Abstract
Elephants are the only living representatives of the Proboscidea, a formerly diverse mammalian order whose history began with the 55-million years (mys) old Phosphatherium. Reported here is the discovery from the early late Paleocene of Morocco, ca. 60 mys, of the oldest and most primitive elephant relative, Eritherium azzouzorum n.g., n.sp., which is one of the earliest known representatives of modern placental orders. This well supported stem proboscidean is extraordinarily primitive and condylarth-like. It provides the first dental evidence of a resemblance between the proboscideans and African ungulates (paenungulates) on the one hand and the louisinines and early macroscelideans on the other. Eritherium illustrates the origin of the elephant order at a previously unknown primitive stage among paenungulates and “ungulates.” The primitive morphology of Eritherium suggests a recent and rapid paenungulate radiation after the Cretaceous-Tertiary boundary, probably favoured by early endemic African paleoecosystems. At a broader scale, Eritherium provides a new old calibration point of the placental tree and supports an explosive placental radiation. The Ouled Abdoun basin, which yields the oldest known African placentals, is a key locality for elucidating phylogeny and early evolution of paenungulates and other related endemic African lineages.
Keywords: Africa-Morocco, Afrotheria, Paenungulata, Placentalia, Proboscidea
The elephant order (Proboscidea) includes some of the most derived and spectacular extant placental mammals. Today, it is represented by only 3 species. However, the fossil record shows that this diversity is relictual, and that the order has a remarkably rich and long history (1). The early history of the proboscideans is endemic to Africa, where they have their oldest record 55 million years ago (mys) at the beginning of the Eocene, with primitive representatives such as Phosphatherium (2). Reported here is the discovery in Morocco of a new, earliest known proboscidean predating Phosphatherium by 5 mys. It demonstrates an early history of elephant relatives into the Paleocene and close to the beginning of the placental radiation (3, 4). It provides the first evidence of a transitional stage between modern ungulates, especially African ungulates, and primitive condylarth-like mammals (Louisininae here) from the beginning of the Tertiary.
The proboscidean reported here was discovered in Sidi Chennane quarries of the Ouled Abdoun phosphate basin, Morocco, 10–20 km south of Grand Daoui quarries where Phosphatherium occurs, in an overlying Ypresian level (1, 5–7). It comes from the “lower bone bed” horizon of local phosphate “bed IIa“ that yielded other mammals, including the earliest hyaenodontids (8) and which lies close to the local Thanetian base above the Danian “bed IIb.” Its Thanetian age is indicated by its stratigraphic position and by the associated elasmobranch fauna (SI Appendix, Table S1). The low position of the lower bone bed in Thanetian beds IIa involves the locally undistinguished Selandian age, i.e., an early Late Paleocene age as old as ca. 60 mys. The early Late Paleocene age of the mammal level of Sidi Chennane phosphate quarries makes them the oldest known placental mammal localities from Africa.
Systematic Palaeontology
Placentalia Owen, 1837
Paenungulata Simpson, 1945
Proboscidea Illiger, 1811
Family indet.
Eritherium azzouzorum n.g., n.sp.
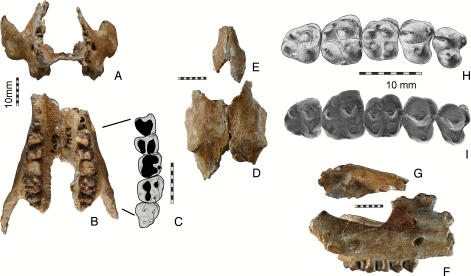
Fig. 1.
Skull and upper dentition of Eritherium azzouzorum n.g., n.sp. (A–G) Holotype, MNHN PM69. (A and B) Anterior part of skull (rostrum) with maxilla and jugals in mesial and ventral views and showing nasal cavity, zygomatic arches and jugal dentition. (C) Left P3–4, M1–3, occlusal sketch. (D and E) Frontals and nasals in dorsal view, specimen MHNT PAL 2006.0.18–20 (Museum National d'Histoire Naturelle de Toulouse). (F and G) Frontal and rostrum (jugal and right maxillary with P3–4, M1–3) in lateral view (G is reversed for reconstruction). (H and I) Right P3–4, M1–3 in occlusal view (H is SEM view of I). (Scale bar, 10 mm.)
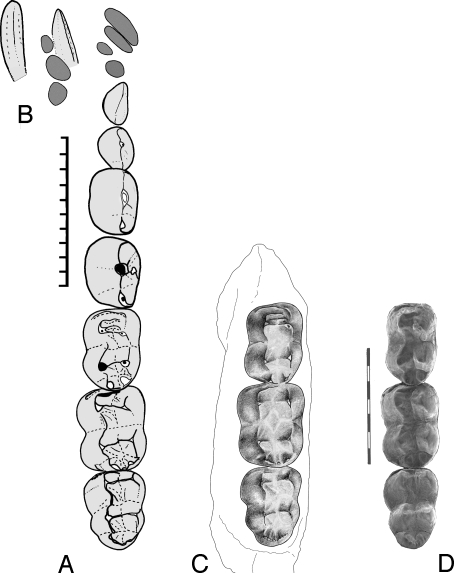
Fig. 2.
Lower dentition of Eritherium azzouzorum n.g., n.sp. (A) Reconstruction of lower tooth row: M3–1, P4–1 and alveoli for C1, I3–1 in occlusal view, based on specimens OCP DEK/GE 307 (M3–1), MNHN PM28 (P4–2, alveoli of C1, I3–1), PM84 [(d)P1]. (B) Sketch of enlarged and styliform I2 in occlusal (dorsal) and lingual views, plus alveoli of (d)P1, C1 and I3 from specimen MNHN PM50. (C and D) Left M3–1, specimen OCP DEK/GE 307, in occlusal view (drawing and SEM photograph). (Scale bar, 10 mm.)
Etymology.
Eritherium (monotypic genus), from eri (g.), early, and therion (g.), beast; azzouzorum, species dedicated to people from Ouled Azzouz village close to Sidi Chennane, who recovered most of the fossils.
Locality and Age.
Morocco, NE Ouled Abdoun basin, Sidi Chennane quarries; phosphate bed IIa, lower bone-bed horizon, early Thanetian (incl. Selandian). Type locality: Quarry A4, N 32° 38′18.04′′, W 06° 42′ 57.10′′.
Holotype.
MNHN [Museum National d'Histoire Naturelle] PM69: Skull rostrum preserving maxilla with P3–4 and M1–3 (length of P3-M3 = 27 mm), Fig. 1 A–G.
Hypodigm.
There are 15 specimens representing upper and lower jugal dentition and skull part, including the holotype, MHNL PAL 2006.0.18–20 (P3–4, M1–3), OCP DEK/GE 307 (M1–3), MNHN PM50 (I2, P2–4, M1–3).
Diagnosis.
Most primitive and smallest known proboscidean, along with Khamsaconus. Dental and cranial morphology closest to Phosphatherium, and to Khamsaconus (known only by one tooth). Main proboscidean synapomorphies: I1 enlarged, larger than I2, I1–2 with high (styliform), labio-lingually compressed, asymmetric, and procumbent crown; I3 strongly reduced; C1 very small; (d)P1 small and simple; molar hypoconulid labial; coronoid retromolar fossa enlarged. Proboscidean synapomorphies with more ambiguous distribution: Orbit anterior rim bordered by maxillary and with high lateral jugal bony blade; no postcingulum and lingual cingulum on M1–3; P3–4 more or less simplified; molar mesoconid present; molar cristid obliqua labial; postentoconulid on M1–2. The combination of these features is distinct from all other ungulates, including primitive hyracoids.
Differs from Phosphatherium by a smaller size (60–70%) and primitive features: Bunodont-lophodont molars, small M33, full eutherian lower dental formula (retention of I3 and (d)P1), maxillary less developed on the orbit and orbit position above P4-M1 level. Other primitive features: Shorter mandibular symphysis; upper premolars with no trace of protoloph and weaker metacone; more developed mesostyle and ectocingulum (upper molars); postmetacristid distinct (lower molars); C1 larger; I1–2 less enlarged and slender; M22 less enlarged with respect to M11; absence of submaxillary fossa. Khamsaconus differs by: Smaller size (50%), large postentoconule, more anterior hypocone with respect to metacone, preprotocrista ending at mesio-lingual basis of paracone, and related reduced paracingulum.
Description.
See characters K1–143 in SI Appendix. The estimated body mass of Eritherium azzouzorum, inferred from allometric relation of tooth size [regression equations from Damuth et al. (9) and Janis (10)], varies between 3 and 8 kg, with a median estimation most comparable to the body weight of the largest extant hyraxes (e.g., Procavia, 4–5 kg).
Characters Study and Relationships with Proboscideans
Among ungulate mammals, the dental and cranial morphology of Eritherium closely recalls the primitive proboscideans Khamsaconus and Phosphatherium. There are also some dental resemblances with primitive hyraxes such as Seggeurius, with louisinine “condylarths” Monshyus and Microhyus, and with primitive macroscelideans such as Chambius. However, detailed comparative anatomical study (characters K1–143, see SI Appendix) and an extended phylogenetic analysis with TNT (11) among lophodont ungulates demonstrate unambiguous relationships with Phosphatherium and proboscideans (Fig. 3). This is supported in unweighted parsimony analysis, with strong Bremer and bootstrap indices, and in the “implied weighting” exact analysis.
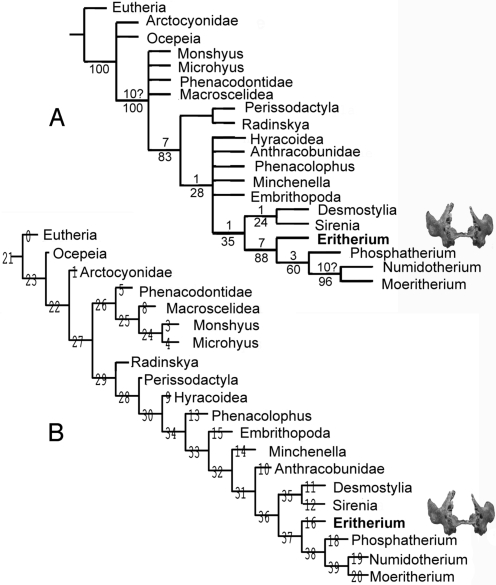
Fig. 3.
Relationships of Eritherium azzouzorum n.g., n.sp. Cladograms resulting from parsimony analysis with TNT program (11) based on modified matrix for Phosphatherium analysis (7) (see SI Appendix). With respect to Phosphatherium analysis (7), several basal taxa are added to test basal relationships of Paenungulata, and Khamsaconus, which TNT “pruned tree” procedure (11) identifies as the most unstable taxon, is excluded from the analysis. (A) Consensus of 14 most parsimonious trees (L = 455 steps, CI = 58.7; CI = 41.8) resulting from exact analysis (nelsen); upper numbers in nodes refer to Bremer indices, lowers refer to bootstrap indices. (B) Standard TNT “implied weighting” analysis (L = 457 steps, RI = 58.4; CI = 41.6) with congruent topology to that resulting from Phosphatherium study (7), in particular with similar primitive position of Anthracobunia and Embrithopoda within Tethytheria. In both cases the proboscidean (basal) relationship of Eritherium azzouzorum n.g., n.sp is well supported.
Several identified proboscidean synapomorphies (see Diagnosis and Table 1) support relationships of Eritherium to Phosphatherium and Proboscidea. Some deserve special comments: C1 is more reduced than in embrithopods (K6). (d)P1 (K9) is smaller and simpler than in sirenians and desmostylians; its reduction fits well the proboscidean evolutionary trend toward its loss, with the remarkable intermediate state of Phosphatherium whose (d)P1 is present in juvenile individuals (specimen OCP DEK/GE 450) but lost in adults. The enlarged I1 (K3), reduced C1 (K6) and (d)P1 (K9), hypoconulid in labial position (K37) and well developed coronoid retromolar fossa (K55) are unambiguous synapomorphies.
Table 1.
Proboscidean synapomorphies of Eritherium azzouzorum n. g., n.sp.
| K | RI | State | Homoplasy | Description |
|---|---|---|---|---|
| 3 | 100 | 0→1* | I1 enlarged and probably high crowned | |
| 5 | 83 | 0→1 | c: Ocepeia | I3 strongly reduced |
| 6 | 77 | 01→2* | C1 very small | |
| 9 | 69 | 01→2 | c: ? | (d)P1 small and simple |
| 14, | 42, | 1→0 | r: Paenungulata/Tethytheria, Daouitherium, | Simplified premolars: P3–4: paraconid and |
| 15, | 42, | 0→1 | Moeritherium, and advanced proboscideans | ectocingulid reduced; P3: metaconid reduced |
| 18, | 25, | 0→1 | ||
| 21 | 50 | 1→0 | ||
| 34 | 20 | 0→1 | r: Numidotherium; c: Hyracoidea | M1–3: Mesoconid |
| 35 | 28 | 01→2 | r: Numidotherium, Daouitherium, Barytherium | M1–3: Cristid obliqua labial |
| 37 | 44 | 0→2* | M1–3: hypoconulid labial | |
| 39 | 33 | 0→1 | r: Numidotherium, Daouitherium, Barytherium | M1–2: Postentoconulid |
| 55 | 62 | 1→2* | Coronoid retromolar fossa enlarged | |
| 86 | 50 | 1→0 | r: Moeritherium | M1–3: No postcingulum |
| 87 | 63 | 3→2 | r: Moeritherium | M1–3: No lingual cingulum |
| 118 | 83 | 0→1 | c: Embrithopoda | Skull: Orbit mesially bordered by maxillary |
| 125 | 75 | 0→1 | c: Sirenia | Skull: Jugal high, with ventral process |
| 11–12 | 33, | 1→2 | 11(1)–12(1): also Desmostylia and Sirenia; r?: | P2 small, simplified and uniradicular |
| 33 | 0→1 | Numidotherium, Daouitherium, Barytherium, Moeritherium |
Proboscidean synapomorphies of Eritherium azzouzorum n.g., n. sp. K, character number (see description in SI Appendix). It should be noted that all characters listed are preserved and observed in available material of Eritherium. r and c, reversion and convergence known in taxa compared; RI: Retention Index.
*Nonhomoplastic state.
Several derived features shared with Phosphatherium, that are distinctive among paenungulates, are strikingly reversed in later proboscideans (Table 1). The simplified P3–4 (K14–15, K18, K21) shared with Numidotherium is distinctive from later proboscideans (Table 1) but also from the inferred generalized paenungulate morphotype. The cladistic analysis suggests indeed that the simplified P3–4 is unexpectedly reversed in Proboscidea with respect to the ancestral paenungulate (molarized) morphotype, and that advanced proboscideans secondarily acquired molarized premolars. Alternative hypothesis of convergent molarization of premolars in several paenungulate lineages cannot, however, be excluded, which would emphasize again the primitive pattern of Eritherium. Other proboscidean features of Eritherium are occasionally known in other paenungulates. Enlarged and procumbent lower incisors (K1–2) are generalized in tethytherians, and some embrithopods share moderately enlarged I1 (K3). However, I1–2 of Eritherium is more proboscidean-like in the procumbent, high (styliform), and asymmetric crown (inferred in I1), and in the larger I1 (alveolus). Strong reduction of I3 (K5) occurs in Ocepeia, but in a less advanced stage; the Eritherium condition foreshadows the loss of this tooth in Phosphatherium and more advanced proboscideans. Proboscidean synapomorphies, such as the high lateral jugal bony blade (K125) and the presence of a molar mesoconid (K34), imply convergences with sirenians and hyracoids, respectively. An alternative interpretation of the evolution of the high jugal (zygoma, K125) in our cladistic analysis is that it is a tethytherian feature secondarily lost in the desmostylians. Other derived features shared with proboscideans, such as reduced preparacrista (K93) and conules (K95), are known in other paenungulates as either convergences or more inclusive synapomorphies. The maxillary extension and related reduction of jugal on the antorbital rim (K118) is a classic proboscidean synapomorphy. The jugal extends further anteriorly in Eritherium than in Phosphatherium, but it is still more reduced than in the eutherian condition. The feature is made less significant with regard to Eritherium plesiomorph condition and with regard to incipient jugal reduction in hyracoids (12). The P2 small, simple, and single-rooted (K11–12) in Eritherium and Phosphatherium also recalls sirenians and desmostylians. P2 is more simplified in Eritherium and Phosphatherium (K11) (2) as a unique and unexpectedly derived trait with respect to other early proboscideans. However, our analysis does not support their autapomorphic grouping, implying either convergence or reversals in proboscideans.
Eritherium also shows remarkable shared primitive features with Phosphatherium by contrast to later proboscideans, such as the retention of C1 and of molar centrocrista and mesostyle. Other striking plesiomorphies of Eritherium are unknown in Phosphatherium and later proboscideans, such as the small size (60–70% of Phosphatherium), bunodont incipiently bilophodont molars, small M33, full eutherian dental formula (retention of I3 and (d)P1), orbit above P4-M1 level, and maxillary less developed on the orbit (K118). Eritherium shares poorly advanced bilophodonty with Khamsaconus (13) and supports its proboscidean relationships (5, 7). However, Khamsaconus is distinctive (see Diagnosis) with some of the shared differences with Phosphatherium, suggesting a more advanced lophodont trend and possible familial distinction. In addition to several recognized primitive tethytherian and paenungulate traits (see SI Appendix), Eritherium allows the identification of additional convergences in these groups, such as the large M33, the submaxillary fossa, and the orbit anterior to P4.
Supraordinal Relationships
The TNT unweighted parsimony analysis including Eritherium yields a very poorly resolved consensus tree mainly resulting from the unstable position of Khamsaconus. Analysis without Khamsaconus shows that, besides the robust proboscidean relationships of Eritherium, basal relationships among paenungulates remain unstable (7), as illustrated by the basal polytomy in the consensus (Fig. 3A). This polytomy is basically related to our poor fossil knowledge of the ancestral morphotype of several orders such as Embrithopoda, Desmostylia, and Anthracobunia. Our analysis supports a Sirenia-Desmostylia clade sister group of Proboscidea within Tethytheria. The standard TNT “implied weighting” analysis yields a topology (Fig. 3B), which is nearly identical to that of Gheerbrant et al. (7).
Eritherium is remarkably reminiscent of the early Tertiary European louisinines Microhyus and Monshyus and early macroscelideans Chambius and Herodotius, in such primitive features as the bunodont incipiently lophodont molars (in addition to the small size, small M33, and full dental formula). This is the initial report of resemblances between the louisinine “condylarths” (Apheliscidae) and the proboscideans. The bunodont incipient lophodont morphotype is derived relative to the eutherian condition, and it is distinct from the perissodactyl pattern. This morphotype is an additional morphological character (14–18) and one of the most remarkable dental characters reported (16, 17) for close relationships of paenungulates, macroscelideans, and louisinines. However, our parsimony analysis does not formally support sister-group relationships of the Macroscelidea plus Louisininae and the Paenungulata by contrast to molecular (19, 20) and recent morphological (14–18, 21) analyses advocating the Afrotheria clade. The recovered topology (Fig. 3) shows a sister-group relationship of Laurasian lophodont ungulates such as perissodactyls to paenungulates, instead of the macroscelideans (and louisinines). Similarly, our analysis does not discriminate clearly Laurasian (e.g., phenacodontids) and African (e.g., Ocepeia) “condylarths” as possible early ungulate representatives of molecular laurasiatherian and afrotherian clades. Fossils gaps, and especially for African taxa, most probably explain poorly resolved cladistic basal relationships of the Paenungulata in our tree (Fig. 3). These gaps are illustrated by our poor knowledge of the ancestral morphotype of several key paenungulates orders; for instance, the ancestral relative size of the last molar in paenungulates is challenged by Eritherium (M33 not enlarged). At lower level in the tree, the morphological and fossil gap is even worse for the phylogenetic analysis of the superordinal clade Afrotheria including Tenrecoidea and Tubulidentata, which are excluded from this study because of the lack of Paleogene data. In this respect, the cladistic study of Eritherium does not help to test the question of the macroscelidean position within Afrotheria. However, Eritherium dental morphology argues for a bunodont-lophodont, i.e., ungulate-like, ancestral morphotype for the Paenungulata, Louisininae, and Macroscelidea, within putative Afrotheria.
Discussion and Implications for the Placental Radiation
Eritherium, Phosphatherium, Daouitherium, and Numidotherium provide the most complete basal evolutionary sequence known among paenungulate and “ungulate” mammals. Such a remarkable early fossil record for the Proboscidea provides evidence for (i) the emergence of a modern ungulate order at a previously unreported primitive stage, still close to the generalized early Tertiary condylarth-like grade, and (ii) one of the most spectacular examples of morphological evolution known in Mammalia (1). The discovery of Eritherium especially reveals a major evolutionary leap in proboscidean evolution at the beginning of the Eocene, with the development of the true lophodonty and the large body size (22). It shows that Paleocene-Eocene (PE) transition is a key period of evolution in Africa for (at least) Proboscidea, as it is classically in Laurasia for several other placental orders (23). In this instance, Africa matches Laurasia in major early evolutionary events of placentals.
The elephant order, that can now be traced back to early Late Paleocene, ca. 60 mys, is one of the earliest known “modern” placental orders, beside few other Paleocene occurrences such as xenarthrans, lipotyphlans, carnivores, euprimates, and rodents (24, 25). The order Proboscidea is one of the earliest known putative Afrotheria, if not the earliest.
The remarkably poorly derived morphology of Eritherium from the inferred paenungulate ancestral morphotype supports a recent proboscidean origin (i.e., recent before Eritherium, ca. 60 mys) and a rapid paenungulate radiation at the Cretaceous-Tertiary (KT) transition, which is also supported by the latest genomic studies (26–28). Rapid paenungulate radiation and fossil gaps may explain poorly resolved interordinal relationships. Such a rapid paenungulate radiation is most consistent with the “conventional” view of the placental adaptive radiation at the beginning of the Tertiary in relation to major KT events, and it may reflect the adaptive response to favourable early Tertiary African conditions, such as the colonization of local free niches (29). Eritherium provides new and outstanding fossil data for calibrating the placental tree. In this regard, morphological phylogenies (3, 4) that place the Paenungulata and Proboscidea in nested or apical position suppose in light of Eritherium either an old, or more likely, a rapid and basically explosive placental radiation; the latter is supported by the phylogeny of Wibble et al. (4) that refutes known pre-Tertiary crown placentals. Paradoxically, molecular phylogenetic topologies (19, 20) advocating a basal position of Afrotheria do not refute the rapid placental radiation based on Eritherium (a low position of Paenungulata and Proboscidea in the molecular placental tree is not consistent with a significant earlier age versus Eritherium of other ordinal divergences). However, a rapid placental and paenungulate radiation at the KT transition obviously does not exclude Cretaceous roots of several basal lineages, especially for stem afrotherians and paenungulates.
Following Phosphatherium, the discovery of Eritherium confirms the long African endemic history of the Proboscidea. Eritherium also provides the most reliable evidence for the African origin of the Paenungulata.
Materials and Methods
Detailed comparative characters study and phylogenetic relationships of Eritherium among lophodont ungulates were developed with the program TNT (11), complemented with Winclada and Nona (e.g., matrix construction and character distribution in the trees). Details of the 143 dental and cranial characters studied and of the phylogenetic analysis are provided in SI Appendix. Determination and biostratigraphic characterization (by H.Cappetta) of the selachian fauna from the fossiliferous level of Eritherium are provided and further commented in SI Appendix.
Supplementary Material
Acknowledgments.
The author thanks F. Escuillié and P. Dalous (Musée d'Histoire Naturelle de Toulouse) for providing material of Eritherium for study and/or for Muséum National d'Histoire Naturelle (MNHN) collection; P. Louis and C. Letenneur for drawings; P. Loubry and C. Lemzaouda for photographs; R. Vacant for preparation and casts; H. Cappetta for determination of the associated selachian fauna and for biostratigraphic comments; and useful comments on the manuscript were provided by E.K. Seiffert, P. Tassy, and an anonymous reviewer. The author also thanks the Geological Survey of the OCP mining centre of Khouribga for help with the field work, especially B. Bouya and M. Amaghzaz for logistic support, and our field collaborators O. Selloum and A. Mazzi, which helped to find and locate material of Eritherium. M. Bichara and S. Meslouh also helped with the field work. Field work was funded by the Centre National de la Recherche Scientifique (Unité Mixte de Recherche 5143) and MNHN (Bonus Qualite Recherche). This work was supported by the Collaboration Agreement with the Ministère de l'Energie et des Mines, the Office Chérifien des Phosphates (OCP) of Morocco, Universities Cadi Ayyad (Marrakech) and Chouaîb Doukkali (El Jadida).
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900251106/DCSupplemental.
References
- 1.Gheerbrant E, Tassy P. Origin and evolution of proboscideans (Translated from French) C R Palevol. 2009;8:281–294. [Google Scholar]
- 2.Gheerbrant E, Sudre J, Cappetta H. A Palaeocene proboscidean from Morocco. Nature. 1996;383:68–71. [Google Scholar]
- 3.Novacek MJ. Mammalian phylogeny: Shaking the tree. Nature. 1992;356:121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- 4.Wible JR, Rougier GW, Asher RJ. Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature. 2007;447:1003–1006. doi: 10.1038/nature05854. [DOI] [PubMed] [Google Scholar]
- 5.Gheerbrant E, Sudre J, Cappetta H, Bignot G. Phosphatherium escuilliei, from the Thanetian of the Ouled Abdoun basin (Morocco), oldest known Proboscidean (Mammalia) from Africa (Translated from French) Geobios. 1998;30:247–269. [Google Scholar]
- 6.Gheerbrant E, et al. The mammal localities of Grand Daoui Quarries, Ouled Abdoun Basin, Morocco, Ypresian: A first survey (Translated from French) Bul Soc Géol Fr. 2003;174:279–293. [Google Scholar]
- 7.Gheerbrant E, et al. New data on Phosphatherium escuilliei (Mammalia, Proboscidea) from the early Eocene of Morocco, and its impact on the phylogeny of Proboscidea and lophodont ungulates (Translated from French) Geodiversitas. 2005;27:239–333. [Google Scholar]
- 8.Solé F, Gheerbrant E, Iarochene M, Amaghzaz M, Bouya B. Further evidence of the African antiquity of hyaenodontid (“Creodonta”, Mammalia) evolution. Zool J Lin Soc. 2009 in press. [Google Scholar]
- 9.Damuth JD. In: Body Size in Mammalian Paleobiology. Damuth J, MacFadden BJ, editors. Cambridge Univ Press; 1990. pp. 229–253. [Google Scholar]
- 10.Janis CM. In: Body Size in Mammalian Paleobiology. Damuth J, MacFadden BJ, editors. Cambridge Univ Press; 1990. pp. 255–300. [Google Scholar]
- 11.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. [Google Scholar]
- 12.Cox PG. Character evolution in the orbital region of the Afrotheria. J Zool. 2006;269:514–526. [Google Scholar]
- 13.Sudre J, Jaeger J-J, Sigé B, Vianey-Liaud M. New data on the condylarths from the Thanetian and Ypresian of the Ouarzazate Basin (Morocco) (Translated from French) Geobios. 1993;26:609–615. [Google Scholar]
- 14.Tabuce R, Coiffait-Martin B, Coiffait PE, Mahboubi M, Jaeger JJ. A new genus of Macroscelidea (Mammalia) from the Eocene of Algeria: A possible origin for elephant-shrews. J Vert Pal. 2001;21:535–546. [Google Scholar]
- 15.Tabuce R, et al. Early Tertiary mammals from North Africa reinforce the molecular Afrotheria clade. Proc Roy Soc B. 2007;274:1159–1166. doi: 10.1098/rspb.2006.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiffert ER. A new estimate of Afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence. BMC Evol Biol. 2007;7:1–13. doi: 10.1186/1471-2148-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asher RJ, Lehman TM. Dental eruption in afrotherian mammals. BMC Evol Biol. 2008;6:14. doi: 10.1186/1741-7007-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zack SP, Penkrot TA, Bloch JI, Rose KD. Affinities of ‘hyopsodontids’ to elephant shrews and a Holarctic origin of Afrotheria. Nature. 2005;434:497–501. doi: 10.1038/nature03351. [DOI] [PubMed] [Google Scholar]
- 19.Murphy WJ, et al. Resolution of the early placental mammal radiation using Bayesian Phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- 20.Madsen O, et al. Parallel adaptive radiations in two major clades of placental mammals. Nature. 2001;409:610–614. doi: 10.1038/35054544. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TJ, Seiffert ER. Afrotherian origins and interrelationships: New views and future prospects. Curr Top Dev Biol. 2004;63:37–60. doi: 10.1016/S0070-2153(04)63002-X. [DOI] [PubMed] [Google Scholar]
- 22.Gheerbrant E, et al. A new large mammal from the Ypresian of Morocco: Evidence of a surprising diversity of early proboscideans. Act Pal Pol. 2002;47:493–506. [Google Scholar]
- 23.Gingerich PD. Environment and evolution through the Paleocene-Eocene thermal maximum. Trend Ecol Evo. 2006;21:246–253. doi: 10.1016/j.tree.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Archibald JD, Rose KD. In: The Rise of Placental Mammals. Rose KD, Archibald JD, editors. Baltimore: Johns Hopkins Univ Press; 2005. [Google Scholar]
- 25.Rose KD. The Beginning of the Age of Mammals. Baltimore: Johns Hopkins Univ Press; 2006. [Google Scholar]
- 26.Rokas A, Carroll SB. Bushes in the tree of life. PLOS. 2006;4:1899–1904. doi: 10.1371/journal.pbio.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proc Nat Acad Sci USA. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardini AT, et al. Chromosome painting among Proboscidea, Hyracoidea, and Sirenia: Support for Paenungulata (Afrotheria, Mammalia), but not Tethytheria. Proc Roy Soc B. 2007;274:1333–1340. doi: 10.1098/rspb.2007.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheerbrant E, Rage J-C. Paleobiogeography of Africa: How distinct from Gondwana and Laurasia? Palaeogeo, Palaeocl, Palaeoecol. 2006;241:224–246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.