Abstract
The pulsatile release of gonadotropin-releasing hormone (GnRH) is critical for mammalian fertility, but the mechanisms underlying the synchronization of GnRH neurons are unknown. In the present study, the full extent of the GnRH neuron dendritic tree was visualized by patching and filling individual GnRH neurons with biocytin in acute brain slices from adult GnRH-green fluorescent protein (GFP) transgenic mice. Confocal analysis of 42 filled GnRH neurons from male and female adult mice revealed that the dendrites of the great majority of GnRH neurons (86%) formed multiple close appositions with dendrites of other GnRH neurons. Two types of interactions were encountered; the predominant interaction was one of vertical dendritic bundling where dendrites were found to wrap around each other in the same axis. The other interaction was one in which a GnRH neuron dendrite intercepted other GnRH neuron dendrites in a perpendicular fashion. Electron microscopy using pre-embedded, silver-enhanced immunogold labeling for both GnRH and GFP peptides in GnRH-GFP transgenic mice, confirmed that GnRH neuron dendrites were often immediately juxtaposed. Membrane specializations, including punctae and zonula adherens, were found connecting adjacent dendritic elements of GnRH neurons. Remarkably, individual afferent axon terminals were found to synapse with multiple GnRH neuron dendrites at sites of bundling. Together, these data demonstrate that GnRH neurons are not isolated from one another but, rather, interconnected via their long dendritic extensions. The observation of shared synaptic input to bundled GnRH neuron dendrites suggests a mechanism of GnRH neuron synchronization.
Keywords: dendrite, fertility, LHRH, mouse, GnRH
Reproduction in mammals is controlled by the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus. Despite GnRH pulsatility being essential for fertility (1), the mechanisms enabling GnRH neurons to synchronize their activity to generate discrete episodes of GnRH secretion remain unknown (2, 3). As a result of the GnRH neurons' migration from the nose into the brain during early development, the cell bodies of GnRH neurons lie scattered throughout the medial septum and hypothalamus (2, 4). Although there is evidence that GnRH neuron nerve terminals in the median eminence exhibit some degree of synchronized activity (5, 6), the mechanism by which the scattered GnRH neuron cell bodies may intercommunicate and synchronize has remained a puzzle.
Recent morphological studies in GnRH transgenic mouse models have revealed that the simple unipolar/bipolar GnRH neurons, identified for many years with immunocytochemistry (7), actually elaborate extremely long dendrites (>1,000 μm) that are covered in spines (8–10). Further, electrophysiological recordings of GnRH neuron dendrites show that many action potentials are initiated in the dendrite and propagate to the cell body (10). These observations have centered attention on the dendrite of the GnRH neuron as being the key neuronal compartment for signal integration and spike generation in these cells.
Given the proposed important role of dendro-dendritic communication in enabling other neuroendocrine neurons to generate episodic activity (11, 12), we questioned here whether dendro-dendritic interactions may also occur between GnRH neurons. While the GnRH neuron cell bodies are clearly remote from one another, we speculated that their long dendrites might interact. Using GnRH neuron cell-filling approaches coupled with light and electron microscopy, we report here that the great majority of GnRH neuron dendrites exhibit multiple close dendritic bundling interactions with other GnRH neuron dendrites and, further, that single afferent nerve terminals form shared synapses with adjacent GnRH neuron dendrites.
Results
Using an acute brain slice preparation, 42 GnRH neurons from adult GnRH-green fluorescent protein (GFP) mice (60–70 days old) were patched and filled with 0.5% biocytin. Only 1 GnRH neuron was filled per coronal brain slice and a total of 8 male and 6 female mice were used. Filled GnRH neurons (males, 23; females, 19) represented a random sample of neurons spread throughout the GnRH neuron distribution. The majority of filled neurons were located in the rostral preoptic area (rPOA) (n = 29, 69%), while 11 were in the medial septum (26%) and 2 in the lateral anterior hypothalamic area (5%).
Confocal imaging of sections dual-labeled for GFP and biocytin showed that 36 of the 42 (86%) filled GnRH neurons had dendrites that exhibited close appositions with multiple other GnRH neuron dendrites identified by their endogenous GFP expression (Fig. 1). Processes were identified as dendrites (rather than axons) by noting either a tapered shape extending from their soma of origin, or through the presence of spines along their length. Two distinct types of dendro-dendritic interaction were noted; a vertical bundling pattern (83%) (Fig. 1) and a horizontal intersecting pattern (17%) (Fig. 2).
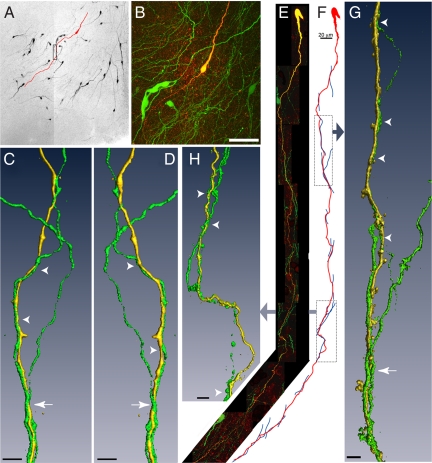
Fig. 1.
Biocytin-filled GnRH neurons exhibiting dendro-dendritic bundling with other GnRH neurons in the verticle orientation. (A) Montage of low power confocal images through a thick slice of the rPOA shows GnRH-GFP neurons (black) and a single GnRH neuron that was filled with biocytin and subsequently labeled with a fluorescent marker (traced in red). (B) High power projection of confocal images of the filled neuron in A, showing endogenous GFP expression in multiple GnRH soma and in a plexus of processes (green) and fluorescently labeled, biocytin-filled GnRH neuron (yellow/red). (C and D) Three-dimensional isosurface rendering of GFP (green) and biocytin-filled (yellow) dendrites (rectangle in A) that bundle together viewed from 2 angles 180° apart. Arrowhead indicates 2 bundled dendrites; arrow shows the bundling of 3 dendrites. (E) Montage of high power confocal image stacks showing another filled GnRH neuron exhibiting dendritic contacts and bundling in the vertical orientation. (F) A schematic traced from confocal image data illustrates 15 apparent close associations between the filled dendrite (red) and other GnRH neuron dendrites (blue). Three-dimensional rendered images of the filled dendrite and apposing dendrites created from high power confocal images are shown for the highlighted regions. (G) Two juxtaposed GnRH neuron dendrites can be seen in the upper portion of the image (arrowheads), and 3 dendrites are found bundling together further down the length of the dendrite (arrow). (H) Points of contact are apparent between the filled dendrite and 2 dendrites that run in parallel with the filled dendrite (arrowheads). (Scale bars: B, 50 μm; C, D, G, and H, 5 μm.)
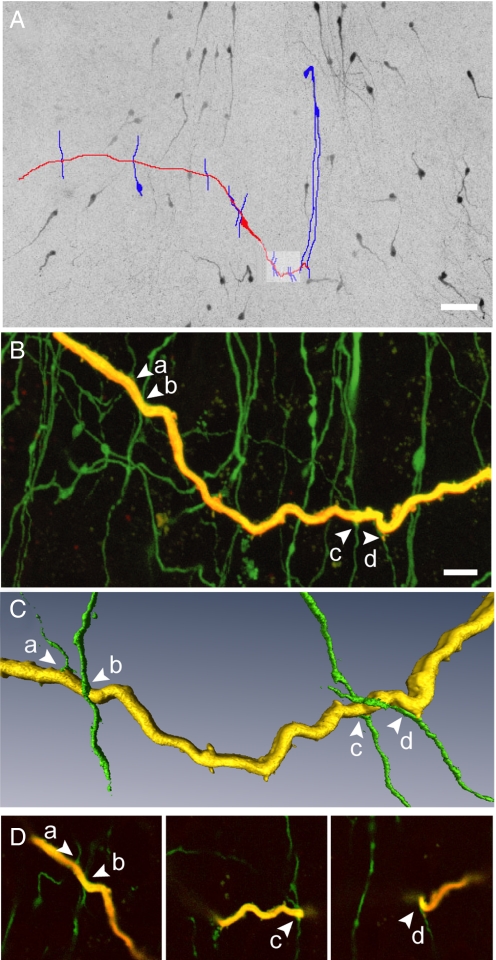
Fig. 2.
Dendro-dendritic associations in the horizontal orientation. (A) Montage of low power confocal images through a thick brain slice through the rPOA shows GnRH-GFP neurons (black) and a single GnRH neuron that was filled with biocytin and subsequently labeled with a fluorescent marker (traced in red). GnRH-GFP neuron dendrites in apparent close apposition with the dendrite of the filled GnRH neuron are traced in blue. (B) High power confocal projection of the area highlighted in panel A showing a portion of the filled dendrite (yellow) and other endogenously fluorescent GnRH processes. Arrows indicate 4 dendrites (a–d) found in close apposition with the filled dendrite. (C) Isosurface rendering of the dendrites in close apposition show the perpendicular nature of these interactions. (D) Individual optical sections (0.32 μm thick) showing the close apposition of dendritic processes. (Scale bars: A, 50 μm; B, 10 μm.)
Vertically-orientated GnRH Neuron Dendrite Bundling.
Fig. 1A shows a representative brain slice from the rPOA containing multiple, scattered GnRH neurons and their processes. As has been noted previously (7) the bipolar shape and processes of GnRH neurons usually exhibit an “inverted Y”-type orientation in the coronal plane; medial septal GnRH neurons have a vertical or 90° orientation while rPOA GnRH neurons have an approximate 45° orientation (Fig. 1A). This topography becomes even more apparent when the full extension of the GnRH neuron dendrite is viewed after biocytin filling (Fig. 1 A and B). A typical bipolar biocytin-filled GnRH neuron is shown in red within the slice in which it was filled (Fig. 1A) and at higher power in a projection of confocal stacks taken through the slice (Fig. 1B). Confocal analysis revealed that GnRH neuron dendrites extending in a similar direction often appeared to be juxtaposed and bundle together. Panels C and D illustrate, by way of a 3-dimensional rendering from high-power confocal images, the bundling of 3 GnRH neuron dendrites (1 filled with biocytin and the other 2 visualized by GFP expression) shown from 2 different angles.
A further example of a GnRH neuron exhibiting vertical dendritic bundling is shown in Fig. 1 E–H. The dendrite of this GnRH neuron was found to extend 1,025 μm until it exited the brain slice, and had 15 close appositions with other GFP-expressing GnRH dendrites (Fig. 1 E and F). Isosurface rendered images of the filled dendrites and portions of the bundling dendrites are shown for 2 parts of the GnRH neuron dendrite. Panel G shows 1 fine dendrite running in parallel to the filled dendrite over 40 μm of dendritic length (arrowheads) with 2 additional GnRH neuron dendrites seen coming together with the filled dendrite in a bundle (arrow). Panel H shows 2 dendrites twisting around the filled GnRH neuron dendrite at different levels (arrowheads).
GnRH neuron dendrites exhibiting vertical bundling were found to have (mean ± SEM) 4.0 ± 0.6 contacts/filled dendrite (range 1–15, n = 30). This was not different between males (4.3 ± 0.6) and females (3.6 ± 1.0) or dependent upon location; MS GnRH neurons (3.5 ± 0.7), rPOA GnRH neurons (4.3 ± 0.9).
Horizontal Interactions between GnRH Neuron Dendrites.
Fig. 2A shows a biocytin-filled GnRH neuron (red) with the less common (17%) pattern of a dendritic extension that runs in the horizontal plane intercepting vertically-orientated GnRH neuron dendrites. In this example, the horizontally extended dendrite was found to intercept 12 other GnRH neuron dendrites; 4 such appositions are depicted at higher power and in individual optical slices (Fig. 2 B–D). GnRH neuron dendrites exhibiting horizontal interactions were found to have (mean ± SEM) 3.3 ± 0.7 contacts/filled dendrite (range 1–12, n = 6).
Electron Microscopy Confirms Direct Dendro-dendritic Appositions between GnRH Neurons.
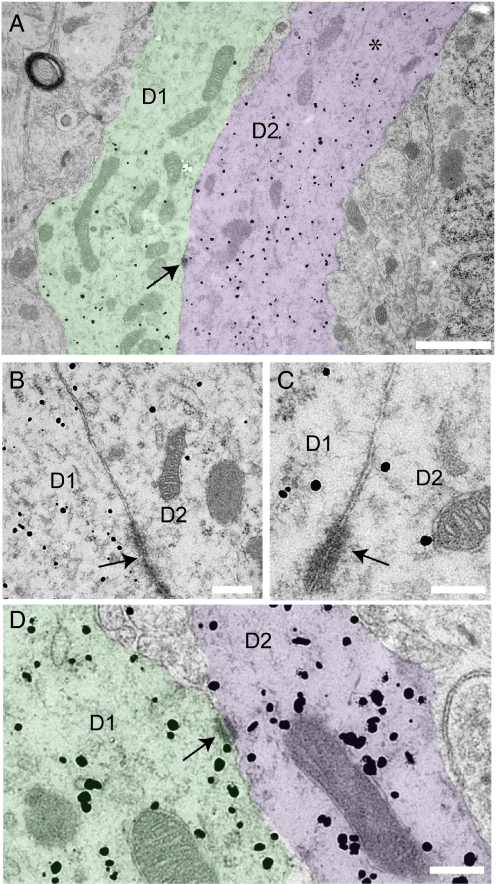
Preembedded, silver-enhanced immunogold labeling for both GnRH and GFP was performed on brain tissue from 2 female GnRH-GFP transgenic mice. We have found that the combination of GnRH and GFP immunolabeling facilitates the analysis of GnRH neuron dendrites as GFP molecules diffuse beyond the distribution of GnRH peptide in GnRH neuron dendrites. Dendritic elements of GnRH neurons were identified in semi-thin sections at the light microscopic level by their light brown label. Identified regions with dendritic labeling were trimmed and cut into serial ultra-thin sections. Electron microscopy showed silver-enhanced gold particle labeling in dendritic compartments, defined by their diameter (larger than axons in the same field), and arrangement of microtubules (asterisks, Figs. 3 and 4). Silver-enhanced gold particles were found associated with dense core vesicles in GnRH neuron axon terminals but also unassociated with vesicles in the soma and dendrites of the cell due to co-labeling with GnRH and GFP. Labeled dendrites were found in close association with one another without any intervening elements in both animals (Fig. 3 A–D). Adjacent GnRH neuron dendrites were often found with plasmalaemma specializations between them, including punctae and zonula adherens, also known as attachment plates (Fig. 3 A–D, arrows). No evidence of gap junction specializations between GnRH neuron dendrites was found. GnRH neuron dendrites cut in the longitudinal plane were found in close association, without any intervening elements, for distances greater than 5 μm.
Fig. 3.
Ultrastructural evidence of bundling GnRH dendrites. (A) Two distinct silver-enhanced immunogold labeled GnRH neuron dendrites (D1 and D2, pseudocolored for clarity) in parallel with membranes juxtaposed. Silver-enhanced immunogold label appears as scattered black dots. Note that both GnRH and GFP were labeled and that the latter does not associate with dense core vesicles. (A) Asterisk indicates an area of loosely arranged microtubules running longitudinally through the labeled dendrites. Arrow indicates a zonula adherens junction between the 2 dendrites. (B and C) Higher power view in adjacent serial sections through the juxtaposed dendrites shown in A, showing additional zonula adherens membrane specializations. (D) Another example of adjacent GnRH neuron dendrites (D1 and D2, pseudocolored for clarity). Arrow indicates punctae adherens junction (arrows) linking the 2 membranes. (Scale bars: A, 1 μm; B–D, 200 nm.
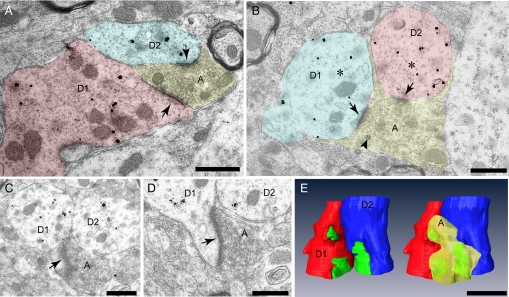
Fig. 4.
Double synapses are present on bundling GnRH dendrites. (A and B) Two examples from different animals of bundling GnRH neuron dendrites in cross section (D1 and D2) receiving shared afferent input (arrows) from single axon terminals (A). (B) Arrowhead indicates a single dense core vesicle amongst numerous clear vesicles in the terminal. Asterisks are positioned amongst typical microtubules, seen in cross section as small circles, in the labeled dendrites. (C–E) Serial ultra-thin images through the labeled, bundled GnRH dendrites shown in B were collected and a 3-dimensional model created (E). Postsynaptic densities (green) were present on the dendritic shaft (B) and on the neck of sessile spines (arrows, C and D). The 3-dimensional, reconstructed dendrites are pictured with and without the shared afferent terminal (yellow).
Bundled GnRH Neuron Dendrites Have Shared Synaptic Inputs.
Juxtaposed dendrites in cross section could be found across multiple serial ultra-thin sections. Examination of apposed dendrites in this plane revealed that single nerve terminals formed synapses with 2 adjacent dendrites (Fig. 4). These “shared synapses” on GnRH neuron dendrites were encountered in tissue from both animals. In 6 juxtaposed dendritic bundles examined, a total of 24 synapses were observed of which 4 were shared synapses. Terminating axons were packed with small clear vesicles (Fig. 4 A–D); however, dense core vesicles were also evident in some synapsing axons (Fig. 4B, arrowhead). Shared synapses were formed with postsynaptic densities on the dendritic shaft as well as on sessile spine necks (Fig. 4 C and D, arrows). A 3-dimensional model of the dendrites depicted in panels B–D was constructed following the collection of images from serial ultra-thin sections (Fig. 4E).
Discussion
We report here that the great majority of GnRH neurons have dendrites that bundle together in a vertical-type orientation with punctae and zonula adherens acting to juxtapose GnRH neuron dendrites. While no morphological evidence was found for gap junctions or direct communication between dendrites, shared synapses were identified at loci of dendritic bundling. These observations provide evidence that the scattered GnRH neuron cell bodies are not in physical isolation from one another but, rather, highly interconnected through dendro-dendritic bundling and associated shared synaptic inputs.
To examine potential dendritic interactions between GnRH neurons, it was necessary to biocytin-fill single GnRH neurons in acute brain slices prepared from GnRH-GFP transgenic mice. This has the advantage of enabling the whole of the dendrite to be visualized while also isolating 1 dendrite from the others so that it can be followed unambiguously. Prior immunocytochemical studies (7), have readily visualized clusters of similarly orientated GnRH-immunoreactive processes but have been unable to define what was dendritic or axonal in nature, and establish the relationship of the immunoreactive elements to each other. An earlier ultrastructural investigation also identified the presence of cytoplasmic bridges between a very few GnRH neuron somata (13) but the examination of GnRH dendrites with electron microscopy has remained problematic due to the relative absence of GnRH peptide in the distal dendrites. In that respect, a key feature of our present ultrastructural studies has been that of combining GnRH and GFP immunocytochemistry in tissue from GnRH-GFP transgenic mice to visualize greater amounts of the GnRH neuron dendrite.
We observed 2 patterns of GnRH neuron dendritic association; vertical bundling and horizontal intersectioning. The horizontal pattern was much less common and consequently, much more difficult to examine at the ultrastructural level. Although the confocal data suggest that some GnRH neurons have dendrites that intersect with multiple vertically-streaming GnRH neuron dendrites, we do not as yet have ultrastructural views of this potential interaction. As such, despite its intriguing nature, it is not possible to speculate on what function the horizontal pattern may have within the GnRH neuron network.
In contrast, the more frequent vertical dendritic bundling has permitted ultrastructural characterization showing the presence of juxtaposed bundled GnRH dendrites with shared synapses. At the confocal level, we found an average of 4, but up to 15, dendro-dendritic appositions per filled dendrite. This indicates that multiple GnRH neurons are interacting through vertical dendro-dendritic bundling streams. We believe, however, that our present work is still an underestimate of the prevalence of dendro-dendritic interactions; first, the full length of the filled dendrite was not always examined, as many dendrites exited the slice before their natural end, and secondly, visualizing un-filled GnRH neuron dendrites is dependent upon endogenous GFP expression which is variable in distal dendritic compartments.
The morphological features revealed here for vertically-bundled GnRH neuron dendrites, including juxtaposed dendritic surfaces and shared synapses onto bundled dendrites, are strikingly similar to that of magnocellular oxytocin neurons of the supraoptic nucleus (11). The cell bodies and dendrites of oxytocin neurons are closely apposed but, under basal conditions, typically encased in extensive glia cell processes (14). However, during periods of enhanced episodic oxytocin secretion, including parturition, lactation (14, 15) and osmotic stimulation (16), oxytocin neuron dendrites are found juxtaposed without intervening glial elements and the number of shared synapses increase markedly. Such features are thought to be related to the ability of oxytocin neurons to exhibit episodic burst firing (11). Another mechanism promoting magnocellular neuron synchronization is that of dendro-dendritic neurotransmitter release whereby release of neurotransmitters from microvesicles in 1 dendrite influence the excitability of adjacent dendrites (12). We have found no ultrastructural evidence for the presence of microvesicles at sites of GnRH dendrite juxtaposition. It is important to note, however, that GnRH neuron dendro-dendritic transmitter release may be state dependent, and it will be of great interest to examine dendro-dendritic interactions in ovariectomized mice that may be expected to exhibit enhanced pulsatile GnRH release.
Taking inference from the neuroendocrine oxytocin neurons, we suggest that the GnRH neuron dendro-dendritic bundling and shared synapses revealed here are involved in the synchronization of GnRH neuron activity. It has recently become apparent that the long GnRH neuron dendrites are important in both spike initiation and the processing of synaptic input in GnRH neurons (8, 10, 17). As such, shared synapses on juxtaposed GnRH neuron dendritic elements would provide a powerful stimulus for synchronizing the electrical activity of multiple GnRH neurons. Whether the dendro-dendritic appositions serve only to allow shared synapses to exist or have other functions is not known. We have not found ultrastructural evidence for gap junctions between GnRH neuron dendrites. Extensive portions of juxtaposed dendrites may also facilitate ephaptic interactions, electrical transmission across juxtaposed membranes without a chemical synapse, as has been suggested for other populations that display bundled dendrites or unmyelinated axons, including oxytocin (11, 15), occularmotor (18), and olfactory neurons (19).
It is commonly believed that pulsatility is an intrinsic property of GnRH neurons (3). Evidence that this pulsatility originates in the GnRH neuron soma is based primarily on data from GnRH-expressing GT1 cell lines where pulsatile GnRH secretion exists in the presence of only GnRH cells (20). Whether the mechanisms underlying GnRH pulsatility from tens of thousands of adjacent immortalized cells are relevant to native GnRH neurons in vivo remains unknown. Neither dendro-dendritic interactions nor shared synapses exist in GT1 cell cultures (21) suggesting that, if our proposal is correct, native GnRH neurons and GT1 cell cultures use different mechanisms to facilitate the synchronization of GnRH neuron activity.
In summary, we report here that GnRH neurons are not isolated from one another as previously thought, but couple through extensive dendro-dendritic bundling. Vertical dendritic bundling was commonly observed between several GnRH neuron elements, suggesting that vertical streams of GnRH neurons interact. This unique pattern of dendro-dendritic interaction may have its origins in the remarkable nose-to-tail manner of GnRH neuron migration into the brain during embryogenesis (4). The observation of shared synapses on bundled GnRH neuron dendrites provides a compelling perspective for understanding GnRH neuron synchronization.
Materials and Methods
Experimental Animals.
Male and female homozygous GnRH-GFP mice (22), were housed under conditions of 12 h of light (lights on at 07:00 h) with ad libitum access to food and water. Daily vaginal smears from female mice were observed and diestrus female mice used. The University of Otago Animal Welfare and Ethics Committee approved all experimentation.
Biocytin Filling of GnRH Neurons.
GnRH-GFP mice between 60–75 days old were killed and 200–300 μm-thick coronal slices containing the medial septum and preoptic area (2–3 per brain) prepared as reported previously (8). Slices were initially examined under fluorescence with a low-power objective to determine the distribution of fluorescent cells. A single, randomly chosen GnRH neuron was then brought into focus using fluorescence for 5–10 sec at high-power before switching to DIC optics. Following patching of the cell, it was then briefly examined under fluorescence again to confirm its fluorescent identity. Patch pipettes (4–6 MΩ) were pulled from thin-wall borosilicate glass capillary tubing on a vertical pipette puller. Biocytin was added at a final concentration of 0.5% to the patch pipette solution (in mM): 130 KCl, 5 NaCl, 0.4 CaCl2, 1 MgCl2, 10 Hepes, and 1.1 EGTA, with the pH adjusted to 7.3. After achieving whole-cell mode, the pipette was kept attached to the GnRH neuron for 5 min to allow diffusion of biocytin. After detaching the pipette from the cell, slices were maintained in the recording chamber for 30 min and then placed in ice-cold 4% paraformaldehyde for 4 h at 4 °C. Only 1 cell was filled in each brain slice.
Immunocytochemistry and Confocal Analysis.
Floating slices were washed in Tris-buffered saline (TBS) at room temperature to remove any residual paraformaldehyde. Slices were then incubated with Texas Red-conjugated avidin (2.6 μL/mL, Vector Laboratories, Inc.) in TBS containing 0.3% Triton X-100 and 0.3% BSA for 4 h at RT in darkness. Slices were then washed, mounted, and coverslipped with Vectashield (Vector Laboratories, Inc.). Forty-two biocytin-filled, fluorescently labeled GnRH neurons were imaged and analyzed on a Zeiss 510 LSM upright confocal laser scanning microscope system using LSM 510 control software (version 3.2) (n = 23 neurons from 8 male mice, n = 19 neurons from 6 diestrus female mice). Stacks of confocal images were captured using the following objectives, 40× Plan Neofluar (numerical aperture 1.3), 63× PlanApochromat (numerical aperture 1.4) with 2× zoom function. A series of images at 0.23–0.36-μm intervals throughout the entire filled neuron were collected for analysis. Images are presented as single optical images or projections of optical image stacks. The brightness and contrast of the images were adjusted in Photoshop (Adobe Systems) to match microscope visualization. Amira software (Visage Imaging) was used for isosurface rendering images from confocal stacks.
Tissue Preparation for Electron Microscopy.
Two female transgenic GnRH-GFP mice were anesthetised and perfused transcardially with 4% paraformaldehyde and 0.5% glutaraldehyde in 0.1 M phosphate buffer saline (PBS). Brains were then removed and postfixed for 1 h. Coronal sections (50 μm) were cut on a vibratome with 0.1 M PBS. Sections from the rostral preoptic area (rPOA) were collected (8–10 sections/brain) and trimmed with a razor blade to the area of interest.
Preembedding, Silver-enhanced Immunogold Labeling.
Trimmed brain sections underwent aldehyde inactivation for 15 min (0.1% sodium tetrahydroborate in 0.1 M PBS), followed by permeablization in 0.1 M PBS containing 0.05% Triton X-100 for 30 min. Tissue was then incubated in blocking solution (10 mM PBS, 150 mM NaCl, 0.5% BSA, 0.1% fish skin gelatin, and 5% goat serum or blocking solution for goat-gold conjugates, Aurion) for 30 min. Sections were rinsed thoroughly in PBS then incubated in a mixture of primary antibodies (LR5 rabbit anti-GnRH 1:10,000, provided by Dr. Robert Benoit; rabbit anti-GFP 1:2,000, Molecular Probes) in incubation buffer (10 mM phosphate buffer, 150 mM NaCl, and 0.1% BSA-C from Aurion) overnight on an orbital shaker at 4 °C. The following day, tissue was washed in incubation buffer (6 × 10 min) and then placed in secondary antibodies [ultra-small gold congugate of f(ab′)/f(ab)2 goat anti-rabbit, Aurion, code 100.366] overnight at 4 °C. After initial washes in incubation buffer (6 × 10 min), tissue was washed in Aurion Enhancement Conditioning Solution (4 × 10 min, Aurion, code 500.055). Silver enhancement was performed with silver enhancement reagents (Aurion R-Gent SE EM, code 500.033) until golden brown GnRH-GFP neurons could be detected in sections with light microscopy. Tissue was then rinsed in Aurion Enhancement Conditioning Solution (4 × 10 min) and subsequently in PBS (2 × 10 min).
Tissue Processing for Electron Microscopy.
Osmication with 0.5% osmium tetroxide (15 min) was performed before tissue dehydration through a graded series of ethanol and propylene oxide. Sections were then flat embedded between 2 silanized glass slides in agar 100 resin and left to polymerize for 48 h at 4 °C. Semi-thin sections (4 μm each) were cut on an ultramicrotome. Each semi-thin section was analyzed by light microscopy after staining with 1% methylene blue, 1% azur 11, and 1% borax. Sections with labeled GnRH cell bodies and processes were re-embedded in agar 100 resin and cut as ultra-thin serial sections (60–90 nm). Ribbons of ultra-thin serial sections were placed on formvar-coated copper slot grids and stained with 1% uranyl acetate and lead citrate.
Ultrastructural Imaging.
Ultra-thin sections were examined using a Philips CM100 transmission electron microscope fitted with MegaView III digital camera. Digital images were analyzed using iTEM software. Anatomical data collected in serial sections was aligned and 3-dimensionally rendered with Amira Imaging software.
Statistics.
Mean (± SEM) values were calculated for each experimental group. Differences within each experiment were tested for significance using a 2-tailed Mann-Whitney test (GraphPad Software; INSTAT). P values <0.05 were considered significant.
Acknowledgments.
We thank Mr. Andrew McNaughton (Otago Centre for Confocal Microscopy), Mr. Allan Mitchell (Otago Centre for Electron Microscopy), and Dr. Jan Schulz (Aurion, Wageningen, The Netherlands) for advice and assistance. This work was supported by New Zealand Health Research Council Funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: Permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;4437:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE. Physiology of the gonadotropin-releasing hormone neuronal network. In: Neill JD, editor. Knobil and Neil's Physiology of Reproduction. 3rd Ed. New York: Raven Press; 2006. pp. 1415–1482. [Google Scholar]
- 3.Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 4.Wray S. Development of luteinizing hormone releasing hormone neurones. J Neuroendocrinol. 2001;13:3–11. doi: 10.1046/j.1365-2826.2001.00609.x. [DOI] [PubMed] [Google Scholar]
- 5.Purnelle G, Gerard A, Czajkowski V, Bourguignon JP. Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons. Neuroendocrinology. 1997;66:305–312. doi: 10.1159/000127253. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen DD. Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology. 1993;58:511–518. doi: 10.1159/000126584. [DOI] [PubMed] [Google Scholar]
- 7.Silverman AJ, Livin I, Witkin JW. The gonadotropin-releasing hormone (GnRH) neuronal systems: Immunocytochemistry and in situ hybridization. In: Knobil E, Neill JE, editors. Physiology of Reproduction. 2nd Ed. New York: Raven Press; 1994. pp. 1683–1709. [Google Scholar]
- 8.Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146:1163–1169. doi: 10.1210/en.2004-1369. [DOI] [PubMed] [Google Scholar]
- 9.Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:3652–3661. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CB, Campbell RE, Herbison AE, Suter KJ. Dendritic action potential initiation in hypothalamic gonadotropin releasing hormone (GnRH) neurons. Endocrinology. 2008;149:3355–3369. doi: 10.1210/en.2008-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodosis DT. Oxytocin-secreting neurons: A physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002;23:101–135. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 13.Witkin JW, O'Sullivan H, Silverman AJ. Novel associations among gonadotropin-releasing hormone neurons. Endocrinology. 1995;136:4323–4330. doi: 10.1210/endo.136.10.7664651. [DOI] [PubMed] [Google Scholar]
- 14.Theodosis DT, Poulain DA, Vincent JD. Possible morphological bases for synchronisation of neuronal firing in the rat supraoptic nucleus during lactation. Neuroscience. 1981;6:919–929. doi: 10.1016/0306-4522(81)90173-1. [DOI] [PubMed] [Google Scholar]
- 15.Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986;17:661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- 16.Chapman DB, Theodosis DT, Montagnese C, Poulain DA, Morris JF. Osmotic stimulation causes structural plasticity of neurone-glia relationships of the oxytocin but not vasopressin secreting neurones in the hypothalamic supraoptic nucleus. Neuroscience. 1986;17:679–686. doi: 10.1016/0306-4522(86)90039-4. [DOI] [PubMed] [Google Scholar]
- 17.Roberts CB, O'Boyle MP, Suter KJ. Dendrites determine the contribution of after depolarization potentials (ADPs) to generation of repetitive action potentials in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. J Comput Neurosci. 2009;26:39–53. doi: 10.1007/s10827-008-0095-5. [DOI] [PubMed] [Google Scholar]
- 18.Bacskai T, et al. Dendrodendritic and dendrosomatic contacts between oculomotor and trochlear motoneurons of the frog, Rana esculenta. Brain Res Bull. 2008;75:419–423. doi: 10.1016/j.brainresbull.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 19.Bokil H, Laaris N, Blinder K, Ennis M, Keller A. Ephaptic interactions in the mammalian olfactory system. J Neurosci. 2001;21:RC173, 1–5. doi: 10.1523/JNEUROSCI.21-20-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner RI, et al. Gonadotropin-releasing hormone neuronal cell lines. Front Neuroendocrinol. 1992;13:95–119. [PubMed] [Google Scholar]
- 21.Liposits Z, et al. Morphological characterization of immortalized hypothalamic neurons synthesizing luteinizing hormone-releasing hormone. Endocrinology. 1991;129:1575–1583. doi: 10.1210/endo-129-3-1575. [DOI] [PubMed] [Google Scholar]
- 22.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]