Abstract
We identified a cis-prenyltransferase gene, neryl diphosphate synthase 1 (NDPS1), that is expressed in cultivated tomato (Solanum lycopersicum) cultivar M82 type VI glandular trichomes and encodes an enzyme that catalyzes the formation of neryl diphosphate from isopentenyl diphosphate and dimethylallyl diphosphate. mRNA for a terpene synthase gene, phellandrene synthase 1 (PHS1), was also identified in these glands. It encodes an enzyme that uses neryl diphosphate to produce β-phellandrene as the major product as well as a variety of other monoterpenes. The profile of monoterpenes produced by PHS1 is identical with the monoterpenes found in type VI glands. PHS1 and NDPS1 map to chromosome 8, and the presence of a segment of chromosome 8 derived from Solanum pennellii LA0716 causes conversion from the M82 gland monoterpene pattern to that characteristic of LA0716 plants. The data indicate that, contrary to the textbook view of geranyl diphosphate as the “universal” substrate of monoterpene synthases, in tomato glands neryl diphosphate serves as a precursor for the synthesis of monoterpenes.
Keywords: plant biochemistry, terpene synthases, cis-prenyltransferases, biochemical diversity, specialized metabolism
Terpenoids constitute a very large class of compounds synthesized by organisms in all kingdoms, with tens of thousands of structures known and a plethora of functions. The vast majority of the terpenoids are synthesized from the C5 building blocks isopentenyl diphosphate (IPP) and dimethyl allyl diphosphate (DMAPP). IPP and DMAPP are condensed, with the loss of one diphosphate group, to form larger prenyl diphosphate intermediates. These prenyl diphosphates serve as substrates for terpene synthases (TPSs), the enzymes that elaborate the backbone of the final terpenoid products (1).
Mono (C10), sesqui (C15), and di (C20) terpenes are very common in plants and serve various physiological and ecological roles. In particular, those containing no oxygen functionalities or only hydroxyl or carbonyl functionalities are generally volatile and are often emitted from plants to attract pollinators or repel herbivores. These also contribute to the aroma and flavor of foods consumed by humans. Consequently, the biosynthesis of these compounds has been extensively investigated in plants. Early studies demonstrated that partially purified monoterpene synthases preferentially used the prenyl diphosphate precursor geranyl diphosphate (GPP), in which the two isoprene units are joined in the trans (E) configuration, for the production of the observed monoterpenes (2, 3). Taken together with labeling studies showing that conversion of GPP to neryl diphosphate (NPP), the cis-isomer of GPP, was not necessary before cyclization (4) and several studies showing use of GPP by recombinant monoterpene synthases (5, 6), it has become widely accepted that GPP is the common substrate for monoterpene biosynthesis. Likewise, sesquiterpene synthases have been found to use the C15-diphosphate intermediate E,E-farnesyl diphosphate (FPP), and diterpene synthases use the C20-diphosphate intermediate E,E,E-geranylgeranyl diphosphate (GGPP) (7). The enzymes that synthesize these intermediates are designated as trans-prenyltransferases, and they consist of a family of structurally related proteins with representatives found in all branches of life (8).
In contrast, the isoprene units of some long-chain plant terpenoids such as rubber and dolichols (the latter group of compounds is present in bacteria, fungi, and animals as well) are linked to each other in the cis (Z) conformation (9, 10). They are synthesized from Z-prenyl diphosphates, which are in turn synthesized by cis-prenyltransferases directly from IPP and DMAPP (although in the case of rubber, the starting substrate may be E,E-FPP and IPP) (11). The cis-prenyltransferases are also structurally related to each other but they are not homologous to the trans-prenyltransferases and appear to use a different mechanism (12). Several cis-prenyltransferases have been characterized in bacteria and yeast (13–15). Bioinformatic analysis of plant sequences identified several proteins with sequence similarity to these cis-prenyltransferases (10, 11). The protein encoded by one Arabidopsis thaliana putative cis-prenyltransferase gene catalyzes the formation of C90 to C130 cis-prenyl diphosphates in vitro (10). It was hypothesized that this enzyme is involved in the biosynthesis of dolichols in vivo, but no direct in planta evidence was presented. The A. thaliana genome contains a total of nine genes with sequence similarity to cis-prenyltransferases, but clear functional assignment for any of them is lacking.
The glandular trichomes found on the surface of the leaves and stems of the cultivated tomato (Solanum lycopersicum) were previously shown to contain a mixture of volatile terpenoids consisting mostly of monoterpenes and sesquiterpenes (16). To date, however, only two monoterpene synthases, designated MTS1 and MTS2, have been characterized in tomato (17). The expression of MTS1 in trichomes was shown to be low under normal conditions but highly induced by the application of methyl jasmonate (12). MTS1 protein was shown to catalyze the formation of linalool from GPP in vitro (17), but consistent with its low level of expression under normal conditions, no linalool is observed in tomato trichomes under such conditions (16). MTS2, which is expressed in stems and roots but not in trichomes, encodes an enzyme that catalyzes the formation of β-phellandrene, β-myrcene, and sabinene from GPP (17).
It was recently reported that the major sesquiterpenes produced in Solanum habrochaites trichomes are synthesized from IPP and DMAPP via a cis-prenyltransferase that makes Z,Z-FPP. This, in turn, is a substrate for a novel sesquiterpene synthase with highest similarity to diterpene synthases in the TPSe subfamily (18). Here, we show that tomato trichomes express mRNA for a gene encoding a cis-prenyltransferase, which catalyzes the formation of NPP. NPP in turn is a substrate for a monoterpene synthase, whose gene is highly expressed in the trichome, to make several monoterpenes including 2-carene, α-phellandrene, α-terpinene, β-phellandrene, limonene, and γ-terpinene. Contrary to the prevailing view of monoterpene biosynthesis, our results indicate that GPP is not the only substrate used by terpene synthases to produce monoterpenes.
Results
Tomato Type VI Glandular Trichomes Make a Number of Monoterpenes, and Gene(s) on Chromosome 8 Are Involved.
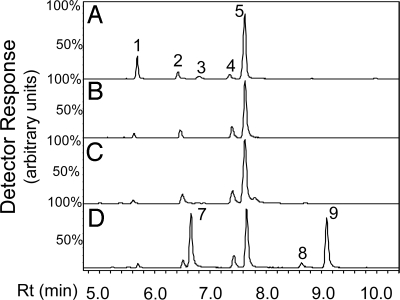
Previous reports indicated that trichomes on the surface of tomato leaves, stems, and green fruit produce monoterpenes and sesquiterpenes (16, 19–21). We analyzed terpene content by using gas chromatography–mass spectrometry (GC-MS) in leaf trichomes by briefly dipping detached leaflets in tert-butyl methyl ether (MTBE). S. lycopersicum cultivar M82 produces a mixture of several monoterpenes, with β-phellandrene being the main volatile (Fig. 1A, Table S1). Solanum pennellii LA0716 produces a different profile of monoterpenes, with α-phellandrene as the main volatile (Fig. 1A, Table S1). To determine the genetic basis for differences in monoterpene biosynthesis between M82 and LA0716, introgression lines (ILs) were screened for changes in terpene accumulation compared to the M82 recurrent parent (22). An IL that has S. pennellii DNA at the top of chromosome 8 (IL8-1-1) accumulated the same monoterpenes as LA0716 (Fig. 1A), whereas ILs from elsewhere in the genome consistently displayed the M82 monoterpene profile (e.g., IL1-4) (Fig. 1A).
Fig. 1.
Monoterpenes obtained from S. lycopersicum (M82) and S. pennellii (LA0716) plants and two isogenic chromosomal substitution lines and analyzed by GC-MS. (A) A leaf was dipped briefly in MTBE and the extract analyzed. (B) Type VI trichomes were collected by hand and placed into a vial containing MTBE. Numbered peaks are as follows: 1, δ-2-carene; 2, α-phellandrene; 3, α-terpinene; 4, limonene; 5, β-phellandrene; 6, γ-terpinene. No linalool was detected in any of the samples. Chromatograms show the detection of m/z = 93. Rt, retention time.
The terpene content of type VI glands from M82, LA0716, and IL8-1-1 as well as from other types of glandular trichomes (type I in M82, type IV in LA0716) was also profiled by using pulled Pasteur pipettes to selectively collect individual glands into MTBE followed by GC-MS analysis. Terpenes were observed only in type VI glands (Fig. 1B).
Type VI Glandular Trichomes of M82 also Make a Number of Sesquiterpenes.
GC-MS analysis of the terpenes found on the surface of the leaf and in isolated glands also showed small amounts of sesquiterpenes. β-Caryophyllene, α-humulene, and δ-elemene were identified in extracts from M82, IL8-1-1, and IL1-4 but not in LA0716 (Table S1). Based on previous work, δ-elemene is probably a decay product of germacrene C produced under elevated temperature of the GC inlet (23). Previous reports indicated that two sesquiterpene synthases genes on chromosome 6, designated SSTLE1 and SSTLE2, are responsible for the synthesis of sesquiterpenes in type VI glands (20, 21).
Identification of Putative Prenyltransferases and Monoterpene Synthases in Tomato Trichome Expressed Sequence Tag (EST) Databases.
To examine the profile of genes expressed in tomato trichomes, a cDNA library was made from isolated stem and petiole trichomes and sequenced by massively parallel pyrosequencing (24). Reads from the GS20 sequencer were assembled into contigs that were searched against various databases at the National Center for Biotechnology Information, by using the BLASTX algorithm. All contig sequence data and BLAST results are stored in a searchable database (http://bioinfo.bch.msu.edu/M82/). Using this database, a search was conducted to find candidate prenyltransferase and monoterpene synthase genes. No genes with significant similarity to GPP synthase (GDPS) subunits were found; therefore, the search was expanded to look for any prenyltransferase sequences. Nineteen sequences (0.0078% of library reads) were found that represent a putative tomato FPP synthase (FPS1; AF048747). Expression of FPS1 in trichomes is consistent with the presence of sesquiterpenes in the type VI glands of cultivated tomato (Table S1). No sequences with similarity to a GGPP synthase (GGDPS) were found.
A large collection of ESTs (1632 reads; 0.67% of the library) were found in a contig encoding a protein that we designated as NDPS1 (for neryl diphosphate synthase 1) (see below) (Figs. S1 and S2). The inferred protein is highly similar (95.4% amino acid sequence identity) to the recently reported Z,Z-FPP synthase from S. habrochaites, as well as to several verified and putative dehydrodolichyl diphosphate synthases (DDPSs) from A. thaliana (Fig. S1). To date, two DDPS genes have been investigated in A. thaliana. At2g23410 was shown to complement a Saccharomyces cereviseae (baker's yeast) rer2 DDPS structural gene mutation, indicating that this A. thaliana protein could catalyze the production of dolichols in yeast (10). At1g11755/lew1 was identified in a mutant screen for plants with a leaf-wilting phenotype under normal growth conditions (25). The lew1 mutation resulted in reduced levels of dolichols in A. thaliana. NDPS1 shows 41.7% and 16.0% amino acid identity with At2g23410 and At1g11755, respectively, and 32.4% and 40.7% identity to DDPS proteins from yeast and Escherichia coli (Fig. S2).
A BLAST search of the database identified several contigs that encode proteins with similarity to known TPSs. Fifty-one sequences (0.021% of total reads) matched to MTS1 (GenBank accession no. AY840091). Two other contigs with a total of 719 sequences (0.29% of total reads) were identical with the tomato sesquiterpene synthase SSTLE1/2 (GenBank accession no. AF279453) from chromosome 6 (20). Finally, a second abundant transcript (741 reads; 0.30%) represented a gene that we designated as PHS1 (for phellandrene synthase 1) (see below) (Fig. S3). This gene encodes a predicted protein with highest similarity to the S. habrochaites sesquiterpene synthase that uses Z,Z-FPP as a substrate (88.9%), to an uncharacterized tobacco terpene synthase (GenBank accession no. AY528645; 60.7%), and to ent-kaurene synthases from various plants (Figs. S4 and S5). All these sequences belong to the TPSe subfamily of terpene synthases (Fig. S5). Compared with most monoterpene synthases, which range from 600 to 650 aa in length, PHS1 and other related TPSs are significantly larger, with PHS1 having 778 aa. This is due to an insertion of ≈100 aa that is related to the ancestral internal element typically found in diterpene synthases (7).
Both NDPS1 and PHS1 Map to Chromosome 8.
To determine whether NDPS1 and PHS1 are involved in specifying the monoterpene profiles seen in M82 and LA0716, we determined the map position of each gene. Surprisingly, the nucleotide sequences of the coding region of the NDPS1 gene in M82 and LA0716 (amplified by PCR) were identical, precluding the use of polymorphisms within the gene for mapping. As an alternative, restriction fragment length polymorphism analysis was performed by using a probe derived from NDPS1 with digested DNA from M82, LA0716, as well as introgression lines IL1-4 and IL8-1-1. The restriction fragment pattern was consistent with NDPS1 being located on the top of chromosome 8 within the IL8-1-1 introgression (Fig. S6). For mapping PHS1, the 3′ end of the gene was amplified and sequenced. The IL8-1-1 sequence was identical with that of LA0716, but different from the sequenced amplified from M82 and other ILs (Fig. S7), indicating that this TPS gene is also located on the top of chromosome 8.
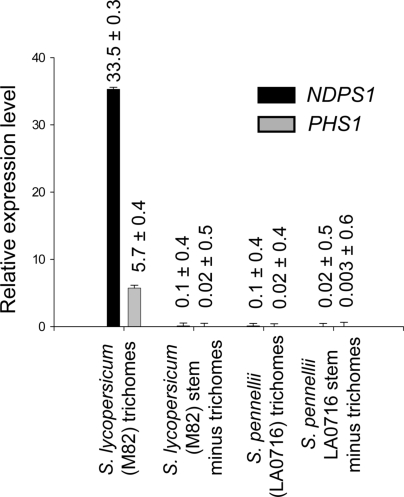
NDPS1 and PHS1 Are Highly Expressed in S. lycopersicum Trichomes.
Only 9 ESTs (0.003%) for NDPS1 and 13 ESTs (0.005%) for PHS1 were found out of the 249,138 ESTs in the TIGR tomato transcript assembly (release 5; http://plantta.jcvi.org/cgi-bin/plantta_release.pl), which does not include any trichome-specific cDNA libraries. The abundance of NDPS1 and PHS1 ESTs from our S. lycopersicum trichome database, combined with the very low frequency of these ESTs in databases of other tissues, suggests that NDPS1 and PHS1 expression is specific to trichomes. Quantitative RT-PCR measurements indicated that the expression of NDPS1 and PHS1 was 333- and 316-fold higher, respectively, in isolated M82 stem trichomes than in M82 stems from which trichomes have been removed (Fig. 2). In addition, although NDPS1 and PHS1 transcripts were present in LA0716 stem trichomes, the expression levels were 324- and 250-fold higher in M82 stem trichomes compared with LA0716 for NDPS1 and PHS1, respectively.
Fig. 2.
Quantitative RT-PCR analysis of NDPS1 and PHS1 gene expression in M82 and LA0716 trichomes. Expression of NDPS1 and PHS1 was measured by qRT-PCR and normalized to expression of EF-1α in total trichomes isolated from stem and petiole tissue and from stem and petiole tissue after removal of the trichomes.
NDPS1 Catalyzes the Formation of NPP from IPP and DMAPP.
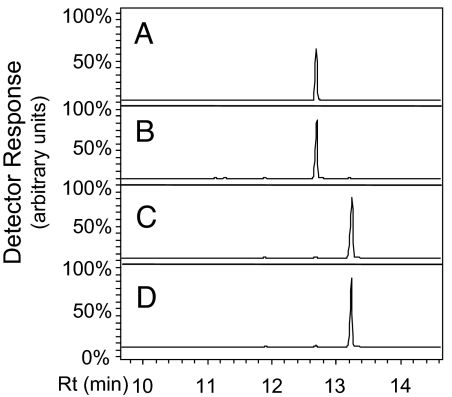
The predicted NDPS1 ORF encodes a protein of 303 aa. Comparison with the S. cereviseae and E. coli homologs (Fig. S2) show that tomato NDPS1, as well as some A. thaliana homologs (e.g., At2g23410, but not At1g11755) have an N-terminal extension sequence of ≈50 aa likely to serve as a transit peptide directing the proteins into the plastids, after which the transit peptide would be removed. Attempts to express the full-length ORF in E. coli resulted in insoluble protein. We therefore constructed an ORF of NDPS1 that began at Ser-45 (with an initiating Met codon in front) and a His-tag extension at the C terminus and expressed this protein in E. coli. The affinity-purified protein (Fig. S8) was assayed for activity with IPP and DMAPP. The product of the reaction was isolated, hydrolyzed with alkaline phosphatase or with HCl, and the resulting prenyl alcohols (prenols) were analyzed by GC-MS. After alkaline hydrolysis, only nerol was detected (Fig. 3 A and B), indicating that the product of the condensation of IPP and DMAPP catalyzed by NDPS1 was neryl diphosphate. As a control, commercially obtained GPP was hydrolyzed in similar conditions, and the product obtained was geraniol (Fig. 3 C and D). In addition, because it was previously reported that acid hydrolysis of GPP produces mostly linalool (26), but acid hydrolysis of neryl diphosphate results in linalool, α-terpineol, and nerol (27), we hydrolyzed the product of the reaction catalyzed by NDPS1 and observed linalool, α-terpineol, and nerol (Fig. S9).
Fig. 3.
In vitro assay for identification of NDPS1 product using GC-MS. (A) Purified NDPS1 was incubated with IPP and DMAPP for 30 min at 30 °C, after which alkaline phosphatase was added to the reaction and the solution incubated overnight and then analyzed. (B) Authentic nerol standard. (C) Authentic geraniol standard. (D) Commercial GPP was treated with alkaline phosphatase as in A. GC-MS chromatograms show the detection of m/z = 93.
Kinetic analysis of the purified NDPS1 showed a Km value for IPP of 152 μM, a Km value for DMAPP of 177 μM, and a turnover rate of 0.2 s−1 (Table 1).
Table 1.
Kinetic parameters of NDPS1 and PHS1
| Enzyme | Substrate | Km, μM | Kcat, s−1 | Kcat/Km, s−1 mM−1 |
|---|---|---|---|---|
| NDPS1 | IPP | 152 ± 68* | 0.2 ± 0.07 | 1.39 ± 0.2 |
| NDPS1 | DMAPP | 177 ± 42 | 0.2 ± 0.03 | 1.04 ± 0.06 |
| PHS1 | NPP | 9.1 ± 0.8 | 4.1 ± 0.2 | 451.5 ± 18.5 |
| PHS1 | GPP | 2,900 ± 300 | 9.9 × 10−10 ± 0.38 × 10−10 | 3.4 × 10−10 ± 0.26 × 10−10 |
*Standard error.
PHS1 Catalyzes the Formation of five Monoterpenes from NPP.
The complete ORF of PHS1 encodes a protein of 778 aa. It has significant similarity to several plant proteins including plastid-localized (28) kaurene synthase (Figs. S4 and S5). The comparison with kaurene synthase strongly suggests that the complete ORF of PHS1 also encodes a plastid transit peptide. We therefore constructed an ORF of PHS1 that began at Met-45 and included a His-tag extension at the N terminus, and expressed this protein in E. coli.
The affinity-purified protein (Fig. S8) was assayed with NPP and the reaction products analyzed by GC-MS. The profile of the monoterpenes produced in this reaction was nearly identical with the profile of the monoterpenes extracted from the surface of the leaves and specifically from type VI trichomes, with β-phellandrene as the main product (Figs. 1 and 4 A and B). The Km value of PHS1 for NPP was determined to be 9.1 μM, with a turnover rate of 4.1 s−1 (Table 1). A coupled reaction in which NDPS1, PHS1, and DMAPP and IPP were incubated together also gave the same pattern (Fig. 4C). In contrast, when incubated with GPP, PHS1 catalyzed the formation of the acyclic myrcene and ocimene as major products in addition to β-phellandrene (Fig. 4D), but the Km value with this substrate was considerably higher and the turnover rate extremely low (Table 1). As a control, MTS1 gave only linalool when incubated with GPP, confirming a previous report (17), and did not catalyze the formation of any monoterpenes when incubated with NPP (Fig. S10).
Fig. 4.
In vitro assays with purified recombinant PHS1 using different substrates. (A) S. lycopersicum (M82) monoterpene profile obtained by dipping a leaf in MTBE. Peaks labeled 1–5 are the same as in Fig. 1A. (B) GC analysis of the in vitro-coupled reaction catalyzed by NDPS1 and PHS1 and using IPP and DMAPP as substrates. (C) GC analysis of products of the reaction catalyzed by PHS1 with NPP as the substrate. (D) GC analysis of products of the reaction catalyzed by PHS1 with GPP as the substrate. Labeled peaks are as follows: 7, myrcene; 8 and 9, ocimene isomers. A small linalool peak (equivalent to the size of α-phellandrene peak (peak 3) in this chromatograph is also observed at retention time (Rt) 17.4 min (not shown here). GC-MS chromatograms show the detection of m/z = 93. Heights of peaks are not comparable between samples.
Discussion
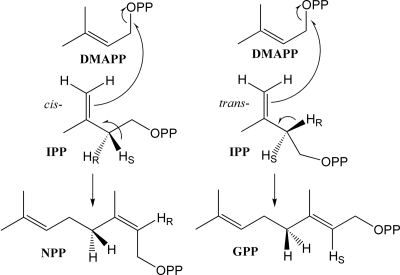
We show here that the trichomes of S. lycopersicum express a gene, NDPS1, which encodes an enzyme catalyzing NPP formation (Fig. 3). NDPS1 produces a prenyl diphosphate with a cis configuration, rather than a trans configuration as does GDPS (Fig. 5), possibly because it extracts a different proton from carbon 4 than GDPS does (12, 29). We also show that NPP can be used by the monoterpene synthase PHS1 in vitro to catalyze the formation of the same monoterpenes also found, and in similar ratios, in the glands of S. lycopersicum (Fig. 4 A–C), suggesting that these compounds are produced in the glands by the action of PHS1 on NPP. Although PHS1 can also use GPP as a substrate in vitro, there is apparently little GPP in the trichomes, PHS1 has a very poor affinity to this substrate (Table 1), and the mixture and ratio of the products resulting from the action of PHS1 on GPP are very different from those seen in planta (Fig. 4 A and D). In particular, the major product of PHS1 with NPP, β-phellandrene, as well as all other products are cyclic, whereas the major portion of the products with GPP are acyclic (i.e., myrcene, ocimene, and linalool).
Fig. 5.
A model showing how GDPS and NDPS could produce their respective products from IPP and DMAPP by extracting a different proton at C4 of the IPP molecule during the condensation reaction (based on ref. 29).
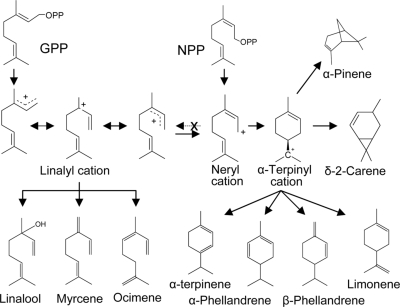
The differences in products formed by PHS1 with NPP and GPP can be explained by the reaction mechanism of terpene synthases (30). As illustrated in Fig. 6, NPP most likely ionizes to a neryl cation, which can further isomerize to an α-terpinyl cation. Further transformations of this cyclic intermediate (30) can eventually yield all observed products of PHS1. When GPP is used as the substrate (albeit a very poor one) of PHS1, the linalyl cation intermediate is formed first (Fig. 6). Although the linalyl cation can undergo trans-cis isomerization, leading to the neryl cation (30), it appears that substantial amounts of the acyclic terpenes myrcene, ocimene, and some linalool, are formed directly from the linalyl cation. Our results also suggest that with PHS1, the neryl cation can be converted only to the α-terpinyl cation but not to the linalyl cation, because no acyclic monoterpenes are obtained in vitro when NPP is used as the substrate, nor are they detected in planta.
Fig. 6.
Proposed mechanism for the synthesis of monoterpenes when PHS1 uses GPP or NPP as substrates. A minor peak of α-pinene is occasionally seen in the in vitro assay of PHS1 with NPP, and a correspondingly small portion of α-pinene is also occasionally seen in the profile of monoterpenes extracted from S. lycopersicum leaves.
Our results may help explain anomalous results into the function of monoterpene synthases from various species. For example, there are reports in which no enzymatic activity was detected when GPP was used as a substrate. In addition, there are cases in which monoterpene products not found in the species under investigation were detected when the enzyme was tested with GPP in vitro (31). The observation that PHS1 converts NPP to the expected monoterpene products and the prevalence of NDPS1-like sequences in plant genomes together suggest that some previously negative or anomalous in vitro results with putative monoterpene synthases could be explained if these enzymes use NPP rather than GPP as the in vivo substrate. Indeed, a report from 1976 (27) presented evidence that cell-free extracts of sage (Salvia officinalis) can convert NPP to several monoterpenes, but to our knowledge no specific enzymes that carry out this reaction have been identified.
S. pennellii LA0716 glands make a somewhat different set of monoterpenes (Fig. 1). We found that S. pennellii and S. lycopersicum NDPS are identical in sequence. We have not yet determined the complete sequence of S. pennellii PHS1, although the partial sequence obtained indicates that it encodes a protein that is not identical with S. lycopersicum. However, because neither NDPS1 nor PHS1 are highly expressed in glands of S. pennellii (Fig. 2), at present we do not know which enzyme is responsible for the production of monoterpenes in the glands of this species, nor whether it uses NPP or GPP.
Recently, Sallaud et al. (18) reported the sequences of the orthologs of S. lycopersicum NDPS1 and PHS1 in S. habrochaites. Both proteins were shown to be highly expressed in the glands and localized in the plastids. Interestingly, although NDPS1 and its S. habrochaites ortholog are 95% identical (Fig. S2), the S. habrochaites protein has a different activity, catalyzing the formation of Z,Z-FPP from IPP and DMAPP. Likewise, although S. lycopersicum PHS1 and the S. habrochaites orthologous protein are 89% identical (Fig. S4), they too have divergent enzymatic activities: the S. habrochaites protein catalyzes the formation of the sesquiterpenes bergamotene and santalene from Z,Z-FPP, rather than monoterpenes from NPP.
Our results, together with those of Sallaud et al. (18), underline the remarkable capacity of plant TPSs, which are believed to be monophyletic (1, 7), to evolve the ability to use different substrates and to synthesize new products. Our results also bring to the fore the evolution of new functions that occurs among prenyltransferases in plants, which belong to at least two distinct families. The identification of reactions in such well-studied specialized metabolic pathways indicates that there is still much to be learned about the diverse biosynthetic strategies of plants.
Materials and Methods
Plant Growth and Conditions.
Tomato seed, S. lycopersicum cv. M82 and S. pennellii LA0716, was obtained from the Tomato Genetic Resource Center (http://tgrc/ucdavis.edu). Seedlings were grown in Jiffy peat pots (Hummert International) in a controlled growth chamber maintained for 16 h in the light (300 μE/m2/s; mixed cool white and incandescent bulbs) at 28 °C and 8 h in the dark at 20 °C.
Terpene and Prenyl Alcohols Analyses.
For analysis of total trichome terpenes, leaflets from the second leaf after the newly emerging leaf of 3-week-old plants were dipped with gentle rocking for 1 min in 750 μL of MTBE containing 10 ng/μL of tetradecane internal standard. For type VI trichome analysis, ≈200 type VI glands from 3-week-old plants (M82, IL8-1-1, IL1-4) and greenhouse grown plants (LA0716) were picked by using a pulled Pasteur pipette into 100 μL of MTBE containing tetradecane internal standard. GC-MS analysis was performed as described in SI Materials and Methods.
cDNA Library Construction and Sequencing.
Isolation of RNA from glandular trichomes, construction of cDNA libraries, and sequence determination and assembly are described in SI Materials and Methods.
Gene Expression Analysis.
Steady-state levels of specific transcripts in tomato trichome and leaves were determined by the quantitative RT-PCR (qRT-PCR) method as described in SI Materials and Methods.
Expression of NDPS1 and PHS1 in E. coli and Purification of the Proteins.
The ORF of NDPS1 and PHS1 without the transit peptides were spliced into the expression vector pEXP5-CT/TOPO (Invitrogen), mobilized into E. coli, and protein produced and purified as described in SI Materials and Methods.
NDPS Enzyme Assay.
For product identification, the reaction was initiated by adding 2.5 μg of affinity-purified His-tagged enzyme (10 μL) in 50 mM Hepes, pH 8, 5% vol/vol glycerol, 5 mM DTT, 100 mM KCl, and 7.5 mM MgCl2, containing 40 μM IPP and 40 μM DMAPP in a final volume of 50 μL. Assays were incubated for 30 min at 30 °C. The reaction was stopped by heating and then treated by either adding 2 units of calf intestinal alkaline phosphatase (alkaline hydrolysis) (Fisher) and incubating overnight, or by adding 5 μL of 2 M HCl (acid hydrolysis). After hydrolysis (either acid or alkaline), glass vials containing the reaction were placed at 42 °C, the gas phase was extracted with a solid-phase microextraction (SPME) fiber (polymethylsiloxane; 100 μm; Supelco) for 15 min and was then injected in GC-MS. Blanks with assay buffer without enzyme and substrates, but with commercial standards, were performed to confirm identity of peaks.
For kinetic studies, a similar protocol was followed, but 1.7 μg of protein were used and [1-14C]IPP (50 μCi/μmol, initial concentration 0.1 mCi/mL and mixed in appropriate amounts with cold IPP) was used as substrate together with DMAPP. The Km value for IPP was determined by using 200 μM DMAPP, whereas the Km value for DMAPP was determined with 200 μM IPP. Reactions were stopped by adding 5 μL of 2 M HCl. Prenols were extracted with 200 μL of ethyl acetate and the 14C counts in the organic phase was analyzed in a scintillation counter (LS6500 Beckman). Negative controls with boiled enzyme revealed no nonenzymatic IPP hydrolysis. IPP and DMAPP were obtained from Echelon Biosciences. [1-14C]IPP was obtained from American Radiolabeled Chemicals.
PHS Enzyme Assay.
For product identification, 2 μg of affinity-purified His-tagged enzyme (10 μL) were incubated in 50 mM Hepes, pH 8, 5% vol/vol glycerol, 5 mM DTT, 100 mM KCl, and 7.5 mM MgCl2, containing 3.5 μM NPP or 10 μM GPP in a final volume of 50 μL. Assays were incubated for 30 min at 30 °C, after which glass vials containing the reaction were placed at 42 °C, the gas phase was extracted with a SPME fiber for 15 min and was then injected in GC-MS. For coupled assay with NDPS1, the assay was first performed as previously described above for NDPS, but products were not hydrolyzed. PHS1 was added after 30 min of incubation in the reaction mixture and was incubated for 30 min longer at the same temperature. Monoterpenes were extracted by using SPME as previously described and analyzed by using GC-MS. Commercial standards were spiked in assay buffer without enzyme or substrates, extracted with SPME, and analyzed by using GC-MS.
For kinetic studies, similar conditions were followed but using 0.5 μg of purified enzyme and NPP or GPP as a substrate. Products were quantified by using SPME. A calibration curve was performed by using the same SPME fiber, to evaluate the linearity range for 2-carene and for tetradecane (used as an internal standard). NPP was a gift from Charles Waechter and Jeffrey Rush, and GPP was purchased from Echelon Biosciences.
Supplementary Material
Acknowledgments.
We thank Drs. Charles Waechter (University of Kentucky, Lexington) and Jeffrey Rush (University of Kentucky, Lexington) for providing neryl diphosphate. Seeds of the S. pennellii introgression lines were generously provided by Dr. Dani Zamir (Hebrew University, Rehovot, Israel). This work was supported by National Science Foundation Grant DBI-0604336.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. SlNDPS1, FJ797956, SlPHS1, and FJ797957).
See Commentary on page 10402.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904113106/DCSupplemental.
References
- 1.Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9:297–304. doi: 10.1016/j.pbi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Croteau R, Felton M, Ronald RC. Biosynthesis of monoterpenes: Preliminary characterization of i-endo-fenchol synthetase from fennel (Foeniculum vulgare) and evidence that no free intermediate is involved in the cyclization of geranyl pyrophosphate to the rearranged product. Arch Biochem Biophys. 1980;200:534. doi: 10.1016/0003-9861(80)90385-9. [DOI] [PubMed] [Google Scholar]
- 3.Croteau R, Karp F. Biosynthesis of monoterpenes: Preliminary characterization of bornyl pyrophosphate synthetase from sage (Salvia officinalis) and demonstration that geranyl pyrophosphate is the preferred substrate for cyclization. Arch Biochem Biophys. 1979;198:512–522. doi: 10.1016/0003-9861(79)90526-5. [DOI] [PubMed] [Google Scholar]
- 4.Croteau R, Felton M. Conversion of [1-3H2,G-14C]geranyl pyrophosphate to cyclic monoterpenes without loss of tritium. Arch Biochem Biophys. 1981;207:460–464. doi: 10.1016/0003-9861(81)90054-0. [DOI] [PubMed] [Google Scholar]
- 5.Colby SM, Alonso WR, Katahira EJ, McGarvey DJ, Croteau R. 4S-limonene synthase from the oil glands of spearmint (Mentha spicata). cDNA isolation, characterization, and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem. 1993;268:23016–23024. [PubMed] [Google Scholar]
- 6.Bohlmann J, Steele CL, Croteau R. Monoterpene synthases from grand fir (Abies grandis). cDNA isolation, characterization, and functional expression of myrcene synthase, (−)-(4S)-limonene synthase, and (−)-(1S,5S)-pinene synthase. J Biol Chem. 1997;272:21784–21792. doi: 10.1074/jbc.272.35.21784. [DOI] [PubMed] [Google Scholar]
- 7.Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: Molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang KC, Ohnuma S. Isoprenyl diphosphate synthases. Biochim Biophys Acta. 2000;1529:33–48. doi: 10.1016/s1388-1981(00)00136-0. [DOI] [PubMed] [Google Scholar]
- 9.Asawatreratanakul K, et al. Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis. Eur J Biochem. 2003;270:4671–4680. doi: 10.1046/j.1432-1033.2003.03863.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunillera N, Arró M, Forés O, Manzano D, Ferrer A. Characterization of dehydrodolichyl diphosphate synthase of Arabidopsis thaliana, a key enzyme in dolichol biosynthesis. FEBS Lett. 2000;477:170–174. doi: 10.1016/s0014-5793(00)01798-1. [DOI] [PubMed] [Google Scholar]
- 11.Oh SK, Han KH, Ryu SB, Kang H. Molecular cloning, expression, and functional analysis of a cis-prenyltransferase from Arabidopsis thaliana Implication in rubber biosynthesis. J Biol Chem. 2000;275:18482–18488. doi: 10.1074/jbc.M002000200. [DOI] [PubMed] [Google Scholar]
- 12.Kharel Y, Koyama T. Molecular analysis of cis-prenyl chain elongating enzymes. Nat Prod Rep. 2003;20:111–118. doi: 10.1039/b108934j. [DOI] [PubMed] [Google Scholar]
- 13.Apfel CM, Takacs B, Fountoulakis M, Stieger M, Keck W. Use of genomics to identify bacterial undecaprenyl pyrophosphate synthetase: Cloning, expression, and characterization of the essential uppS gene. J Bacteriol. 1999;181:483–492. doi: 10.1128/jb.181.2.483-492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, et al. The yeast RER2 gene, identified by endoplasmic reticulum protein localization mutations, encodes cis-prenyltransferase, a key enzyme in dolichol synthesis. Mol Cell Biol. 1999;19:471–483. doi: 10.1128/mcb.19.1.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurokawa T, Ogura K, Seto S. Formation of polyprenyl phosphates by a cell-free enzyme of Micrococcus lysodeikticus. Biochem Biophys Res Comm. 1971;45:251–257. doi: 10.1016/0006-291x(71)90077-5. [DOI] [PubMed] [Google Scholar]
- 16.Li L, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Schie C, Haring M, Schuurink R. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol. 2007;64:251–263. doi: 10.1007/s11103-007-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallaud C, et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21:301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fridman E, et al. Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant Cell. 2005;17:1252–1267. doi: 10.1105/tpc.104.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hoeven RS, Monforte AJ, Breeden D, Tanksley SD, Steffens JC. Genetic control and evolution of sesquiterpene biosynthesis in Lycopersicon esculentum and L. hirsutum. Plant Cell. 2000;12:2283–2294. doi: 10.1105/tpc.12.11.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besser K, et al. Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol. 2008;149:499–514. doi: 10.1104/pp.108.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshed Y, Zamir D. A genomic library of Lycopersicon pennellii in L. esculentum: A tool for fine mapping of genes. Euphytica. 1994;79:175–179. [Google Scholar]
- 23.Colby SM, Crock J, Dowdle-Rizzo B, Lemaux PG, Croteau R. Germacrene C synthase from Lycopersicon esculentum cv. VFNT cherry tomato: cDNA isolation, characterization, and bacterial expression of the multiple product sesquiterpene cyclase. Proc Natl Acad Sci USA. 1998;95:2216–2221. doi: 10.1073/pnas.95.5.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margulies M, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, et al. Dolichol biosynthesis and its effects on the unfolded protein response and abiotic stress resistance in Arabidopsis. Plant Cell. 2008;20:1879–1898. doi: 10.1105/tpc.108.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tholl D, Croteau R, Gershenzon J. Partial purification and characterization of the short-chain prenyltransferases, geranyl diphosphate synthase and farnesyl diphosphate synthase, from Abies grandis (grand fir) Arch Biochem Biophys. 2001;386:233–242. doi: 10.1006/abbi.2000.2212. [DOI] [PubMed] [Google Scholar]
- 27.Croteau R, Karp F. Biosynthesis of monoterpenes—enzymatic conversion of neryl pyrophosphate to 1,8-cineole, alpha-terpineol, and cyclic monoterpene hydrocarbons by a cell-free preparation from sage (Salvia officinalis) Arch Biochem Biophys. 1976;176:734–746. doi: 10.1016/0003-9861(76)90217-4. [DOI] [PubMed] [Google Scholar]
- 28.Sun T-p, Kamiya Y. Regulation and cellular localization of ent-kaurene synthesis. Physiol Plant. 1997;101:701–708. [Google Scholar]
- 29.Ogura K, Koyama T. Enzymatic aspects of isoprenoid chain elongation. Chem Rev. 1998;98:1263–1276. doi: 10.1021/cr9600464. [DOI] [PubMed] [Google Scholar]
- 30.Croteau R. Biosynthesis and catabolism of monoterpenoids. Chem Rev. 1987;87:929–954. [Google Scholar]
- 31.Jia JW, Crock J, Lu S, Croteau R, Chen XY. (3R)-Linalool synthase from Artemisia annua L.: cDNA isolation, characterization, and wound induction. Arch Biochem Biophys. 1999;372:143–149. doi: 10.1006/abbi.1999.1466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.