworth Center's Resource for Visualization of Biological Complexity is an NIH Biomedical Technology Research Center supported by grant P41-RR01219 from NCRR/PHS. Julie Anderton-Skinner (Merck, Inc) made the Δp66 mutant, Jennifer Coburn (Medical College of Wisconsin) provided the P66 antisera and Michael Marko (Wadsworth Center) provided helpful discussions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901858106/DCSupplemental.
References
- 1.Burgdorfer W, et al. Lyme disease—A tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Benach JL, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 3.Johnson RC. The spirochetes. Annu Rev Microbiol. 1977;31:89–106. doi: 10.1146/annurev.mi.31.100177.000513. [DOI] [PubMed] [Google Scholar]
- 4.LaRocca TJ, Benach JL. The important and diverse roles of antibodies in the host response to Borrelia infections. Curr Top Microbiol Immunol. 2008;319:63–103. doi: 10.1007/978-3-540-73900-5_4. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SE, Benach JL. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005;3:411–420. doi: 10.1038/nrmicro1149. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SE, Benach JL. Cutting edge: The spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol. 2001;167:3029–3032. doi: 10.4049/jimmunol.167.6.3029. [DOI] [PubMed] [Google Scholar]
- 7.Bockenstedt LK, Barthold S, Deponte K, Marcantonio N, Kantor FS. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect Immun. 1993;61:2104–2107. doi: 10.1128/iai.61.5.2104-2107.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- 9.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur J Immunol. 2001;31:1674–1684. doi: 10.1002/1521-4141(200106)31:6<1674::aid-immu1674>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Meri T, Cutler SJ, Blom AM, Meri S, Jokiranta TS. Relapsing fever spirochetes Borrelia recurrentis and B. duttonii acquire complement regulators C4b-binding protein and factor H. Infect Immun. 2006;74:4157–4163. doi: 10.1128/IAI.00007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochi SK, Johnson RC. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988;56:314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadziene A, et al. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect Immun. 1994;62(5):2037–2045. doi: 10.1128/iai.62.5.2037-2045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaRocca TJ, Katona LI, Thanassi DG, Benach JL. Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J Immunol. 2008;180:6222–6228. doi: 10.4049/jimmunol.180.9.6222. [DOI] [PubMed] [Google Scholar]
- 15.Coleman JL, Rogers RC, Rosa PA, Benach JL. Variations in the ospB gene of Borrelia burgdorferi result in differences in monoclonal antibody reactivity and in production of escape variants. Infect Immun. 1994;62:303–307. doi: 10.1128/iai.62.1.303-307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker M, et al. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem. 2005;280:17363–17370. doi: 10.1074/jbc.M412842200. [DOI] [PubMed] [Google Scholar]
- 17.Katona LI, Ayalew S, Coleman JL, Benach JL. A bactericidal monoclonal antibody elicits a change in its antigen, OspB of Borrelia burgdorferi, that can be detected by limited proteolysis. J Immunol. 2000;164:1425–1431. doi: 10.4049/jimmunol.164.3.1425. [DOI] [PubMed] [Google Scholar]
- 18.Benach JL, Coleman JL, Golightly MG. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988;140:265–272. [PubMed] [Google Scholar]
- 19.Bergstrom S, Bundoc VG, Barbour AG. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 20.Viboud GI, Bliska JB. Measurement of pore formation by contact-dependent type III protein secretion systems. Methods Enzymol. 2002;358:345–350. doi: 10.1016/s0076-6879(02)58100-3. [DOI] [PubMed] [Google Scholar]
- 21.McNeil PL, Steinhardt RA. Plasma membrane disruption: Repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 22.Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin alphavbeta3. Proc Natl Acad Sci USA. 2003;100:7301–7306. doi: 10.1073/pnas.1131117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skare JT, et al. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 25.Polonelli L, et al. Antibody complementarity-determining regions (CDRs) can display differential antimicrobial, antiviral and antitumor activities. PLoS ONE. 2008;3:e2371. doi: 10.1371/journal.pone.0002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radolf JD, et al. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun. 1995;63:2154–2163. doi: 10.1128/iai.63.6.2154-2163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler CM, et al. Nonprotein antigens of Borrelia burgdorferi. J Infect Dis. 1993;167:665–674. doi: 10.1093/infdis/167.3.665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.