Abstract
Using Drosophila as a model system, we identified here a stringent requirement for Mre11-Rad50-Nbs (MRN) function in telomere protection during early embryonic development. Animals homozygous for hypomorphic mutations in either mre11 or nbs develop normally with minimal telomere dysfunction. However, they produce inviable embryos that succumb to failure of mitosis caused by covalent fusion of telomeric DNA. Interestingly, the molecular defect is not the absence of MRN interaction or of Mre11 nuclease activities, but the depletion of the maternal pool of Nbs protein in these embryos. Because of Nbs depletion, Mre11 and Rad50 (MR) are excluded from chromatin. This maternal effect lethality in Drosophila is similar to that seen in mice carrying hypomorphic mrn mutations found in human patients, suggesting a common defect in telomere maintenance because of the loss of MRN integrity.
Keywords: maternal lethality, telomere capping, targeted mutagenesis, ALTD
The Mre11-Rad50-Nbs (MRN) complex is multifunctional in genome maintenance (1). In DNA double strand break (DSB) repair and damage checkpoint activation, MRN resects DNA ends in cooperation with other proteins (2, 3), and activates the ataxia telangiectasia-mutated (ATM) checkpoint kinase (4). Biochemical studies suggest an additional structural role of MRN in genome maintenance through tethering of DNA ends (5). The Nbs subunit serves to recruit an Mre11 and Rad50 (MR) complex to the nucleus (6–8).
The mechanism of MRN function at telomeres is poorly understood. MRN is partially responsible for maintaining a 3′ DNA overhang at telomeres (9, 10), and this structure is important for loading telomerase activities and the CDC13 capping protein in yeast (11–13). More recently, a direct role in capping was proposed for yeast MRX (14). In Drosophila where telomeres are not elongated by a telomerase, loss of MRN leads to telomere fusion (15–19). At Drosophila telomeres, MRN may function to maintain a chromatin structure appropriate for loading of the capping machinery (20).
The regulation of MRN function in development is also poorly understood. Mammalian mrn-null mutations lead to cell lethality (21). Human hypomorphic mutations in mre11 and nbs cause AT-like disorder (LD) and Nijmegen breakage syndrome (NBS), respectively. Female mice that are homozygous for such mutations produce embryos that die within a few cell divisions after fertilization, suggesting that MRN is important for early animal development (8, 22, 23). To our knowledge, the reason for this requirement remains undetermined.
We used the more amenable Drosophila model to dissect the requirements for MRN during early embryo development. We discovered that animals with hypomorphic mutations in either mre11 or nbs develop normally. However, these females produced inviable embryos that suffered gross chromosome segregation defects during the early cell cycles. We developed new molecular and cytological methods that identified the cause of this mitotic catastrophe as telomere uncapping leading to telomere association. We show that this association is accompanied by covalent linkage of telomeric DNA. In the developing mutant embryos, MR proteins are excluded from chromatin because of the depletion of Nbs protein. We suggest that the evolutionarily conserved requirement for MRN during early development is to prevent telomere fusion.
Results
Hypomorphic mre11 and nbs Mutations Cause Maternal Effect Lethality.
The cell divisions that occur before the activation of zygotic expression during Drosophila embryogenesis have many features that are common in early development of other animals, such as being rapid and having no Gap phases (24). We hypothesized that these unique features might impose a stringent requirement for MRN function. MRN-null embryos from heterozygous crosses develop normally owing to the maternal contribution of wild type protein. Germ-line clones that are null for rad50 cannot be generated efficiently (25). Therefore, the study of MRN in embryogenesis would be facilitated by hypomorphic alleles that allow the survival of homozygous mutant females.
We chose to study mre1158S, the most thoroughly studied mre11 hypomorphic mutation. It is caused by a single histidine to tyrosine substitution at an invariant residue that is essential for the nuclease activity of Mre11 in both yeast and humans (26, 27). Although mre1158S is the strongest point mutation in various in vitro assays, many of the cellular defects that it causes in yeast are intermediate to those of the null mutation (28). We identified his230 in Drosophila Mre11 as equivalent to his213 in yeast and his217 in human Mre11 (Fig. S1), and generated the his to tyr change at the endogenous mre11 locus by ends-in gene targeting (SI Materials and Methods). For nbs, we isolated a hypomorphic allele during our development of the site-specific integrase mediated repeated targeting (SIRT) method (29). The nbs2K allele is predicted to encode an N-terminally truncated Nbs protein (SI Materials and Methods).
Adult flies homozygous for mre1158S or transheterozygous for mre1158S and the mre11Δ35K1-null allele (hemizygous for mre1158S) are viable, and recovered at a Mendelian ratio. Similar results were obtained for animals homozygous or hemizygous for nbs2K. This is in contrast to mre11 or nbs-null mutants, which die late in development as pupae because of telomere uncapping with an average of 2.5 telomere associations per nucleus (15, 16). We observed that both mre1158S and nbs2K animals display mild telomere-capping defects: 0.2 telomere associations per mre1158S nucleus (n = 118), and 0.3 associations per nbs2K nucleus (n = 122), compared with the wild-type level of 0.04 (15). Also, both mre1158S and nbs2K males are fertile.
Despite the normal appearance of homozygous or hemizygous mre1158S or nbs2K females, they laid embryos that did not hatch (>10,000 embryos counted), even when they were mated to wild-type males. Therefore, both mre1158S and nbs2K mutations cause maternal effect lethality. For simplicity, we refer to these embryos as mre1158S or nbs2K embryos, but note that they have a wild-type copy of the paternal mre11 and nbs genes. The lethality of these embryos is caused by a defect in the maternal contribution of MRN.
Maternal Effect Lethality Is Caused by Nuclear Division Defects.
Analyses of DAPI stained mutant embryos suggest that lethality is caused by failure of chromosome segregation (Fig. 1). Early embryos (those examined before cycle 7) appeared to be mostly normal, with occasional nuclei connected by chromosomal bridges (8%, n = 454) (Fig. 1B). As the embryos developed, more nuclei appeared connected by bridges, and nuclei with abnormal DNA content became abundant. Sister nuclei separation failed in 69 out of 228 mitoses; likely, the result of unresolved chromosome bridges. Some of these polyploid nuclei apparently attempted to divide in the next mitosis, creating multiple-lobed nuclei (Fig. 1B). To improve understanding of the chromosome segregation defect, we performed live imaging analyses of mre1158S embryos, using GFP-tagged histones to visualize chromosomes. A movie showing nuclear divisions of such an embryo is presented in Movie S1 and Movie S2. Two hundred nuclei were monitored for 2 or more divisions. Mitotic bridges were observed in 38% of anaphases and telophases.
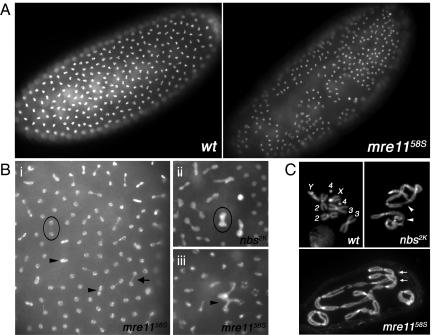
Fig. 1.
Chromosome segregation defects in mre1158S and nbs2K embryos. All panels are pictures of DAPI staining. (A) Low magnification pictures showing 2 similarly staged embryos from wild-type (wt) and mre1158S mothers. In the mutant, nuclei are missing from large areas of the embryo cortex. (B) Close-up pictures of mre1158S and nbs2K embryonic nuclei. (i) The arrow points to one of many mre1158S nuclei connected by chromosome bridge(s). Arrowheads point to 2 of many polyploid nuclei that probably resulted from failed mitoses. The nucleus in the black circle has lagging chromosome(s). (ii) A polyploid nbs2K nucleus is circled. (iii) The arrowhead points to a multilobed mre1158S nucleus possibly having had multiple spindles. (C) Mitotic chromosome spreads from embryonic nuclei. In the wt nucleus, all chromosomes are labeled. In the nbs2K nucleus, arrowheads point to single telomere fusions. In the mre1158S nucleus, arrows point to 2 double fusions each between 2 pairs of sister telomeres. This nucleus was polyploid.
Drosophila embryos can clear abnormal nuclei by “sinking” them interiorly (30, 31). Most late stage mre1158S or nbs2K embryos had large nuclei-free areas (Fig. 1A), and their interior was filled with abnormally large and highly condensed nuclei. They rarely developed signs of gastrulation. We concluded that abundant chromosome bridging led to excessive loss of nuclei and the death of those embryos.
Telomere Fusions Cause Mitotic Catastrophe.
We offer unresolved telomere associations as the most likely mechanism leading to chromosome bridging. Previously, telomere associations in mrn-null mutants were visualized in mitotic chromosome spreads from larval neuroblasts. To provide evidence for telomere associations in embryos, we developed a mitotic squashing technique, and observed mitotic configurations indicative of frequent telomere associations in mutant nuclei (Fig. 1C). Telomere associations were unequivocally identified in 95.3% of mre1158S nuclei (n = 257), and 93.4% of nbs2K nuclei (n = 91).
The occurrence of unresolved mitotic bridges suggests that these telomere associations probably involve covalent DNA linkage. To provide direct evidence, we developed a specialized PCR protocol to isolate telomere fusion junctions, taking advantage of the fact that telomere associations in mre11 or nbs mutants are not associated with significant loss of telomeric DNA (15, 16).
The ends of Drosophila chromosomes are populated by 3 terminal specific retro-transposons (32). They attach to chromosome ends by the 3′ end of the transcript, resulting in tandem arrays of retro-elements in directed repeats. A telomere fusion can result in a pair of telomere-pointing primers facing each other, leading to a PCR product that would not be produced from wild-type samples (Fig. 2A). Using a series of telomere-pointing primers from the conserved GAG region of the most abundant telomere element, HeT-A, we recovered PCR products from 0- to 2-h mre1158S or nbs2K embryos, but such products were rarely recovered from the wild-type controls (Fig. 2B). Sequencing of 9 independent clones from mre1158S and 10 from nbs2K samples revealed that all represented “head-to-head” attachment of HeT-A elements, as predicted for telomere fusions, and appeared to be the products of nonhomologous end joining repair of DSBs (Fig. 2C). Therefore, covalent telomere fusions were abundant in mutant embryos. The 6 clones sequenced from wild-type samples were all linear amplifications of a HeT-A region primed from a single HeT-A primer. These products showed no evidence of telomere fusion, although the mechanism leading to the formation of these products is unknown.
Fig. 2.
Telomeres in mre1158S and nbs2K embryos engage in covalent fusions. (A) Schematic representation for isolating telomere fusion junctions. HeT-A retro-transposons are depicted as block arrows in gray. They attach to chromosomal DNA (black) unidirectionally. Black and white arrowheads denote a pair of end-facing primers. They can anneal to multiple positions along the HeT-A arrays. (Top) Wild-type situation in which the PCRs are not expected to be productive. (Middle) Telomere fusion in which some of the primer pairs can lead to the formation of PCR products. (Bottom) Several features of a HeT-A element: 3′ UTR, gag ORF, and 5′ UTR. The approximate positions are indicated for the 5 end-facing primers (arrowheads). (B) Agarose gel pictures show PCR products obtained using wild-type (+), mre1158S (−; Upper), or nbs2K (−; Lower) DNA templates from embryos. The primer combinations are listed at the top. m, marker DNA with sizes in kilobase. The band marked by * might correspond to nucleotides 2081–3496 in GenBank entry U06920.2 for HeT-A, with 3496 corresponding to the first nucleotide in primer 3. (C) Sequences of fusion junctions from mre1158S (Upper) and nbs2K (Lower). The numbers are nucleotide numbers from U06920.2. Three strands (in the 5′ to 3′ direction) are shown for each junction, with the actual sequences connected by the fusion underlined. The rest of the sequences are those predicted from U06920.2. In each fusion, the top strand came from one telomere that fused with the other telomere shown in the bottom strand, giving rise to the fusion product denoted in the middle strand. The top fusion used the overlapping T (in bold) for repair. The bottom fusion had an a (in small case) as a filler base for repair.
MR Are Excluded from Chromatin in the Mutant Embryos.
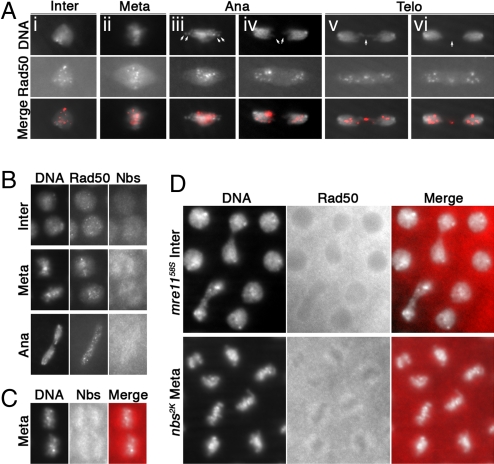
To gain insight into the molecular defect of telomere dysfunction, we studied the cellular distribution of MRN in syncytial embryos using antibodies against each subunit. Immunostaining analyses using MR antibodies produced very similar, if not identical, distribution patterns (Fig. 3A; Fig. S2). In wild-type embryos, MR showed strong staining indicative of abundant maternal contributions of these proteins. We observed MR foci throughout the cell cycle, but they were especially prominent on condensed chromosomes. MR foci did not appear to be localized to telomeres in wild-type cells (Fig. 3A). For example, MR foci are not seen frequently on the dot-like chromosome 4. Also, MR foci are often absent from lagging telomeres in anaphase or telophase. A nontelomeric localization of Rad50 was observed in larval nuclei (17). In wild-type cells, Nbs does not colocalize extensively with Mre11 or Rad50 (Fig. 3 B and C). Instead, Nbs is evenly distributed throughout the interphase nucleus, and is underrepresented on metaphase chromatin, whereas Rad50 forms bright foci.
Fig. 3.
Rad50 forms large nuclear foci in wild-type, but not mutant embryos. DNA (DAPI) and antibody signals are shown as grayscale pictures in separate panels. In merged pictures, antibody signals are shown in red. (A) Rad50 distribution in wild-type cells. (iii) Arrows indicate the dot-like chromosome 4s without Rad50 signals. (iv) Arrows indicate 2 lagging chromosome ends with no Rad50 signals. (v) The arrow points to 2 juxtaposing chromosome ends with strong Rad50 signals. (vi) The arrow points to an area with strong Rad50 signals that appear not to be associated with chromatin. (B) Nbs does not colocalize with Rad50 in wild type cells. (C) Nbs is excluded from mitotic chromatin in wild-type cells. (D) Rad50 is excluded from chromatin in an mre1158S cell in interphase (Upper) and an nbs2K cell in metaphase (Lower). Cell-cycle phases are indicated as follows: Inter, interphase; Meta, metaphase; Ana, anaphase; and Telo, telophase.
MR was absent from interphase chromatin in both mre1158S and nbs2K embryos, although cytoplasmic staining remained (Fig. 3D; Fig. S2). MR was also absent from chromatin during metaphase (Fig. 3D; Fig. S2), suggesting that nuclear membrane breakdown before mitosis was insufficient to restore MR binding to chromatin in the mutants. Thus, mutations in 2 different genes (mre11 and nbs) both led to the exclusion of MR from chromatin.
Maternal Nbs Is Depleted in nbs2K and mre1158S Mutants.
The Nbs subunit regulates nuclear localization of MR in mammalian cell. We examined whether Drosophila cells employ a similar mechanism. We did not detect Nbs in mre1158S or nbs2K nuclei by immunostaining, and used Western blot analyses to document the extent of this Nbs reduction. There was a greatly reduced level of Nbs, but normal amounts of MR in extracts from mre1158S or nbs2K adult females, suggesting that the initial maternal store of Nbs is probably less than the wild-type level. In both mre1158S and nbs2K embryos, the amount of Nbs was below the level of detection, even after enrichment by IP. Although MR proteins were present at near normal levels in the extracts, they were absent from anti-Nbs immuno-precipitates (Fig. 4A).
Fig. 4.
Biochemical characterization of MRN during development. Western blot analysis results using extracts from syncytial embryos produced from wt, mre1158S (58S), or nbs2K (2K) mothers (A), extracts from pupae of wild-type, mre1158S homozygotes, or nbs2K homozygotes from heterozygous crosses (B), or extracts from pupae of wild-type, mre11Δ homozygotes (mΔ), or nbsΔ homozygotes (nΔ) (C). The antibodies used are indicated at the left. Arrowheads indicate the positions of the protein of interest except for Tubulin. Other bands are recognized by the antibodies nonspecifically. ∗∗, Presumed truncated Nbs protein from nbs2K homozygous extracts; WCE, whole-cell extract. IP with the antibody indicated.
The nbs2K mutation encodes an N-terminally truncated protein (Fig. 4B Center; Fig. S1), and similarly truncated proteins are unstable in yeast and mammals (7, 33). Thus, the absence of Nbs in extracts from nbs2K embryos was expected. However, Nbs depletion was not expected for mre1158S embryos, because these embryos were from females with both wild-type copies of the nbs gene on a chromosome separate from the mre11 locus. It is possible that defective MRN interactions in mre1158S might have led to Nbs depletion, because the mutation disrupts such interactions in yeast (26). However, results from our IP experiments did not support this hypothesis (Fig. 4A). In mre1158S extracts, interactions between Mre1158S and Rad50 were about as efficient as those between normal MR proteins in wild-type extracts. In nbs2K extracts, interactions between MR were also proficient. Therefore, defective MR interaction was not observed in the mutant extracts. Nbs depletion has prevented us from assaying the efficiency of Mre11-Nbs interaction in the mutant extracts.
Residual MRN Function in Postembryonic Tissues of mre11 and nbs Mutants.
Contrary to the severe capping defect in the embryos, mre1158S and nbs2K postembryonic animals developed mild telomere dysfunction, and were viable. We investigated possible mechanisms for this difference by Western blot and IP analyses. As already indicated in Figs. 3 and 4A, there is abundant maternal deposition of MRN in wild-type syncytial embryos. This maternal supply probably runs out during the third instar larval stage. Two observations support this hypothesis. First, telomere uncapping in mre11Δ35K1-null mutants first occurs during larval development (SI Materials and Methods). Second, maternal Mre11 and Nbs proteins are absent in pupal extracts from respective deletion mutants (Fig. 4C). In mre1158S pupal extracts, the levels of Rad50 and the mutant Mre1158S proteins were slightly reduced, whereas Nbs level was reduced to a much greater extent (Fig. 4B Left). Similar to the situation observed in embryo extracts, the interactions between Mre1158S and Rad50 were efficient in pupal extracts (Fig. 4B Right). Interestingly, the residual Nbs proteins seemed to able to interact with both Mre1158S and Rad50, because we detected a very small amount of MR in anti-Nbs IPs (Fig. 4B Center). We surmise that this residual amount of Nbs, possibly in complex with MR, might be sufficient to provide telomere protection and to support viability.
We detected a protein that migrates faster than wild-type Nbs in nbs2K extracts (marked with 2 asterisks in Fig. 4B), which might correspond to the N-terminally truncated protein as our antibody recognized C-terminal epitopes in Nbs. This protein was much less abundant than Nbs in wild-type cells, consistent with our hypothesis that the truncation destabilized the mutant protein. Similar to the situation in mre1158S, this residual level of Nbs in nbs2K might be sufficient to maintain telomere integrity. We tested whether the remaining Nbs function in nbs2K could be reduced further by the mre1158S mutation, and found that mre1158S and nbs2K double mutants were lethal as pupae. They suffered a more severe telomere dysfunction than either single mutant (1.4 fusions per nucleus, n = 81).
Discussion
We have used Drosophila to study the functions of MRN in a developmental context. We discovered that mre11 and nbs hypomorphic mutations yielded maternal effect lethality caused by telomere fusions resulting in failure of mitosis. This loss of telomere protection is probably caused by the depletion of the maternal Nbs pool and the subsequent exclusion of MR from chromatin.
Regulation of MR Function by Nbs.
The abnormal localization of MR in nbs2K embryos can be explained by the depletion of maternal Nbs due to a destabilizing nbs mutation. Based on this result, we suggest that the presence of Nbs is essential for MR loading to chromatin, and that this loading is unlikely to occur during mitosis, because Nbs is excluded from mitotic chromatin in normal cells (Fig. 3C). The situation is less clear for mre1158S embryos. The H230Y mutation might have led to the exclusion of MR from chromatin and a separate defect that caused Nbs destabilization. An alternative hypothesis is that the exclusion of MR from chromatin is secondary to Nbs depletion, similar to the situation in nbs2K. We cannot distinguish between these 2 possibilities.
The mre1158S mutation in this study was modeled after the yeast mutation, but the 2 have different effects on MRN integrity. The yeast Mre1158 protein does not interact with either Rad50 or Xrs2 (the yeast Nbs equivalent), but those defective interactions do not lead to Xrs2 depletion in vivo (26). However, we observed Nbs depletion in mre1158S animals despite efficient interactions of Mre1158S with Rad50 in embryonic and pupal extracts (Fig. 4). This difference may be organism specific, and an explanation of how mre1158S compromises MRN integrity in Drosophila will require further investigation.
In contrast to syncytial embryos from mre1158S or nbs2K homozygous mothers, mre1158S or nbs2K mutant embryos from heterozygous parents have sufficient maternal contributions to support proliferation passed the critical point of zygotic activation. Subsequently, zygotic nbs expression may be sufficient for maintaining a low level of Nbs in postembryonic cells to prevent telomere uncapping in these mutant animals. However, other developmental factors could have also contributed to the different degrees of telomere dysfunction in mre1158S and nbs2K animals during embryonic versus postembryonic development. For example, although Nbs is essential for MR binding to chromatin in embryonic cell cycles, other proteins might, in late stages of development, partly fulfill the function of recruiting MR. This proposition would be consistent with previous results showing that nbs1 mutants have a partial defect in chromatin retention of Rad50 during metaphase in larval neuroblasts (18).
Other Possible Defects in mre1158S and nbs2K Embryos.
Our results from mitotic chromosome spreads and telomere fusion PCR analyses suggest that telomere fusion is abundant in mre1158S and nbs2K embryonic nuclei, and is probably the major cause for mitotic failures. However, there may be other possibilities, because we showed nuclear MR foci that were not at the telomere (Fig. 3). Consistently, we did not detect interactions between MRN and the HOAP capping protein (34, 35) in coIP experiments. Future work decoding the chromosomal addresses for these MR foci may implicate other defects in chromatin architecture. It is conceivable that other types of chromosome fusions occur such as those between DSBs that persist because of MRN dysfunction, or between DSBs and uncapped telomeres.
MRN is also required for meiotic DSB repair and mrn mutations disrupt meiotic processes in animals (8, 25, 36, 37). In Drosophila females, defects in meiotic DSB repair lead to the activation of a checkpoint that disrupts axis formation in oocytes, giving rise to embryos with a “spindle” phenotype (38). Female mre1158S and nbs2K mutants lay abundant embryos, and these embryos display no spindle phenotype. Also, meiotic progression in mre1158S females is normal with a delay in the repair of some DSBs (K. McKim, personal communication). Therefore, we have no evidence to suggest that mitotic catastrophes in mre1158S and nbs2K embryos are a manifestation of a gross defect in meiotic DSB repair.
MRN controls various checkpoints that arrest the cell cycle in response to DNA damage (21). The rapid embryonic cycles in Drosophila are atypical, and lack conventional checkpoints, because replication inhibitors or X-ray irradiation are unable to arrest cell-cycle progression (30, 39). However, loss of MRN could activate other specialized cell-cycle control mechanisms operating during the early cell divisions. For example, mitotic failure in mre1158S and nbs2K embryos could activate CHK2 in subsequent cell cycle(s) leading to nuclei displaced from the embryo cortex (31). Also, the mitotic spindle checkpoint might also be activated because of abnormal chromosome behaviors in mutant embryos. Silva et al. (40) identified mitotic defects in embryos that were devoid of maternal ATM. Because of the intimate functional relationship between ATM and MRN, it is possible that telomere uncapping was also responsible for those defects.
Critical Requirement for MRN in Early Development Is Conserved in Animals.
The maternal effect lethality uncovered in our study is similar to that described for mre11ATLD and nbsΔB mice, which suggests that the requirement for MRN in early development is conserved in animals. Theunissen et al. (23) speculated that a synergistic effect of defective checkpoint and recombination functions was responsible for the rapid loss of cell proliferation in those mice. We suggest that telomere dysfunction, because of the instability of the maternal Nbs supply, might be the major contributing cause for maternal lethality.
In mrn and other telomere uncapping mutants in Drosophila, one of the most striking consequences of chromosome segregation failure is the generation of polyploid cells (Fig. 1). This observation suggests that the mitotic apparatus might not exert enough force to overcome multiple covalent linkages between DNA molecules (41). Interestingly, many chicken DT40 cells and mouse B-lymphocytes become polyploid after a gradual loss of MRN (42, 43), suggesting that these polyploid cells might have occurred as a result of telomere fusion. Although telomere elongation in Drosophila is independent of the telomerase function common in most other animals, the telomere capping function of Drosophila MRN is probably a conserved feature.
Materials and Methods
Primer sequences are listed in Table S1. Information about primers used for sequencing is available on request. Fly stocks and antibody information, as well as a detailed protocol for extract preparations, are provided in SI Materials and Methods.
Mitotic Spreads of Larval and Embryonic Nuclei.
Mitotic spreads from larval neuroblasts were performed as described (44). For embryonic mitotic spreads, the larval protocol and a protocol for metabolic labeling of embryos (45) were combined and modified as follows. Embryos (0–1 h) were collected onto a Netwell insert with 74-μm mesh (Costar, Corning). Embryos were dechorionated with 50% bleach for 90 s, and washed extensively with embryo wash buffer (0.7% NaCl/0.05% Triton X-100). Embryos were dried with blotting paper, then submerged in a small amount of octane that just covered the embryo layer. All subsequent treatments were performed on a rotating platform, and embryos were agitated periodically using a pipette. Embryos were treated for 5 min in octane, dried, and rehydrated in embryo wash for 1 min with agitation. Embryos were treated with 0.05 mM colchicine in 0.7% NaCl for 20 min, followed by 5 min in a hypotonic solution of 0.5% sodium citrate. The hypotonic solution was substituted with embryo wash, the embryos were transferred to V-vials (Wheaton), and the aqueous solution was removed as much as possible; 1 mL of a methanol, acetic acid, and water mix (11:11:2) was added to the embryos, and they were fixed for at least 5 min on a rotator, and stored at 4 °C until used for chromosome spreading. A drop of the fixative carrying embryos was placed on a slide. To avoid overcrowding of mitotic figures, <10 embryos were loaded per slide. A clean siliconized coverslip was placed on the embryos. The embryos were squashed first gently, then hard. All remaining steps were performed according to the protocol for larval neuroblasts (44).
Isolation of Telomere Fusion Junctions.
As templates, we used DNA from 0- to 2-h embryos from mre1158S or nbs2K females that had been mated with their heterozygous male siblings. Similarly staged embryos from matings between heterozygous male and female siblings were used as controls. Embryos were homogenized in a buffer (100 mM Tris, pH 8.8/200 mM NaCl/5 mM EDTA/0.2% of SDS) with a final concentration of 5 ng/μL of proteinase K freshly added, and left at room temperature for 2 h or at 4 °C overnight. DNA was phenol extracted, and iso-propanol precipitated. Between 0.2 and 1 μg of genomic DNA was used in a 50 to 100 μL PCR using Extaq (Takara) with the following PCR program: 98 °C 30 s, 68 °C 5–8 min for 40 cycles followed by a single 72 °C 10-min step. Primers were HeT-A453rev (primer 1), HeT-A1196rev (primer 2), HeT-A1751rev (primer 3), HeT-A1997rev (primer 4), and HeT-A2615rev (primer 5). The combinations 1 + 4, 2 + 4, 3 + 4, and 3 + 5 were used for junction isolation (Fig. 2). PCR products were cloned by TOPO TA cloning and sequenced.
Whole-Mount DAPI and Antibody Staining of Embryos.
DAPI and Immunostaining of whole-mount embryos were performed essentially as described using boiling as the initial fixation method (46). Anti-Rad50 sera from either rabbit or guinea pig were used at a 1:200 dilution. Anti-Mre11 and anti-Nbs sera from guinea pigs were used at a 1:100 dilution. Secondary antibodies (Invitrogen) were used at a 1:500 dilution.
Supplementary Material
Acknowledgments.
We thank Dr. Paul Goldsmith's group at National Cancer Institute (NCI) for purifying anti-Rad50 antibodies; Drs. Kami Ahmad (Harvard University, Cambridge, MA) and Maurizio Gatti (University of Rome, Rome, Italy) for reagents; members of the Rong lab for comments on the manuscript; and Drs. Maxine Singer, Michael Lichten, and Dhruba Chattoraj at NCI for their efforts in improving this manuscript. This work was supported by the NCI Intramural Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902707106/DCSupplemental.
References
- 1.Assenmacher N, Hopfner KP. MRE11/RAD50/NBS1: Complex activities. Chromosoma. 2004;113:157–166. doi: 10.1007/s00412-004-0306-4. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins BB, Paull TT. The p. furiosus mre11/rad50 complex promotes 5′ strand resection at a DNA double-strand break. Cell. 2008;135:250–260. doi: 10.1016/j.cell.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science. 2004;304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 5.Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem Cell Biol. 2007;85:509–520. doi: 10.1139/O07-069. [DOI] [PubMed] [Google Scholar]
- 6.Desai-Mehta A, Cerosaletti KM, Concannon P. Distinct functional domains of nibrin mediate Mre11 binding, focus formation, and nuclear localization. Mol Cell Biol. 2001;21:2184–2191. doi: 10.1128/MCB.21.6.2184-2191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maser RS, Zinkel R, Petrini JH. An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat Genet. 2001;27:417–421. doi: 10.1038/86920. [DOI] [PubMed] [Google Scholar]
- 8.Difilippantonio S, et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nat Cell Biol. 2005;7:675–685. doi: 10.1038/ncb1270. [DOI] [PubMed] [Google Scholar]
- 9.Larrivée M, LeBel C, Wellinger RJ. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 2004;18:1391–1396. doi: 10.1101/gad.1199404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai W, Sfeir AJ, Hoshiyama H, Shay JW, Wright WE. The involvement of the Mre11/Rad50/Nbs1 complex in the generation of G-overhangs at human telomeres. EMBO Rep. 2006;7:225–230. doi: 10.1038/sj.embor.7400600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diede SJ, Gottschling DE. Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr Biol. 2001;11:1336–1340. doi: 10.1016/s0960-9822(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 12.Frank CJ, Hyde M, Greider CW. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol Cell. 2006;24:423–432. doi: 10.1016/j.molcel.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Goudsouzian LK, Tuzon CT, Zakian VA. S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol Cell. 2006;24:603–610. doi: 10.1016/j.molcel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Foster SS, Zubko MK, Guillard S, Lydall D. MRX protects telomeric DNA at uncapped telomeres of budding yeast cdc13–1 mutants. DNA Repair. 2006;5:840–851. doi: 10.1016/j.dnarep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Bi X, Wei SC, Rong YS. Telomere protection without a telomerase; the role of ATM and Mre11 in Drosophila telomere maintenance. Curr Biol. 2004;14:1348–1353. doi: 10.1016/j.cub.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 16.Bi X, et al. Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance. Proc Natl Acad Sci USA. 2005;102:15167–15172. doi: 10.1073/pnas.0504981102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciapponi L, et al. The Drosophila Mre11/Rad50 complex is required to prevent both telomeric fusion and chromosome breakage. Curr Biol. 2004;14:1360–1366. doi: 10.1016/j.cub.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Ciapponi L, Cenci G, Gatti M. The Drosophila Nbs protein functions in multiple pathways for the maintenance of genome stability. Genetics. 2006;173:1447–1454. doi: 10.1534/genetics.106.058081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oikemus SR, et al. Epigenetic telomere protection by Drosophila DNA damage response pathways. PLoS Genet. 2006;2:e71. doi: 10.1371/journal.pgen.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong YS. Telomere capping in Drosophila: Dealing with chromosome ends that most resemble DNA breaks. Chromosoma. 2008;117:235–242. doi: 10.1007/s00412-007-0144-2. [DOI] [PubMed] [Google Scholar]
- 21.Stracker TH, Theunissen JW, Morales M, Petrini JH. The Mre11 complex and the metabolism of chromosome breaks: The importance of communicating and holding things together. DNA Repair. 2004;3:845–854. doi: 10.1016/j.dnarep.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Williams BR, et al. A murine model of Nijmegen breakage syndrome. Curr Biol. 2002;12:648–653. doi: 10.1016/s0960-9822(02)00763-7. [DOI] [PubMed] [Google Scholar]
- 23.Theunissen JW, et al. Checkpoint failure and chromosomal instability without lymphomagenesis in Mre11(ATLD1/ATLD1) mice. Mol Cell. 2003;12:1511–1523. doi: 10.1016/s1097-2765(03)00455-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee LA, Orr-Weaver TL. Regulation of cell cycles in Drosophila development: Intrinsic and extrinsic cues. Annu Rev Genet. 2003;37:545–578. doi: 10.1146/annurev.genet.37.110801.143149. [DOI] [PubMed] [Google Scholar]
- 25.Mehrotra S, McKim KS. Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet. 2006;2:e200. doi: 10.1371/journal.pgen.0020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usui T, et al. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 27.Paull TT, Gellert M. A mechanistic basis for Mre11-directed DNA joining at microhomologies. Proc Natl Acad Sci USA. 2000;97:6409–6414. doi: 10.1073/pnas.110144297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SE, Bressan DA, Petrini JH, Haber JE. Complementation between N-terminal Saccharomyces cerevisiae mre11 alleles in DNA repair and telomere length maintenance. DNA Repair. 2002;1:27–40. doi: 10.1016/s1568-7864(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 29.Gao G, McMahon C, Chen J, Rong YS. A powerful method combining homologous recombination and site-specific recombination for targeted mutagenesis in Drosophila. Proc Natl Acad Sci USA. 2008;105:13999–14004. doi: 10.1073/pnas.0805843105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raff JW, Glover DM. Nuclear and cytoplasmic mitotic cycles continue in Drosophila embryos in which DNA synthesis is inhibited with aphidicolin. J Cell Biol. 1988;107:2009–2019. doi: 10.1083/jcb.107.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takada S, Kelkar A, Theurkauf WE. Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell. 2003;113:87–99. doi: 10.1016/s0092-8674(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 32.Pardue ML, Debaryshe PG. Drosophila telomeres: A variation on the telomerase theme. Fly. 2008;2:101–110. doi: 10.4161/fly.6393. [DOI] [PubMed] [Google Scholar]
- 33.Shima H, Suzuki M, Shinohara M. Isolation and characterization of novel xrs2 mutations in Saccharomyces cerevisiae. Genetics. 2005;170:71–85. doi: 10.1534/genetics.104.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shareef MM, et al. Drosophila heterochromatin protein 1 (HP1)/origin recognition complex (ORC) protein is associated with HP1 and ORC and functions in heterochromatin-induced silencing. Mol Biol Cell. 2001;12:1671–1685. doi: 10.1091/mbc.12.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cenci G, Siriaco G, Raffa GD, Kellum R, Gatti M. The Drosophila HOAP protein is required for telomere capping. Nat Cell Biol. 2003;5:82–84. doi: 10.1038/ncb902. [DOI] [PubMed] [Google Scholar]
- 36.Chin GM, Villeneuve AM. C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev. 2001;15:522–534. doi: 10.1101/gad.864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherry SM, et al. The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr Biol. 2007;17:373–378. doi: 10.1016/j.cub.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa V, Kimm N, Lehmann R. A maternal screen for genes regulating Drosophila oocyte polarity uncovers new steps in meiotic progression. Genetics. 2007;176:1967–1977. doi: 10.1534/genetics.106.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogarty P, et al. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- 40.Silva E, et al. ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr Biol. 2004;14:1341–1347. doi: 10.1016/j.cub.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 41.Bloom KS. Beyond the code: The mechanical properties of DNA as they relate to mitosis. Chromosoma. 2008;117:103–110. doi: 10.1007/s00412-007-0138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi-Iwai Y, et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reina-San-Martin B, Nussenzweig MC, Nussenzweig A, Difilippantonio S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc Natl Acad Sci USA. 2005;102:1590–1595. doi: 10.1073/pnas.0406289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pimpinelli S, Bonaccorsi S, Fanti L, Maurizio G. Preparation and analysis of Drosophila mitotic chromosomes. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Plainview, NY: Cold Spring Harbor Lab Press; 2000. pp. 3–23. [Google Scholar]
- 45.Shermoen A. BrdU labeling of chromosomes. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Plainview, NY: Cold Spring Harbor Lab Press; 2000. pp. 57–65. [Google Scholar]
- 46.Rothwell WF, Sullivan W. Fluorescent analysis of Drosophila embryos. In: Sullivan W, Ashburner M, Hawley RS, editors. Drosophila Protocols. Plainview, NY: Cold Spring Harbor Lab Press; 2000. pp. 141–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.