Abstract
A single nucleotide polymorphism (SNP) in the human μ-opioid receptor gene (OPRM1 A118G) has been widely studied for its association in a variety of drug addiction and pain sensitivity phenotypes; however, the extent of these adaptations and the mechanisms underlying these associations remain elusive. To clarify the functional mechanisms linking the OPRM1 A118G SNP to addiction and analgesia phenotypes, we derived a mouse model possessing the equivalent nucleotide/amino acid substitution in the Oprm1 gene. Mice harboring this SNP (A112G) demonstrated several phenotypic similarities to humans carrying the A118G SNP, including reduced mRNA expression and morphine-mediated antinociception. We found additional phenotypes associated with this SNP including significant reductions of receptor protein levels, morphine-mediated hyperactivity, and the development of locomotor sensitization in mice harboring the G112 allele. In addition, we found sex-specific reductions in the rewarding properties of morphine and the aversive components of naloxone-precipitated morphine withdrawal. Further cross-species analysis will allow us to investigate mechanisms and adaptations present in humans carrying this SNP.
Keywords: analgesia, morphine, μ-opioid receptor, sex differences, SNP
MOPR (μ-opioid receptors) are integrally involved in the modulation of several pathways including pain, stress, and drug reward. Genetic mutations of the MOPR alter endogenous and exogenous opioidergic function, thus influencing behavior. A single nucleotide polymorphism (SNP) in exon 1 of the μ-opioid receptor gene (OPRM1), in which an adenine-to-guanine substitution (A118G) exchanges an asparagine for an aspartic acid at a putative N-glycosylation site (N40D), is common in persons of European (15−30%) and Asian ancestry (49–60%), with lower prevalence in African American and Hispanic populations (1–3). The A118G SNP has been associated with an altered vulnerability to opioid addiction (4–6), a decreased response to opioid-induced analgesia (7, 8), and an enhanced response to therapies for alcohol (9, 10) and nicotine addiction (7, 11). However, some association studies report divergent effects (12, 13), and sex-specific associations (14–16), underscoring the need to understand the functional significance of this SNP.
Examination of the A118G variant in heterologous expression systems has yielded inconsistent results. Initial in vitro studies indicated that expression of the human G118 MOPR variant in AV-12 cells increases the binding affinity of β-endorphin to 3-fold higher than that of the human A118 MOPR and results in higher potency for activation of G protein-coupled potassium channels (17), suggesting a gain of function of the receptor. However, other studies report no differences in agonist binding, functional coupling, or desensitization (18). Using an allelic expression assay, Zhang and colleagues (19) found a 1.5-fold reduction in allele-specific mRNA expression in postmortem brain tissue and also a 10-fold reduction in protein levels in CHO cells expressing the G118 variant, supporting a loss of function of the receptor. More recent data support this claim, showing lower surface receptor expression, decreased forskolin-induced cAMP activation, and lower agonist-induced MOPR activation in cell culture systems expressing the G118 allele (20). Discrepancies in the in vitro findings established the rationale for generating a mouse model to examine the molecular, pharmacological, and behavioral significance of this polymorphism in humans. Thus, we generated a mouse possessing the equivalent SNP (A112G), which corresponds to a similar amino acid (N38D) substitution. Because of high homology between mouse and human sequences at the nucleotide (86.9%) and amino acid level (92.3%), similar gene expression levels between human and mouse (Genomics Institute, Novartis Research Foundation; http://symatlas.gnf.org), and conserved chromosomal synteny (http://genome.ucsc.edu/cgi-bin/hgTracks), we generated the mouse equivalent of the human SNP rather than replacing the mouse Oprm1 gene with the human OPRM1 gene in exon 1.
Results
Generation of the A112G Mouse.
The derivation of the Oprm1 A112G mouse was accomplished using a bacterial artificial chromosome (BAC) containing the entire Oprm1 locus derived from C57BL/6 mouse DNA (PAC/BAC Resource) [supporting information (SI) Fig. S1]. Mating A112G heterozygous mice produced offspring of each genotype (A/A, 32.6%; A/G, 47.7%; and G/G, 19.7%; n = 700). Although G/G births were below expected Mendelian rates (χ2 = 24.6), to our knowledge there were no noticeable deficits in overall size or health, nor were there differences in rates of perinatal mortality between genotypes or sexes. A/A and G/G homozygous mice of both sexes were used for all molecular, biochemical, and behavioral assays.
MOPR Expression and Function.
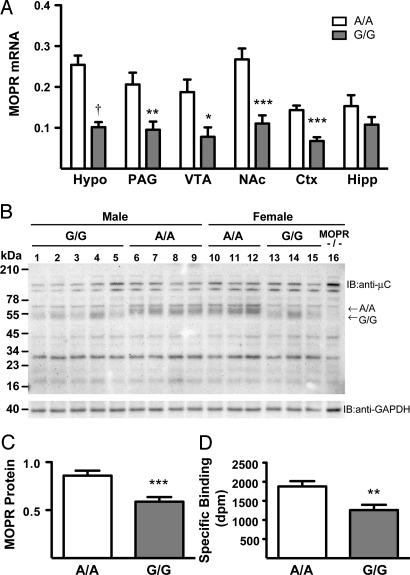
We evaluated the expression and function of the MOPR using a variety of molecular and pharmacological techniques. MOPR mRNA was reduced in G/G mice in several brain regions related to pain, stress, and reward (main effect of genotype, F1,77 = 71.018, P < 0.0001; Fig. 1A). Using primers designed to anneal to different regions of the Oprm1 gene both 5′ and 3′ of the modified SNP, we found similar reductions in mRNA (primer × genotype interaction, F2,42 = 3.416, P = 0.04; Fig. S2). The A-to-G substitution in these mice eliminates 1 of the 4 putative N-glycosylation sites; thus, the observed decrease in MOPR protein size in G/G mice may reflect the reduction in the extent of N-linked glycosylation (Fig. 1B). In addition to a lower molecular weight, total MOPR protein levels were reduced in G/G animals in the thalamus, a region highly enriched in these receptors (t20 = 3.881, P = 0.0009; Fig. 1C). Whole brain saturation binding using [3H]DAMGO showed decreases in receptor number (Bmax) in G/G animals (effect of genotype F1,8 = 8.161, P = 0.02; Table 1). These data are in accordance with previous studies showing decreased cell-surface or total [3H]DAMGO binding in AV-12 and HEK293 cells stably expressing the G118 variant (18, 20). Kd values of [3H]DAMGO for the MOPR were similar among the 4 groups of mice (Table 1). Analysis of brain region-specific binding using a single concentration of [3H]DAMGO (3 nM) revealed decreased specific receptor binding in the thalamus of G/G animals compared with their A/A counterparts (t20 = 3.170, P = 0.005; Fig. 1D). Using whole brain membranes, we determined the binding affinities of β-endorphin, morphine, and naloxone by competitive inhibition of [3H]DAMGO binding and found no alterations between genotypes or sexes (Table 1).
Fig. 1.
MOPR expression is decreased in A112G knock-in mice. (A) MOPR mRNA, as measured by real-time RT-PCR and normalized against TATA binding protein (TBP), in the periaqueductal gray (PAG), hypothalamus (Hypo), ventral tegmental area (VTA), nucleus accumbens (NAc), and cortex (Ctx) (mean ± SEM, n = 7–8; *, P < 0.05; **, P < 0.01; ***, P < 0.001; †, P < 0.0001 compared to A/A, Bonferroni/Dunn). (B) A representative immunoblot of MOPR in membranes prepared from thalami of A/A mice, G/G mice, and MOPR−/− mice and probed with the MOPR antibody shows decreased molecular weight of MOPR protein in G/G mice. (C) Quantification of MOPR immunoreactivities, normalized against GAPDH (mean ± SEM, n = 11; ***, P < 0.001 compared to A/A). (D) Binding of [3H]DAMGO (3 nM) in thalamus membranes (0.1 mg/tube). Data are presented as specific binding/tube (dpm) for each sample run in duplicate (mean ± SEM, n = 11; **, P < 0.01 compared to A/A).
Table 1.
Expression levels and ligand binding affinities of MOPR in A/A and G/G mice
| [3H] DAMGO |
β-Endorphin |
Morphine |
Naloxone |
||
|---|---|---|---|---|---|
| Bmax, fmol/mg protein | Kd, nM | Ki, nM | |||
| A/A male | 158 ± 11.7 | 0.29 ± 0.03 | 1.9 ± 0.06 | 2.4 ± 0.16 | 2.9 ± 0.04 |
| A/A female | 182 ± 5.0 | 0.26 ± 0.03 | 2.1 ± 0.28 | 2.7 ± 0.25 | 3.2 ± 0.12 |
| G/G male | 142 ± 13.7* | 0.33 ± 0.02 | 1.6 ± 0.11 | 2.9 ± 0.23 | 2.7 ± 0.32 |
| G/G female | 114 ± 22.5* | 0.33 ± 0.03 | 1.8 ± 0.18 | 2.8 ± 0.31 | 3.0 ± 0.27 |
Kd and Bmax values were calculated from saturation binding of [3H]DAMGO using whole brain membranes. Competitive inhibition by β-endorphin, morphine, and naloxone of [3H]DAMGO (1.0 nM) binding was conducted to determine Ki values. For each independent experiment, 2 mouse brains were pooled (0.2–0.4 mg membrane proteins/tube) for 1 saturation curve and 3 competition curves. Each value represents the binding for 3 independent experiments performed in duplicate (mean ± SEM;
*, P < 0.05 compared to A/A).
Behavioral Responses to Acute Morphine Administration.
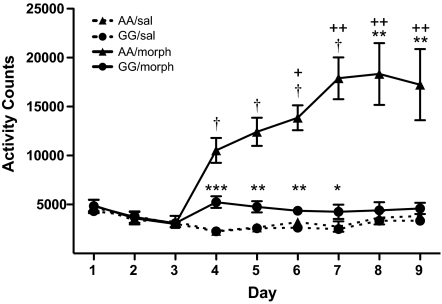
In C57BL/6 mice, acute morphine elevates locomotor activity (21); accordingly, we observed a robust increase in locomotor activity in A/A mice over the course of a 120-min session following morphine administration. In contrast, G/G mice failed to exhibit morphine-mediated hyperactivity (time × treatment × genotype interaction, F11,649 = 11.108, P < 0.0001; Fig. S3a). There was no difference in locomotor activity between genotypes during the 30-min baseline test or following saline administration, suggesting that the alterations in activity are specific to morphine effects and not reflective of a general locomotor deficit. Additionally, there was no difference in activity between males and females in either genotype or treatment group (treatment × genotype interaction, F1,59 = 16.076, P = 0.0002; Fig. S3b). Because morphine can have hypolocomotor actions at high doses, it is possible that the decrease in activity in the G/G animals could result from a heightened sensitivity to morphine. Thus, we evaluated the locomotor response to a low dose of morphine (1 mg/kg). Neither of the genotypes or sexes displayed elevated activity in response to a low-dose morphine administration, indicating that the A112G SNP does not confer an enhanced sensitivity (Fig. S3 c and d). Morphine has been shown to elicit enhanced locomotor-activating effects (behavioral sensitization) with repeated, intermittent administrations (22, 23). Indeed, A/A animals showed behavioral sensitization following repeated morphine injections, while G/G animals did not (day × genotype × treatment interaction, F6,138 = 9.688, P < 0.0001; Fig. 2). Under these conditions, in which animals habituated to the testing chambers, morphine elevated locomotor activity in the G/G animals, although this response was greatly reduced compared to A/A animals (Fig. 2).
Fig. 2.
Morphine-mediated hyperlocomotion is blunted in G/G mice. Saline was administered to all groups on days 1–3 and morphine (10 mg/kg) was administered on days 4–9 (saline control groups received saline injections on all 9 days). Results are presented as total activity counts for the 120-min postinjection test (mean ± SEM, n = 6–7; *, P < 0.05; **, P < 0.01; ***, P < 0.001; †, P < 0.0001 compared to saline-injected controls; +, P < 0.01; ++, P < 0.0001 compared to the average of days 1–3, Bonferroni/Dunn).
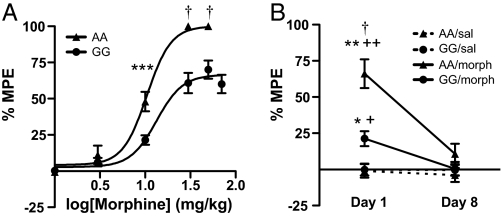
Opiate analgesics are widely used for pain management, but individual differences in opiate sensitivity can alter effective treatment. Clinical findings demonstrate that individuals carrying the G118 allele report greater pain sensation (8) and require higher doses of morphine to alleviate pain following surgery (7, 8, 24). Therefore, we used the hot-plate assay to evaluate basal nociceptive responses and morphine-mediated antinociception in mice with the A112G SNP. Using a cumulative dosing paradigm, in which animals were injected with increasing doses of morphine and evaluated for morphine-mediated antinociception at 30-min intervals (25), G/G mice showed a significantly lower maximal possible effect of morphine (% MPE) at higher doses (genotype × dose interaction, F1,63 = 5.348, P = 0.02; Fig. 3A). There were no baseline differences in hind-paw lick latency, suggesting that the G/G mice do not have a decreased pain threshold. However, when testing at a higher temperature (58 °C), a difference was detected in baseline jumping behavior (main effect of genotype, F1,63 = 5.348, P = 0.02, Fig. S4a) along with a decrease in morphine-mediated antinociception (main effects of genotype, F1,30 = 24.310, P < 0.0001 and sex, F1,30 = 4.356, P = 0.05; Fig. S4b). Following 7 days of twice daily morphine injections (10 mg/kg), all animals showed a reduced effect of morphine (day × genotype × treatment interaction, F1,48 = 4.801, P = 0.03; Fig. 3B), suggesting that although the acute antinociceptive properties of morphine are diminished, tolerance to repeated administration remains intact.
Fig. 3.
Morphine-mediated antinociception is decreased in G/G mice while tolerance to repeated exposure remains intact. (A) Morphine-mediated antinociception, as measured by hind-paw lick latency on a 55 °C hot-plate assay using a cumulative-dosing paradigm, was significantly reduced in G/G mice. Results are presented as percentage of maximal possible effect (MPE) [(morph jump latency − saline jump latency)/(total time − saline jump latency) × 100] (mean ± SEM, n = 18; ***, P < 0.001; †, P < 0.0001 compared to G/G mice). (B) Tolerance to morphine-mediated (10 mg/kg) hot-plate antinociception was present in both A/A and G/G mice. Results are presented as percentage of maximal possible effect (MPE) [(morph jump latency − baseline jump latency)/(total time − baseline jump latency) × 100] (mean ± SEM, n = 12–14; *, P < 0.05; **, P < 0.01 compared to saline-treated controls; +, P < 0.05; ++, P < 0.01 compared to day 8; †, P < 0.0001 compared to G/G mice treated with morphine, Bonferroni/Dunn).
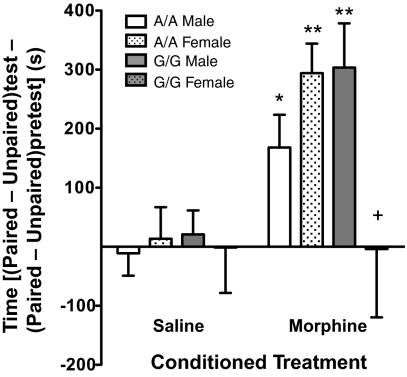
The use of morphine as an analgesic is limited by the abuse liability of the drug engendered by its ability to activate the reward pathway. In mice, the rewarding properties of morphine can be demonstrated through the development of a conditioned place preference to environments paired with morphine. As expected, A/A animals showed a robust preference for morphine-paired environments. G/G males showed a preference for morphine-paired environments equivalent to that of the A/A mice. In contrast, G/G females did not show a preference for the morphine-paired environment (treatment × genotype × sex interaction, F1,44 = 3.958, P = 0.05; Fig. 4). The variable effect of the A118G SNP in males and females has been reported in clinical studies of nicotine addiction and pain response (14–16), but not for opioid reward (26).
Fig. 4.
Female G/G mice failed to show a conditioned place preference to morphine-paired environments (10 mg/kg). Results are presented as the difference in time spent in drug-paired environments compared to nondrug-paired environments on the test day minus the difference in time from the preconditioning day (mean ± SEM, n = 6–8; *, P < 0.05; **, P < 0.01 compared to saline-treated controls; +, P < 0.05 compared to morphine-treated A/A females and G/G males, Bonferroni/Dunn).
Behavioral Responses to Withdrawal from Chronic Morphine Exposure.
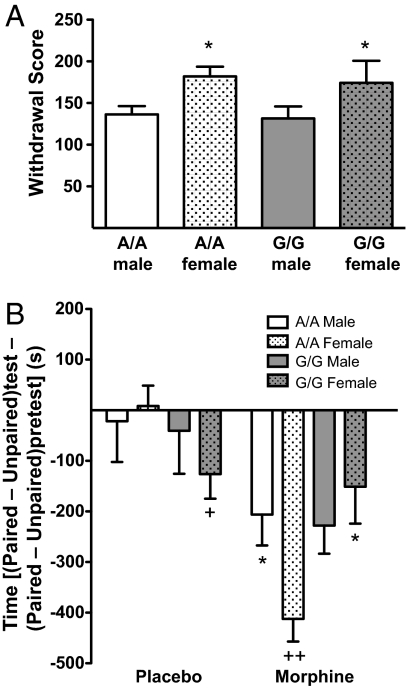
Chronic morphine exposure can cause both physical and psychological dependence. Following chronic morphine administration, male and female mice of both genotypes demonstrated physical dependence, as measured by the presence of somatic signs following naloxone-precipitated morphine withdrawal (main effect of sex, F1,18 = 7.537, P = 0.01, with no contribution of genotype; Fig. 5A). Psychological dependence was measured using a similar conditioning paradigm as was used to evaluate reward. Animals were implanted with s.c. morphine or placebo pellets 3 days before receiving a single naloxone (0.1 mg/kg, s.c.) administration in 1 chamber of a 2-chamber conditioning apparatus. All mice avoided environments associated with naloxone-precipitated morphine withdrawal (main effect of treatment, F1,76 = 20.206, P < 0.0001; Fig. 5B). Further analysis, however, shows that A/A females spent significantly less time on the side of the chamber paired with naloxone-precipiated morphine withdrawal than did G/G females (chronic treatment × genotype × sex interaction, F1,76 = 4.810, P = 0.03; Fig. 5B). In contrast, there were no place aversion differences between male A/A and G/G mice. Interestingly, the placebo-treated G/G females also avoided naloxone-paired environments compared to placebo-treated A/A females, while there were no differences in males between genotypes. Together, these studies demonstrate that, in contrast to the physical withdrawal signs, the psychological aversion associated with acute withdrawal in morphine-dependent mice is altered by the G112 allele in females only.
Fig. 5.
Disassociation of the physical and affective components of naloxone-precipitated morphine withdrawal. (A) A/A and G/G mice displayed similar somatic signs of naloxone-precipitated (0.1 mg/kg) withdrawal. Results are presented as the withdrawal score calculated by summing the total number of occurrences of jumping, paw tremor, genital licking, backing up, gnawing, ptosis, resting tremor, diarrhea, and teeth chatter (mean ± SEM, n = 5–6; *, P < 0.05 compared to male A/A and G/G). (B) Naloxone-precipitated morphine withdrawal-induced place aversions were reduced in G/G females. Additionally, placebo-treated G/G females displayed aversion to naloxone-paired environments compared to A/A females. Results are presented as the difference in time spent in drug-paired environments compared to nondrug-paired environments on the test day minus the difference in time from the preconditioning day (mean ± SEM, n = 8–12; *, P < 0.05 compared to morphine-treated A/A females; +, P < 0.05; ++, P < 0.0001 compared to placebo-treated A/A females, Bonferroni/Dunn).
Discussion
The A118G SNP has been implicated in a variety of pain sensitivity and drug addiction phenotypes in humans. Specifically, carriers of the G118 allele show an elevated sensitivity to pain and a reduced analgesic response to opioid administration. Additionally, the G118 allele has been associated with increased efficacy of treatments for alcohol and nicotine dependence. An understanding of the mechanisms underlying these alterations is essential for developing alternative pain therapies for carriers of the G allele or treatments for addiction that take advantage of the apparent benefit conferred by this SNP. To clarify the functional mechanisms linking the OPRM1 A118G to some of these phenotypes, we developed a knock-in mouse that possesses the mouse-equivalent SNP in the MOPR gene (Oprm1 A112G).
Functional knock-in technology using Cre-loxP homologous recombination allows for the generation of mouse models of human mutations or polymorphisms (27). To prevent interference with normal transcriptional control, most models have removed the selection marker resulting in a residual loxP site in the targeted gene, which has not been shown to alter expression (28). In the present mouse model, the G112-targeted allele did reduce both mRNA and protein expression in some brain regions; however, SNPs in transcribed regions, specifically the A118G SNP, have been shown to affect mRNA processing and turnover (19, 29). Thus, while we cannot rule out the potential effect of the loxP site as contributing to reductions in mRNA and protein, the fact that these mice displayed similar molecular and behavioral phenotypes to human carriers of the G118 allele provides evidence that this SNP indeed has functional consequences and that this mouse could serve as a valuable tool in identifying the effects of these changes.
It has been contested whether the A118G SNP confers a gain or loss of function. Studies reporting elevations in biochemical or behavioral traits [e.g., increases in maternal attachment in primates (30) or cortisol responses in humans (31)] typically cite elevations in β-endorphin binding (17) as a potential mechanism. Alternatively, studies reporting deficits in behavior [e.g., decreased nicotine reward (16)] typically cite decreases in MOPR expression (19) as explanation for the effects. In the present study, we found evidence suggesting that the consequences of this SNP cannot be evaluated as a simple gain or loss of function. We did not find evidence suggesting altered affinity to MOPR agonists, although we did corroborate studies showing decreased MOPR expression by demonstrating decreases in mRNA and protein levels. In line with decreased MOPR levels, G/G mice showed deficits in the hyperlocomotor and antinociceptive actions of acute morphine administration; however, not all behaviors showed deficits despite these reductions. This is most evident in the conditioned reward and aversion studies in which only females demonstrated an altered behavioral response. On the other hand, physical morphine withdrawal signs were similar between genotypes for both sexes. Previous studies have demonstrated a disassociation between the physical and aversive components of precipitated morphine withdrawal (32, 33), suggesting that the alterations caused by this SNP is dependent on the circuitry involved.
Distinctions between genotypes do not appear to be dependent on the timing or duration of morphine administration. G/G mice showed a significant, albeit diminished, antinociceptive effect of acute morphine treatment. This effect decreased with repeated morphine exposure, demonstrating that these mice develop tolerance similar to A/A mice. Furthermore, G/G mice showed a significant, yet diminished, hyperlocomotor effect of acute morphine treatment. However, repeated morphine exposure did not increase this response, demonstrating that G/G mice do not develop locomotor sensitization. Therefore, the mechanisms underlying the development of tolerance and sensitization may be differentially influenced by the Oprm1 SNP. Loss of the delta-opioid receptor, for instance, results in elevated sensitization and diminished tolerance to morphine (34). Although we did not investigate changes in the expression and function of other opioid receptors, it is possible that compensatory upregulation or altered dimerization of these receptors could contribute to some of the altered behaviors of the G/G mice.
Morphine has varying potencies in males compared with females, depending on the assay (for review, see ref. 35). Recently, differences in MOPR receptor levels in rat brain have been identified as essential for sex differences in morphine analgesia (36). Estrogen modulation of MOPRs is supported by in vivo positron emission tomography (PET) imaging studies with [11C] carfentanil. Premenopausal women have ≈25% greater availability of MOPRs than men in cortical and subcortical areas (37, 38), a difference that disappears after menopause (37). The sex-differences we observed in morphine reward and withdrawal, however, cannot be explained by altered levels of MOPRs, as these reductions were equivalent across sexes. To further explore hormonal modulation in this phenotype, studies requiring estrogen depletion of females or feminization of males harboring this SNP will be required. To date, human genetic studies have not been designed a priori with adequate power to examine the sex-dependent effects of the OPRM1 A118G SNP on behavior. The current data suggest such analysis is warranted.
Genetic association studies in psychiatry and addiction are plagued by nonreplications. However, there is a critical mass of positive studies linking the OPRM1 A118G SNP with opioid, alcohol, and nicotine dependence, and subsequent treatment responses. Data obtained from the A112G mice provide compelling evidence that this type of a translational cross-species model is important for complete functional characterization of genetic variants. Future studies using this mouse model could serve as a valuable tool in determining the mechanisms underlying responses to a variety of drugs of abuse and in developing personalized therapies based on genotype.
Materials and Methods
Animals.
All mice (8–15 weeks, 18–30 g) were group housed and maintained on a 12-h/12-h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. For a complete description of the derivation of Oprm1tm1Jabl mice, see SI Materials and Methods. All experimental testing sessions were conducted between 8:00 a.m. and 5:00 p.m., with animals randomly assigned to treatment conditions and tested in counterbalanced order. Both male and female mice were used in all studies except for morphine locomotor sensitization, in which only males were used. Male and female data were combined when there were no statistical contributions of sex. Separate, näive cohorts were used for behavioral experiments, except for acute locomotor (Fig. S3), hot-plate (Fig. S4), and physical withdrawal (Fig. 5A) studies, in which 1 cohort of animals was used for all 3 experiments conducted in the order listed and separated by at least 1 week. Separate cohorts of animals were used and the data combined for the following experiments: CPP (Fig. 4), CPA (Fig. 5), acute locomotor activity (Fig. S3), and 58 °C hot-plate (Fig. S4), as there were no statistical differences within groups measured between cohorts.
Drugs.
For acute drug administration, morphine sulfate was obtained from the NIDA Drug Supply Program and naloxone hydrochloride was obtained from Sigma Aldrich. Both drugs were dissolved in 0.9% saline and administered s.c. at a volume of 0.1 mL/10 g body weight. Morphine dependence was achieved by s.c. implanting a single placebo (cellulose) or morphine (25 mg morphine base) pellet (NIDA Drug Supply Program) in the dorsal surface of mice under general isoflurane anesthesia for 3 days before testing.
Quantitative Real-Time PCR.
For RNA isolation and cDNA synthesis, mice were killed by cervical dislocation and brains were rapidly removed and dissected on ice. Brains were first sliced using a mouse brain matrix into 1-mm slices. Specific regions were identified and macrodissected using their approximate mouse stereotaxic coordinates (AMYG and HIPP, bregma −1.2 mm; BNST and CTX, bregma +0.26 mm; NAc, bregma +1.10 mm; VTA, bregma −3.64 mm). The hypothalamus was removed from the ventral side of the brain before placement in the mouse brain matrix. RNA was isolated from brain tissue using TRIzol/chloroform in conjunction with an RNeasy Mini kit (Qiagen). cDNA was synthesized using Oligo dT primer (Operon) and SuperScript II reverse transcriptase (Invitrogen). Taqman QPCR multiplex reactions were assembled using the Taqman Universal PCR Master Mix (Applied Biosystems) along with 300 nM primers (final concentration). All quantitative real-time polymerase chain reactions (QPCR) were run using the Stratagene MX3000 and MXPro QPCR software with cycling parameters set at 95 °C for 10 min followed by 40 cycles of 95 °C (30 s) and 60 °C (1 min). All reactions were performed in triplicate and the median cycle threshold was used for analysis. The mRNA levels of target genes were normalized to the housekeeping gene, TATA binding protein (TBP). Primers used in Fig. 1 were found 3′ of the knock-in and spanned exon 1 and exon 2 (5′: caccatcatggccctctatt; 3′: caaaatgaagactgccacca).
Brain Membrane Preparation.
Frozen mouse whole brains or thalami were homogenized in ≈8-volume 25 mM Tris-HCl buffer/pH 7.4 containing 1 mM EDTA and 0.1 mM PMSF on ice and then centrifuged at ≈100,000 g for 30 min. Pellets were twice rinsed with 25 mM Tris-HCl buffer and resuspended in 0.32 M sucrose in 50 mM Tris-HCl/pH 7. Suspended membranes were passed through a 26.5 G needle 5 times and then frozen at −80 °C.
Western Blot.
Membranes were prepared from thalami of A/A mice, G/G mice, and MOPR knock-out mice. The MOPR knock-out mice used were originally developed in the lab of John Pintar by disruption of exon 1 of the Oprm1 gene through homologous recombination (39). Membrane proteins were loaded (15 μg per lane) for SDS/PAGE and Western blot was performed with the MOPR antibody, anti-μC [against the MOPR (383–398) peptide] (1:5,000, final 0.26 μg/mL), followed by goat anti-rabbit IgG conjugated with HRP (1:5,000), and then reacted with enhanced chemiluminescence (ECL) Western blotting detection reagents (40). Images were captured with a FujiFilm LAS-1000 Imaging System. After a brief wash, the same blot was then incubated with mouse anti-GAPDH-HRP-conjugated (1:10,000) (Abcam) followed by ECL reagents. Quantification of MOPR-immunoreactivities was carried out by densitometry analysis with the ImageGauge software for Fuji Imaging System. MOPR immunoreactivity in each lane was normalized against that of GAPDH.
MOPR Ligand Binding.
Binding assays were performed as previously described (41) with some modifications. Mouse brain membranes were incubated at room temperature for 30 min with 100 mM NaCl and 100 μM GDP in 50 mM Tris-HCl buffer (pH 7.4) containing 1 mM EDTA (TE buffer) and washed 3 times with the TE buffer. Binding was performed in TE buffer containing 5 mM MgCl2 for ≈2.5–3 h at room temperature to convert receptors to high affinity states. Saturation binding of [3H]DAMGO to MOPRs in whole brain membranes was performed with 7 concentrations of [3H]DAMGO (ranging from 0.1 to 8.0 nM), and Kd and Bmax values were determined by nonlinear regression curve fit with 1 site binding (GraphPad Prism). For binding to MOPR in thalamus membranes, a single concentration of [3H]DAMGO was used (3 nM). Competitive inhibition of [3H]DAMGO (1.0 nM) binding by β-endorphin, morphine, or naloxone was performed in the absence or presence of various concentrations of each ligand. Ki values were determined by nonlinear regression curve fit of 1 site competition (GraphPad Prism). Nonspecific binding was measured in the presence of naloxone (1.0 μM). Each binding assay was carried out in duplicate in a final volume of 0.5 mL with 0.2–0.4 mg protein/tube for whole brain binding or 0.1 mg protein/tube for thalamus binding. Incubations were terminated by filtration through Whatman GF/B filters under vacuum.
Locomotor Activity.
Locomotor activity was analyzed in a “home cage” activity monitoring system (MedAssociates). The testing cage, which was identical in dimension to the home cage, was placed in a photobeam frame (30 × 24 × 8 cm) with 2 levels of sensors arranged in an 8-beam array strip. A small amount of fresh bedding was scattered on the cage floor. Locomotor sensitization: Animals were tested every 2–3 days for 120 min following an injection of saline or morphine (10 mg/kg, s.c.). On treatment days 1–3, all animals were administered saline. On days 4–9, animals received either morphine or saline, according to their treatment group. Beam break data were read into MedAssociates personal computer-designed software and monitored at 10-min intervals.
Hot-Plate.
The nociceptive threshold for analgesia was examined with a hot-plate analgesia meter (Columbus Instruments). The hot-plate provided a constant 55 °C (Fig. 3) or 58 °C (Fig. S4) surface, temperatures low enough to avoid harming the mice, but high enough to be uncomfortable for a saline-treated animal. A small plastic cage surrounding the hot-plate prevented the animal from leaving the plate surface. Cumulative dosing (Fig. 3A): Animals were first placed on the hot-plate and the latency to lick the hind-paw or jump was recorded. Upon displaying 1 of these behaviors or upon reaching the predetermined cut-off time (60 s), the animals were immediately removed from the hot-plate, injected with the first dose of morphine, and returned to their home cage. Animals were retested on the hot-plate and immediately injected with the next morphine dose at 30-min intervals. Animals received doses of 0, 1, 2, 7, 20, and 20 mg/kg; any animals that did not complete the 60-second trial without licking or jumping at the highest dose received an additional 20 mg/kg injection at the usual dosing schedule. Tolerance (Fig. 3B): Animals were injected twice daily for 8 days with morphine (10 mg/kg) or saline, according to treatment group, and tested for antinociception 30 min following the a.m. injection. The latency to lick the hind-paw or jump was recorded and a maximum test duration set at 60 seconds.
Conditioned Place Preference.
Place-conditioning boxes consisted of 2 chambers (20 × 20 × 20 cm), one with stripes on the wall and a metal grid floor and the other with gray walls and a metal patterned floor. A partition with an opening separated the 2 chambers in each box, but allowed access to either side of the chamber. This partition was closed off during the pairing days. Preconditioning phase (day 1): Animals were placed in the boxes and allowed to roam freely throughout both chambers for 15 min; time spent in each chamber was recorded. These data were used to separate the animals into groups of approximately equal bias. Conditioning phase (days 2–9): Animals received 8 days of once-a-day pairings in which an animal was injected with morphine (10 mg/kg) or saline and then immediately placed into 1 chamber for 30 min. On the following day, animals were injected with either saline or morphine, depending on what they had received on the previous day, and placed in the opposite chamber. Drug pairings were divided such that half of the animals received morphine injections on odd days while the other half received morphine on even days; nondrug paired animals received saline injections throughout the conditioning phase. Drug-paired chambers were randomized among all groups. Testing phase (day 10): Animals were all given a saline injection and allowed to roam freely between the 2 chambers; the amount of time spent in each chamber was recorded.
Conditioned Place Aversion.
The CPA test was performed similarly to the CPP test with the following differences. Preconditioning phase (day 1): Three days following implantation of morphine or placebo pellets, animals were placed in the boxes and allowed to explore both chambers to test for preexisting biases. Conditioning phase (days 2–3): On day 2, all animals were injected with saline and confined to 1 chamber for 30 min. On day 3, all animals were injected with naloxone (0.1 mg/kg, s.c.) and confined to the opposite chamber for 30 min. Test phase (day 4): Animals were injected with saline and allowed to explore both chambers; the amount of time spent in either chamber was recorded.
Somatic Withdrawal Signs.
Three days following implantation of morphine or placebo pellets, animals were placed on cotton pads inside of a clear plastic cylinder with an open top. Animals were allowed to habituate for 30 min before receiving a s.c. injection of naloxone (0.1 mg/kg). The number of occurrences of jumping, paw tremor, genital licking, backing up, and gnawing was recorded. Additionally, the presence or absence of the following symptoms was recorded in 5-min bins for the 30-min test: ptosis, resting tremor, diarrhea, and teeth chatter. Withdrawal scores were calculated as the sum of all occurrences of somatic signs displayed during the 30-min test.
Analysis.
When comparing multiple effects, analyses were performed using 2- or 3-way analysis of variance (ANOVA) tests (repeated measures tests were used when comparing multiple time points) with significant F-values reported in the text. Bonferroni/Dunn post hoc tests were used to compare significant interactions between main effects. When comparing differences between genotypes for only 1 effect, unpaired t-tests were used with significant t-values reported in the text. Statistical significance was set at P ≤ 0.05.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants R01-DA-011649 (to J.A.B.), P50-CA-084718 (to J.A.B. and C.L.), R01-DA-17302 (to L-.Y.L.-C.), P30-DA-13429 (to L.-Y.L.-C.), and T32-DA-007241–15 (to S.D.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901800106/DCSupplemental.
References
- 1.Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: Population studies and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4(5):476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 2.Bergen AW, et al. Mu opioid receptor gene variants: Lack of association with alcohol dependence. Mol Psychiatry. 1997;2(6):490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- 3.Tan EC, Tan CH, Karupathivan U, Yap EP. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport. 2003;14(4):569–572. doi: 10.1097/00001756-200303240-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 5.Drakenberg K, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA. 2006;103(20):7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Wildenberg E, et al. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.Chou WY, et al. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50(7):787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- 8.Sia AT, et al. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109(3):520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- 9.Anton RF, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 11.Lerman C, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4(3):184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- 12.Glatt SJ, et al. Evaluation of OPRM1 variants in heroin dependence by family-based association testing and meta-analysis. Drug Alcohol Depend. 2007;90(2–3):159–165. doi: 10.1016/j.drugalcdep.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Kendler KS, Chen X. The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct. 2006;2:28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillingim RB, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6(3):159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J. 2007;7(5):353–361. doi: 10.1038/sj.tpj.6500432. [DOI] [PubMed] [Google Scholar]
- 16.Ray R, et al. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 2006;188(3):355–363. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- 17.Bond C, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89(3):553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 20.Kroslak T, et al. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103(1):77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 21.Crawley JN, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 22.Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46(2):213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mickiewicz AL, Dallimore JE, Napier TC. The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropsychopharmacology. 2009;34(4):874–886. doi: 10.1038/npp.2008.111. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Gibby CC, et al. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130(1–2):25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sora I, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA. 1997;94(4):1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Compton P, Geschwind DH, Alarcon M. Association between human mu-opioid receptor gene polymorphism, pain tolerance, and opioid addiction. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):76–82. doi: 10.1002/ajmg.b.20057. [DOI] [PubMed] [Google Scholar]
- 27.Roebroek AJ, et al. Mutant Lrp1 knock-in mice generated by recombinase-mediated cassette exchange reveal differential importance of the NPXY motifs in the intracellular domain of LRP1 for normal fetal development. Mol Cell Biol. 2006;26(2):605–616. doi: 10.1128/MCB.26.2.605-616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson AD, et al. Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: Detection through allelic expression imbalance in human target tissues. Pharmacogenet Genomics. 2008;18(9):781–791. doi: 10.1097/FPC.0b013e3283050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr CS, et al. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105(13):5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong RY, et al. The micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31(1):204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- 32.Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. Neuropsychopharmacology. 1993;9(4):303–311. doi: 10.1038/npp.1993.66. [DOI] [PubMed] [Google Scholar]
- 33.Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: A quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271(3):1391–1398. [PubMed] [Google Scholar]
- 34.Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34(4):887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16(5):376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 36.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28(52):14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156(6):842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 38.Zubieta JK, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuller AG, et al. Retention of heroin and morphine-6 beta-glucuronide analgesia in a new line of mice lacking exon 1 of MOR-1. Nat Neurosci. 1999;2(2):151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 40.Huang P, et al. Brain region-specific N-glycosylation and lipid rafts association of the rat mu opioid receptor. Biochem Biophys Res Commun. 2008;365(1):82–88. doi: 10.1016/j.bbrc.2007.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu-Chen LY, Yang HH, Li S, Adams JU. Effect of intracerebroventricular beta-funaltrexamine on mu opioid receptors in the rat brain: Consideration of binding condition. J Pharmacol Exp Ther. 1995;273(3):1047–1056. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.