Abstract
Three polarized aromatic guest molecules (pyrene-4,5-dione, 1) form a triple-layered stack in the box-shaped cavity of an organic pillared coordination cage in water. The cavity size strictly limits the number of stacked planar guests but does not restrict guest orientation, and thus enables the study of discrete stacks of polarized guests and their preferred conformations. Crystallographic study shows that the guest molecules in the cavity are rotated 120° with respect to each other, canceling the net dipole moment rather than the local dipole moment. The unique conformation of a discrete, triple stack of 1 sharply contrasts to the standard head-to-tail conformation in infinite stacks of 1.
Keywords: π–π interactions, aromatic molecule, coordination cage, dipole moment, self-assembly
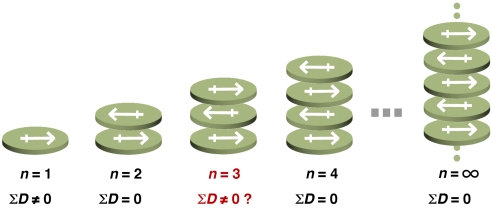
The large dipole moment (6.1–6.7 D) of pyrene-4,5-dione (1) (1–3) induces an infinite, head-to-tail columnar stacking in the crystalline state (4). This alternating stacking motif should also be present in finite stacks and, when an even number of 1 form a stack, the offset stacking will result in a net dipole moment of zero. However, if an odd number of 1 stack in a finite manner, the head-to-tail stacking cannot fully cancel the dipole moments. As for n = 3, if the first 2 molecules form an offset pair, then the third molecule should result in a residual overall dipole moment (Fig. 1). The stepwise, alternating stacking of polar aromatic molecules has, however, never been examined because of insufficient methodology to precisely control the number of stacking units. The self-assembled cavity (5–12) of the box-shaped, organic pillared coordination cage 2 (Fig. 2) is capable of containing a predetermined number of aromatic stacks and provides a method to examine the behavior of the head-to-tail stacking of 3 molecules 1. Accordingly, we examined the self-assembly of an inclusion complex, 2⊃(1)3, and studied the guest orientation. Diffraction study shows the unique way of the highly polarized molecule to cancel the net dipole moments rather than local dipole moments.
Fig. 1.
Prediction of the net dipole moment of discretely and infinitely layered polarized molecules.
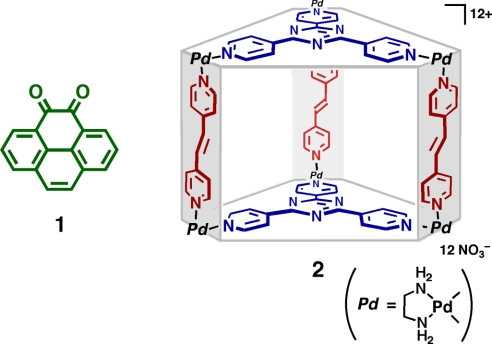
Fig. 2.
Chemical structures of pyrene-4,5-dione (1) and organic pillared coordination cage 2.
Results and Discussion
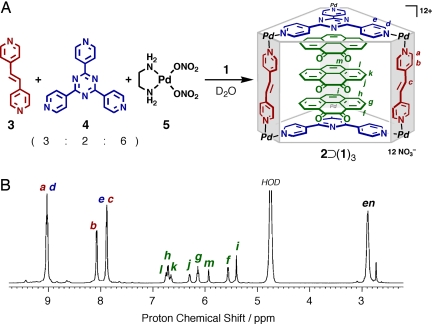
The inclusion complex, 2⊃(1)3, was quantitatively formed by self-assembly (13). NMR analyses (1D, COSY, NOESY, and DOSY) confirmed the formation of a single product where ratio of 2 and 1 is 1:3. All of the signals could be assigned (Fig. 3) and were observed at the same diffusion coefficient in DOSY (see SI), indicating a stable host-guest complex on the NMR time scale. Two sets of signals are observed for dione 1 in a 2:1 ratio and both are considerably shifted upfield (Δδ = −1.13 to −2.96 ppm) as a result of shielding from the nearby π-systems. The structure of 2⊃(1)3 is stable enough in both aqueous solution and solid states.
Fig. 3.
Synthesis and characterization of complex 2(1)3. (A) Schematic representation of a self-assembly of 2⊃(1)3. (B) 1H NMR spectrum (500 MHz, 300 K) of triple-stacked 2⊃(1)3 complex in D2O.
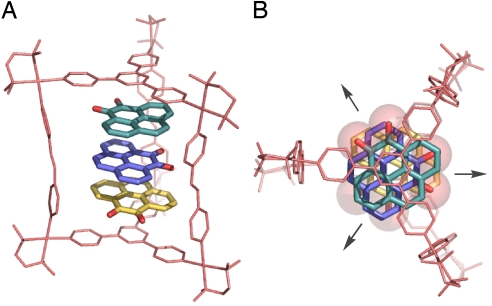
Absolute structural evidence was provided by X-ray analysis of the bright orange crystals of the TMEDA derivative 2′⊃(1)3. The crystal structure confirmed the expected 2′⊃(1)3 structure in which 3 molecules of 1 are stacked (≈3.3 Å) within the cavity of cage 2′ (Fig. 4).
Fig. 4.
X-ray crystal structure of 2′⊃(1)3. (A) Side view. (B) Top view. Solvent molecules (H2O) and counter anions (NO3−) are omitted for clarity.
Unexpectedly, the 3 guest molecules in the cavity are not stacked in a head-to-tail fashion but rather they are twisted by 120° with respect to each other. As a result of the higher (D3h) symmetry, the net dipole moment is fully cancelled. No significant interactions are observed between 1 and any species existing outside the cage (e.g., counter ions or water of crystallization). Because negatively charged carbonyl oxygen atoms of 1 and cationic Pd(II) centers of 2 are not in a close proximity, they do not seem to interact. Therefore, the guest orientation is directed neither by crystal packing nor by the host-guest electrostatic interaction but by the polar guests' own nature to find their optimal way of stacking. We believe that the stacking manner of 1 in the cavity of 2 is the most preferable for n = 3.
The cancellation of the dipole moments among the 3 guests generates dipole-dipole interaction as an attractive force. It seems that the (1)3 structure is stabilized by the dipole-dipole interaction and thus the 2⊃(1)3 complex is formed efficiently. In fact, we failed to obtain 2⊃(G)3 complexes with nonpolarized aromatic molecules (G) such as colonene, pyrene, and perylene, even though the size of the cavity fits that of the layered trimer (G)3 structures. It is well known that the interaction in the donor-donor-donor arrays of electron rich molecules is rather repulsive and they apt to make offset (herringbone-type) stacks (18).
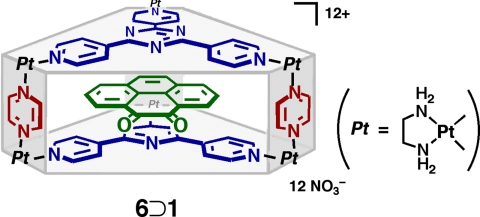
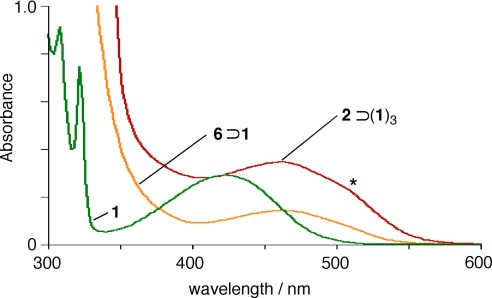
The UV-visible spectra of 2⊃(1)3, free 1, and 6⊃1 (where cage 6 is the analogous cage with pyrazine pillars (6); 14) were compared (Fig. 5, 6). The carbonyl n-π* transitions of the encapsulated diones in cage 2⊃(1)3 and 6⊃1 are considerably red-shifted (Δλ = 40 nm) compared with the free 1 because of π-stacking with the electron deficient triazine panel 4. Additionally, the spectra of complex 2⊃(1)3 exhibits a strong shoulder at 510 nm (asterisk in Fig. 6) that is not present in the isolated stack 6⊃1. Presumably, this transition is characteristic to the π-π interactions between the diones and a similar band is also found in solid 1 (λmax = 525 nm) (see SI).
Fig. 5.
Chemical structure of 6⊃1.
Fig. 6.
UV-visible spectra (room temperature, 1.0 mM) of 2⊃(1)3, 6⊃1 in H2O and of 1 in CH3CN.
In summary, we confirmed that an odd number of polarized aromatic molecules have a unique way of stacking that results in a negligible overall, rather than local, dipole moment. Our results demonstrate that dipole interaction is an important factor in the formation of stable aromatic stacking structures. The pillared cage 2 enables the precise control of stacking units but does not restrict the orientation of the guests, providing opportunities to study the hitherto unexplored properties of discretely stacked aromatic compounds in solution (15–17).
Materials and Methods
General.
The 1H, 13C{1H} NMR, and other 2D NMR spectra were recorded on a Bruker DRX-500 (500 MHz) spectrometer. TMS (CDCl3 solution) in a capillary served as external standard (δ 0 ppm). IR measurements (ATR) were carried out using a DIGILAB Scimitar FTS-2000 instrument. UV-visible spectral data were recorded on a SHIMADZU UV-3150. Melting points were determined with a Yanaco MF-500 V micro melting point apparatus. Diffraction measurements were made using a Bruker APEX CCD diffractometer. Solvents and reagents were purchased from TCI Co., Ltd.; WAKO Pure Chemical Industries, Ltd.; and Sigma–Aldrich. Deuterated H2O was acquired from Cambridge Isotope Laboratories, Inc. and used as supplied for the complexation reactions and NMR measurements.
2⊃(1)3.
A mixture of pillar-like ligand 3 (5.5 mg, 30 μmol), panel-like ligand 4 (6.3 mg, 20 μmol), (en)Pd(NO3)2 (5; 17.4 mg, 60 μmol), and pyrene-4,5-dione (1) (7.0 mg, 30 μmol) in D2O (1.0 mL) was stirred at 60 °C for 3 h to give clear orange solution. 1H NMR analysis of the solution revealed the formation of 2⊃(1)3 quantitatively. After filtration of the resulted orange solution, the resulting solution was dried by vacuum freeze-drying equipment. 2⊃(1)3 was isolated as an orange powder (32.8 mg, 91% yield). 1H NMR (500 MHz, D2O, 27 °C):δ 9.02 (d, J = 6.5 Hz, 12H), 9.01 (d, J = 6.5 Hz, 12H), 8.06 (d, J = 6.5 Hz, 12H), 7.87 (d, J = 6.5 Hz, 12H), 7.86 (s, 6H), 6.74 (d, J = 7.5 Hz, 2H), 6.70 (d, J = 7.5 Hz, 4H), 6.64 (d, J = 6.5 Hz, 2H), 6.28 (d, J = 6.5 Hz, 2H), 6.13 (t, J = 7.5 Hz, 4H), 5.92 (s, 2H), 5.55 (d, J = 7.0 Hz, 4H), 5.39 (s, 4H), 2.87 (br, 24H); 13C{1H} NMR (125 MHz, D2O, TMS):δ 177.2 (Cq), 176.6 (Cq), 167.9 (Cq), 152.2 (CH), 151.7 (CH), 147.5 (Cq), 143.6 (Cq), 136.4 (CH), 136.0(CH), 132.4 (CH), 129.6 (Cq), 129.3 (Cq), 128.7 (CH), 128.7 (CH), 127.7 (CH), 127.2 (CH), 126.0 (Cq), 126.0 (CH), 125.7 (CH), 124.7 (CH), 124.7 (CH), 124.6 (Cq), 46.8 (CH2); DOSY (m2/s): logD = −9.85; IR (ATR, cm−1): 3410(br), 3200(br), 3080(br), 1670, 1614, 1519, 1383, 1061, 831, 804; m.p.: ≈230 °C (decomposed); elemental analysis calculated for C132H126N42O42Pd6·20(H2O): C, 39.92; H, 4.21; N, 14.81. Found: C, 39.76; H, 4.02; N, 15.00.
X-Ray Crystal Data of 2′⊃(1)3.
C156H174N38O65.5Pd6, Mr = 4259.74, crystal dimensions 0.15 × 0.15 × 0.05 mm3, monoclinic space group P21/c, a = 28.563 (4) Å, b = 16.085 (2) Å, c = 41.635 (5) Å, β = 94.598 (3)°, V = 19067 (4) Å 3, Z = 4, ρcalcd = 1.487 g·cm−3, F- (000) = 8704, radiation, λ(MoKα) = 0.71073 Å, T = 80 (2)K, reflections collected/unique 141967/47437 (Rint = 0.1607). The structure was solved by direct methods (SHELXL-97) and refined by full-matrix least-squares methods on F2 with 2584 parameters. R1 = 0.1246 (I > 2σ(I)), wR2 = 0.4073, GOF 0.966; max/min residual density 4.611/−1.703 eÅ−3.
6⊃1.
Powder of 1 (4.6 mg; 20 μmol, 2 eq. per 6) was added to a D2O solution (1.0 mL) of cage 6 (31 mg; 10 μmol) and the suspended mixture was stirred at 60 °C for 30 min. After filtration of the resulted orange solution, the 1H NMR spectrum revealed the quantitative formation of 6⊃1complex. The solution was evaporated and dried by vacuum freeze-drying equipment to give a orange powder of 6⊃1 complex (3.1 mg; 9.2 μmol) in 92% yield. 1H NMR (500 MHz, D2O, 27 °C): 9.59 (s, 12H), 9.08 (d, J = 5.5 Hz, 12H), 8.14 (br, 12H), 7.53 (d, J = 7.5 Hz, 2H), 7.86 (t, J = 7.5 Hz, 2H), 6.30 (s, 2H), 6.20 (d, J = 7.5 Hz, 2H), 2.89 (d, J = 5.5 Hz, 12H), 2.84 (d, J = 5.5 Hz, 12H); 13C{1H} NMR (125 MHz, D2O, TMS):δ 177.9 (Cq), 167.8 (Cq), 153.5 (CH), 151.3 (CH), 144.3 (Cq), 135.9 (CH), 131.1 (CH), 130.1 (Cq), 128.9 (CH), 127.8 (Cq), 127.4 (CH), 125.6 (CH), 47.7 (CH2); DOSY (m2/s): logD = −9.80; IR (ATR, cm−1): 3420(br), 3040(br), 1512, 1383, 823, 816, 722, 705; m.p.: ≈230 °C (decomposed).
Supplementary Material
Acknowledgments.
This work was supported by a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science (to Y.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 703950).
This article contains supporting information online at www.pnas.org/cgi/content/full/0810319106/DCSupplemental.
References
- 1.Vollmann H, Becker H, Corell M, Streeck H, Langbein G. Pyrene and its derivatives. Justus Liebigs Ann Chem. 1937;531:1–159. [Google Scholar]
- 2.Young ERR, Funk RL. A practical synthesis of pyrene-4,5-dione. J Org Chem. 1998;63:9995–9996. [Google Scholar]
- 3.Hu J, Zhang D, Harris FW. Ruthenium(III) chloride catalyzed oxidation of pyrene and 2,7-disubstitued pyrenes: An efficient, one-step synthesis of pyrene-4,5-diones and pyrene-4,5,9,10-tetraones. J Org Chem. 2005;70:707–708. doi: 10.1021/jo048509q. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Enkelmann V, Negri F, Müllen K. Rational design of helical columnar packing in single crystals. Angew Chem Int Ed. 2004;43:1972–1975. doi: 10.1002/anie.200353245. [DOI] [PubMed] [Google Scholar]
- 5.Steed J W, Atwood J L. Supramol Chem. England: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 6.Leininger S, Olenyuk B, Stang PJ. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem Rev. 2000;100:853–908. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]
- 7.Warmuth R, Yoon J. Recent Highlights in hemicarcerand chemistry. Acc Chem Res. 2001;34:95–105. doi: 10.1021/ar980082k. [DOI] [PubMed] [Google Scholar]
- 8.Hof F, Craig SL, Nuckolls C, Rebek J., Jr Molecular encapsulation. Angew Chem Int Ed. 2002;41:1488–1508. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Fiedler D, Leung DH, Bergman RG, Raymond KN. Selective molecular recognition, C–H bond activation, and catalysis in nanoscale reaction vessels. Acc Chem Res. 2005;38:349–358. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]
- 10.Fujita M, Tominaga M, Hori A, Therrien B. Coordination assemblies from a Pd(II)-cornered square complex. Acc Chem Res. 2005;38:371–380. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 11.Vriezema DM, et al. Self-Assembled Nanoreactors. Chem Rev. 2005;105:1445–1490. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]
- 12.Amijs CHM, van Klink GPM, van Koten G. Metallasupramolecular architectures, an overview of functional properties and applications. Dalton Trans. 2006:308–327. doi: 10.1039/b505354d. [DOI] [PubMed] [Google Scholar]
- 13.Yoshizawa M, et al. Related organic pillared cages: Discrete stacking of large aromatic molecules within organic-pillared coordination cages. Angew Chem Int Ed. 2005;44:1810–1813. doi: 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]
- 14.Kumazawa K, Biradha K, Kusukawa T, Okano T, Fujita M. Multicomponent assembly of a pyrazine-pillared coordination cage that selectively binds planar guests by intercalation. Angew Chem Int Ed. 2003;42:3909–3913. doi: 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]
- 15.Yoshizawa M, Kumazawa K, Fujita M. Room-temperature and solution-state observation of the mixed-valence cation radical dimer of tetrathiafulvalene, [(TTF)2]+·, within a self-assembled cage. J Am Chem Soc. 2005;127:13456–13457. doi: 10.1021/ja053508g. [DOI] [PubMed] [Google Scholar]
- 16.Ono K, Yoshizawa M, Kato T, Fujita M. Three-metal-center spin interactions through the intercalation of metal azaporphines and porphines into an organic pillared coordination box. Chem Commun. 2008:2328–2330. doi: 10.1039/b801701h. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi Y, Yoshizawa M, Fujita M. Engineering stacks of aromatic rings by the interpenetration of self-assembled coordination cages. J Am Chem Soc. 2008;130:5832–5833. doi: 10.1021/ja077783+. [DOI] [PubMed] [Google Scholar]
- 18.Hunter CA, Lawson KR, Perkins J, Urch CJ. Aromatic interactions. J Chem Soc Perkin Trans. 2001;2:651–669. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.