Abstract
Improving the potency, breadth, and durability of neutralizing antibody responses to HIV are major challenges for HIV vaccine development. To address these challenges, the studies described evaluate in rabbits the titers, breadth, and epitope specificities of antibody responses elicited by HIV envelope subunit vaccines adjuvanted with MF59 with or without CpG oligodeoxynucleotide (ODN). Animals were immunized with trimeric o-gp140ΔV2 derived from subtype B HIV-1SF162 or subtype C HIV-1TV1, or proteins from both strains. Immunization with SF162 or TV1 with MF59/CpG elicited higher titers of binding and neutralizing antibodies to SF162 than monovalent immunization with MF59 alone (P<0.01). Bivalent immunization increased binding and neutralizing antibody titers over single envelope immunization in MF59 (P<0.01). Bivalent immunization also improved neutralization breadth. Epitope mapping indicated neutralizing activity in rabbits was directed to V3 and V4. Overall, our data suggests that a multivalent vaccination approach with MF59 and CpG can enhance humoral responses to HIV-1.
Keywords: HIV-1, neutralization, adjuvants, MF59, CpG, multivalent
Introduction
Subunit vaccines consisting of highly purified viral proteins have become increasingly prominent, but are often not adequately immunogenic. An attractive approach currently receiving increased attention is to improve the immunogenicity of subunit vaccines by formulation with immune stimulatory adjuvants. These adjuvant formulations serve to accelerate, prolong and/or enhance the quality and magnitude of immune responses to co-administered antigen preparations. Adjuvants have been classified as either immune potentiators or delivery systems (Pashine et al., 2005) and some appear to serve both roles. Immune potentiators were defined as compounds able to trigger innate immune responses and generate robust and long-lasting adaptive responses. Use of a delivery system might then serve to target the antigen and immune potentiator to the desired cells for optimal stimulation. Since adjuvants can be defined by and work through differing mechanisms of action, the key is to select an adjuvant(s) based on the route of immunization as well as the type of response preferred, such as Th1- or Th2-type actions. The desired effect can be accomplished via a variety of mechanisms. Some adjuvants are able to associate with and facilitate transport and delivery of antigen to an antigen presenting cell (APC). Others are able to influence antigen processing and presentation by APCs, and therefore MHC specificity. Adjuvants can also increase the half life of antigens in vivo leading to a prolonged immune response. Therefore, an ideal vaccine would consist of an optimized formulation of antigen, immune potentiator, and delivery system. Specifically, the present study focuses on the capability of adjuvant combinations to enhance the immunogenicity of HIV-1 envelope subunit vaccines, with respect to the generation of potent and broad humoral responses.
Previous studies indicated that adjuvant formulations can dramatically influence the types of antibodies that are generated in response to HIV and other antigens. In one study, administration of an HIV-1 gp120 envelope (Env) protein formulated with alum elicited a Th2 response and IgG1 antibodies (Javaherian et al., 1990). However, the same vaccine antigen with the immune potentiator IL-12 induced a Th1 response and IgG2 and IgG3 antibody isotypes. The inclusion of immune potentiators has the capability to increase not only serum antibody levels, but also antibodies with improved functional attributes. For example, serum anti-Env avidity was increased when using granulocyte-macrophage-colony stimulating factor (GM-SCF) as an immune potentiator (Lai et al., 2007). GM-CSF was also found to be able to influence the neutralizing breadth of antibodies (Robinson et al., 2006). In this study, only serum from animals that included GM-CSF as part of the formulation were able to neutralize the isolate 89.6P.
Synthetic oligodeoxynucleotides (ODN) containing unmethylated deoxycytosine-deoxyguanosine (CpG) motifs, or CpG ODNs, are TLR9 agonists that have also been shown to be effective in enhancing antibody potency as immune potentiators. In the case of the highly efficacious hepatitis B surface antigen (HBsAg) vaccine, a CpG plus alum combination was able to increase antibody avidity to HBsAg and also increased the proportion of high avidity antibodies (Siegrist et al., 2004). CpG is of particular interest as it can directly activate not only dendritic cells (DC), but B cells as well. In particular, CpG preferentially acts on B cell populations that predominantly express TLR9, those being activated and memory B cells. CpG enacts the innate immune response via enhancing the activation and presentation function of DCs. This makes CpG an especially desirable component of a vaccine given that strong stimulation of the innate immune response appears important for the evolution and generation of robust adaptive immune responses. This response then could be maximized by colocalization and efficient delivery of CpG and antigen to DCs by using the oil-emulsion MF59, which has been shown to be internalized by DCs and macrophages into endosomal vesicles and to cause secretion of cytokines known to recruit immune cells from the blood into tissue as well as increase endocytic uptake (Dupuis et al., 1998; Dupuis et al., 2001; Seubert et al., 2008). The combination of CpG and MF59 with antigen could therefore enhance endosomal uptake and facilitate the interaction of CpG with TLR9 in the endocytic vesicles and enhance antigen presentation by DCs. Enhanced DC activation and maturation may lead to enhanced antibody responses and increase the size of the memory B cell pool. The current study seeks to determine whether the formulation of MF59 and the immune potentiator CpG 7909, administered with well-characterized HIV-1 trimeric (o-)gp140 V2 envelope proteins would serve to enhance humoral responses in rabbits over antigen formulated using the potent MF59 alone.
Beyond the issues related to elicitation of potent and durable immunity against HIV by virtue of using optimal adjuvant formulations, a major obstacle to achieving a viable HIV vaccine is the enormous degree of sequence diversity encountered in circulating virus strains. Moreover, known conserved conformational epitopes that could prove to be useful targets for inclusion in a vaccine are cryptic, discontinuous, and relatively non-immunogenic. Thus, one promising approach to overcome these obstacles presented by HIV diversity and cryptic epitope recognition might be to utilize a vaccine consisting of more than one envelope sequence. In fact, a bivalent approach has already been investigated in humans for immunogenicity (Kim et al., 2003; Thongcharoen et al., 2007). Such a vaccine, when used in combination with a potent adjuvant formulation could induce broader and stronger antibody responses than those which can be generated through the use of a single antigen in a weaker adjuvant. Multivalent approaches including from 2 to over 50 separate antigens have been investigated with some demonstrated increases in neutralization breadth over monovalent immunization in direct comparisons (Chakrabarti et al., 2005; Cho et al., 2001; Ljungberg et al., 2002; Seaman et al., 2005; Seaman et al., 2007; Wang et al., 2006). These, along with additional multivalent studies, however, have shown mainly increases in neutralizing breadth to tier 1 viruses with somewhat limited success against more relevant tier 2 isolates (Azizi et al., 2008; Pal et al., 2005; Pal et al., 2006; Wang et al., 2008; Zhan et al., 2005). Only a subset of these studies have investigated the differences in antibody epitopes between the polyvalent and monovalent approaches (Chakrabarti et al., 2005; Rollman et al., 2004). We first described the immunogenicity and protective efficacy of the SF162 o-gp140ΔV2 glycoprotein in monkeys (Barnett et al., 2001; Cherpelis et al., 2001) and recently described the purification and characterization of the clade C TV1 o-gp140ΔV2 Env (Srivastava et al., 2008). To further assess the potential for boosting antibody potency and breadth, as well as the epitopes involved, we also investigated the effect of bivalent Env vaccines consisting of the trimeric proteins SF162 o-gp140ΔV2 and TV1 o-gp140ΔV2 and compared these to approaches containing a single envelope immunogen.
Results
Immunization of rabbits with trimeric o-gp140ΔV2 proteins derived from subtype B and C HIV-1 strains in MF59 with or without CpG ODN elicits high titer antigen-specific antibody responses
Groups of rabbits were immunized with trimeric SF162 o-gp140ΔV2 (subtype B) and TV1 o-gp140ΔV2 (subtype C) Env proteins either separately or together in MF59 with or without the addition of CpG ODN as described in Methods. Serum envelope titers were measured throughout the course of immunization. All immunization formulations, including those comprised of SF162 Env as well as those that contained TV1 Env, were able to elicit high titers of SF162 Env-specific antibodies as determined by ELISA using the SF162 o-gp140ΔV2 protein (Fig. 1A, top panel). Serum titers to SF162 Env were not significantly different following a third or fourth protein administration (P>0.05). As a group, rabbits immunized with the homologous SF162 Env in MF59 with CpG (SF162/MF59/CpG) elicited the highest titers of binding antibodies. This vaccine, elicited significantly higher titers than the vaccine formulated without CpG, SF162/MF59 (P=0.0015, p4). Interestingly, immunization with the subtype C TV1 Env also resulted in the generation of SF162-specific antibody responses, albeit at lower titers than those generated by the homologous antigen (Fig. 1A, top panel). As found for SF162 immunization, the group immunized with TV1 Env in MF59/CpG (TV1/MF59/CpG) also showed increased SF162 titers over those rabbits immunized with TV1 plus MF59 (TV1/MF59) (P=0.0019, p4). The combination of SF162 and TV1 Env antigens also elicited high titers of SF162-specific antibodies. For the bivalent groups, the inclusion of CpG did not significantly improve the antibody response over using MF59 alone (P=0.47, p4). However, bivalent immunization did elicit higher titers than the TV1 monovalent groups (P=0.0098 group 3 vs. 5 and group 4 vs. 6, p4).
Fig. 1.
Vaccine induced envelope-specific antibody responses in rabbit sera. Rabbits were immunized with SF162 o-gp140ΔV2, TV1 o-gp140ΔV2, or a combination of both envelopes in MF59 in the presence or absence of CpG. Two weeks following the third (p3) and fourth (p4) immunizations, individual sera were analyzed via ELISA and the group geomean values calculated. (A) Titers of serum antibodies to SF162 gp140ΔV2 (top panel) and TV1 gp140ΔV2 (bottom panel). (B) Ratio of serum antibodies to native versus denatured SF162 gp140ΔV2. (C) Avidity of SF162 gp140ΔV2-specific antibodies.
TV1 Env-specific antibodies were also elicited by all immunization regimens (Fig. 1A, bottom panel). Just as the SF162/MF59/CpG group elicited the highest SF162 titers, the homologous TV1/MF59/CpG group elicited the highest titers of TV1 o-gp140ΔV2 antibodies. TV1/MF59/CpG serum titers were significantly enhanced as compared to the TV1/MF59 group (P=0.0013, p4). Similar to that found above, the addition of CpG to the SF162 formulation increased titers to the TV1 Env as compared to SF162/MF59 (P<0.0001, p4). However, CpG did not significantly enhance TV1 binding titers in the bivalent Env regimen (P=0.12, p4). Bivalent immunization showed improved TV1-specific titers as compared to immunizing with SF162 Env alone (P=0.0011 group 1 vs. 5, P=0.0004 group 2 vs. 6, p4) but did not enhance serum titers over the TV1 monovalent approach (P=0.32 group 3 vs. 5, P=0.22 group 4 vs. 6, p4).
To evaluate the conformation dependency of antibody binding, we compared the total serum antibody titers to those measured against denatured and reduced SF162 Env. The ratios of total antibody titers to linear antibody responses were calculated and are shown in Fig. 1B. Following a fourth immunization, the ratio of total/linear antibodies decreases for all groups, except for rabbits receiving the SF162/MF59/CpG vaccine. This indicates that for the other five vaccines, a fourth administration leads to an increase in antibodies that recognized linear epitopes. Interestingly, the CpG-formulated vaccines also elicited higher levels of these linear epitope antibodies. Whether the reason for this is due to a direct effect of CpG binding to the antigen or an effect upon immune recognition of the antigen or some other mechanism of action remains to be determined.
To obtain an estimate of antibody maturation, the avidity of SF162 Env-specific serum antibodies was measured using an ammonium thiocyanate ELISA (Fig. 1C). Antibody avidity was similar following three or four immunizations (P>0.05 all groups), although some increases were observed for the monovalent regimens between these two time points, while antibody avidity decreased using bivalent protein administration. Four immunizations with SF162/MF59/CpG elicited serum antibodies with the highest avidity. Formulation with CpG increased avidity when SF162 was used as the antigen (P=0.014) but not when TV1 (P=0.79) or the combination of Envs (P=0.72) was employed. Although immunizing with TV1 yielded SF162-specific antibodies with moderate avidity, this was improved by including SF162 in the vaccine (P=0.022 group 3 vs. 5, P=0.015 group 4 vs. 6). However, the bivalent approach yielded antibodies that were not distinguishable in avidity to those elicited by SF162 (P=0.60 group 1 vs. 5, P=0.13 group 2 vs. 6).
Immunization of rabbits with trimeric o-gp140V2 proteins in MF59 with CpG ODN results in enhanced neutralizing activity against SF162
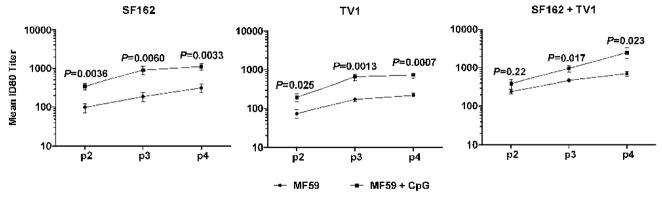
Sera from each immunization group was tested for the capacity to neutralize the SF162 pseudovirus in the TZM-bl assay (Li et al., 2005; Montefiori, 2004). Each immunization group elicited high levels of neutralizing antibodies to SF162 (Fig. 2). Substantial increases in the geomean 80% neutralization titers (ID80 titers) were observed between the second and third protein administrations that were statistically significant for all groups (p<0.05) except SF162/MF59 (p=0.14). An additional fourth immunization further increased neutralizing titers, albeit at levels that were only significantly higher for the SF162/TV1/MF59 group (p=0.048). Following the fourth immunization, the bivalent regimen using MF59 and CpG elicited the highest SF162 neutralization titers.
Fig. 2.
Neutralization of SF162 by immunized rabbit sera. Two weeks following the second (p2), third (p3), and fourth (p4) immunizations, sera were assayed in TZM-bl cells for neutralizing activity against SF162. Individual sera were assayed and the geometric mean 80% (ID80) titers against SF162 are shown for sera immunized with SF162 (left panel), TV1 (center panel), or both proteins (right panel). P values for the differences between groups immunized in MF59 (●) or MF59 + CpG (■) are listed for each time point.
The inclusion of CpG led to increased neutralizing antibody activity for all three immunization regimens. In rabbits where SF162 Env was used as the lone protein antigen, the addition of CpG increased the ID80 titers from 3.3–4.4-fold as compared to using MF59 alone (Fig. 2, left panel). This increase was statistically significant at each time point tested, with the largest increase observed when comparing the p2 sera. Formulating with CpG also led to higher SF162 neutralization titers when the heterologous TV1 Env was used as antigen. These increases were also found to be significant at each time point examined (Fig. 2, middle panel). In contrast, the inclusion of CpG did not significantly increase neutralization titers when the bivalent regimen was used following two administrations (P=0.22) (Fig. 2, right panel). However, following the third and fourth immunizations, the addition of CpG did increase the SF162 ID80 titers significantly (P=0.017 and P<0.023, respectively) over MF59 alone.
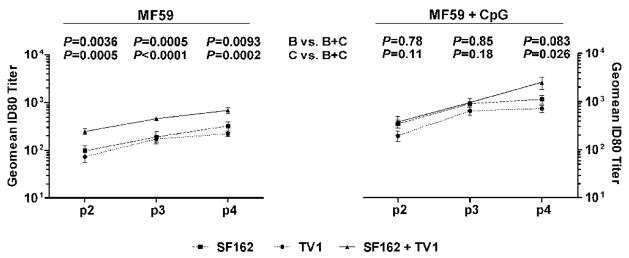
Immunization with a combination of subtype B and C envelope in MF59 elicits high levels of SF162 neutralizing titers
The combination of SF162 and TV1 Envs in MF59 resulted in higher titers of SF162 neutralization than when either single Env was used with MF59, despite the fact that in the bivalent regimen only half the amount of each protein (12.5 μg) was administered as compared to the monovalent cases (25 μg). This increase in ID80 SF162 titers was observed as early as p2 protein boost (Fig. 3, left panel). For each time point examined, the bivalent approach yielded statistically significant higher ID80 levels than using either SF162 or TV1 Env alone in MF59. Interestingly, this effect was not observed when the immune potentiator CpG was included (Fig. 3, right panel). SF162 ID80 titers, in this case, were not significantly improved until following the fourth protein administration and then only compared to the monovalent SF162/MF59/CpG regimen.
Fig. 3.
Bivalent immunization increases SF162 neutralization titers. Geometric mean ID80 neutralization titers against SF162 are shown for the indicated time points from immunizations with MF59 (left panel) or MF59 + CpG (right panel) with SF162 (■), TV1 (●), or both proteins (▲). P values for the differences between monovalent and bivalent immunization are listed above the graphs. (B) SF162, (C) TV1.
Neutralizing activity of SF162-specific antibodies elicited by envelope immunization maps primarily to V3 and V4 regions
Since sera from each group of immunized rabbits contained high levels of antibodies to SF162 Env, we next wanted to determine the contribution due to variable loop-specific antibodies. Serum from each immunization group was analyzed for V4 and V3 binding antibodies via peptide ELISA. Rabbits from all immunization groups exhibited recognition of a cyclic V4 peptide (Table 1). Each antigen and adjuvant combination elicited similar, albeit relatively low, V4 titers. In a similar manner, serum samples were analyzed against two different V3 peptides. The first peptide, termed V3 tip (V3t), consisted of a short region of the SF162 V3 loop that included the GPGR motif with flanking sequences (see materials and methods). The second peptide (V3c) encompassed the entire V3 sequence and was cyclized via cysteine residues at each end. Recognition of the V3 tip peptide was observed for all immunized groups (Table 1). Large increases in V3 antibodies were not observed between the third and fourth administrations, therefore focus was placed on the post fourth time point. In general, the inclusion of CpG had little effect on the generation of V3 antibodies. The increase in V3 antibodies for CpG-containing groups over non-CpG groups correlated with the increase in overall titers observed for these groups. Interestingly, TV1/MF59 groups contained a similar titer of V3 tip antibodies as the homologous SF162/MF59 sera. In addition, bivalent immunization generated a higher titer of V3 tip antibodies than either protein alone in MF59. As for V3 cyclic recognition, the bivalent approach with CpG elicited higher titers than the SF162/MF59/CpG or TV1/MF59/CpG regimens.
Table 1.
Peptide mapping of rabbit sera following four immunizations (p4).
| Serum Titers (log)a | Inhibition of Neutralization (%)b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immunization Group | V3t | V3c | V4 | V1t | V1c | V3t | V3c | V4 | V5 | V3s | 4E10 | b12 |
| SF162 (MF59) | 3.7 | 4.4 | 2.8 | 2 | −1 | 20 | 46 | 28 | 4 | 0 | −6 | 0 |
| SF162 (MF59+CpG) | 4.2 | 4.7 | 2.8 | 11 | 11 | 35 | 53 | 37 | 6 | 0 | −8 | 4 |
| TV1 (MF59) | 3.9 | 4.5 | 2.7 | 4 | 0 | 73 | 76 | 24 | 10 | 1 | −3 | 2 |
| TV1 (MF59+CpG) | 4.1 | 4.7 | 2.9 | −1 | 1 | 76 | 95 | 24 | 10 | 2 | −8 | 3 |
| SF162+TV1 (MF59) | 4.1 | 4.6 | 2.7 | 1 | −1 | 68 | 74 | 38 | 26 | 0 | −2 | 3 |
| SF162+TV1 (MF59+CpG) | 4.2 | 4.9 | 3.0 | −1 | 2 | 35 | 44 | 16 | 8 | −2 | −6 | 1 |
Peptide-specific titers of serum antibodies were measured by ELISA.
Values indicate the percentage of HIV-1 neutralization that is inhibited by the presence of the indicated peptide at a serum dilution that corresponds to the ID70. Data are from a representative experiment performed in duplicate. (t) tip, (c) cyclic, (s) scramble.
To determine the contribution of antibody specificity to the underlying differences between the immunization groups in SF162 neutralizing capacity, epitope mapping was carried out. Using a modification of the TZM-bl assay, pooled group sera (p4) were incubated with peptides corresponding to specific regions of the SF162 Env and/or epitopes of the monoclonal antibodies 4E10 and IgGb12 (Table 1). Both SF162 and TV1 antigens were capable of generating SF162 V3-specific neutralizing responses. High levels of neutralization inhibition were observed using both the V3t and V3c peptides. Interestingly, neutralization by sera from the TV1/MF59/CpG immunized rabbits could be almost entirely blocked using the V3c peptide. This indicates that the TV1 Env was capable of eliciting antibodies that efficiently recognized the V3 region of SF162. Outside of the GPGR/Q motif, the V3 loops of these two Envs differ by six amino acids, with only one considered a non-conservative substitution (see sequences in Methods). Whether this increase is due to the conformational nature of the V3 sequence or simply the inclusion of additional epitopes in the larger peptide remains unclear. However, in the case of SF162 neutralization, this does not seem to be a unique trait as we also observed increases when using the V3c peptide against SF162 immunized rabbit sera. Each group also contained neutralizing antibodies that recognized the regions corresponding to V4. Some groups also contained low levels of V5 neutralizing antibodies. Only the SF162/MF59/CpG immunized group exhibited inhibition by V1 peptides above background levels. When comparing groups immunized with the same Env component(s), the inclusion of CpG resulted in some shifting of the neutralizing epitopes. For example, when SF162 Env was the immunogen, the CpG-containing group recognized V1 as compared to the SF162/MF59 group. For the monovalent TV1 Env groups, the immune potentiator CpG 7909 resulted in neutralizing activity that was increased in V3 cyclic recognition, but not V3 tip, as compared to the TV1/MF59 group. Inclusion of CpG in the bivalent regimen, however, led to a decrease in V3, V4, and V5 neutralizing antibodies. No immunization regimens elicited a significant amount of neutralizing antibodies with specificity for the 4E10 or b12 epitopes (Table 1). All groups were also negative for neutralizing activity that was blocked by the control V3 scramble peptide.
Inclusion of subtype C envelope leads to an increase in neutralization breadth over immunization with subtype B alone
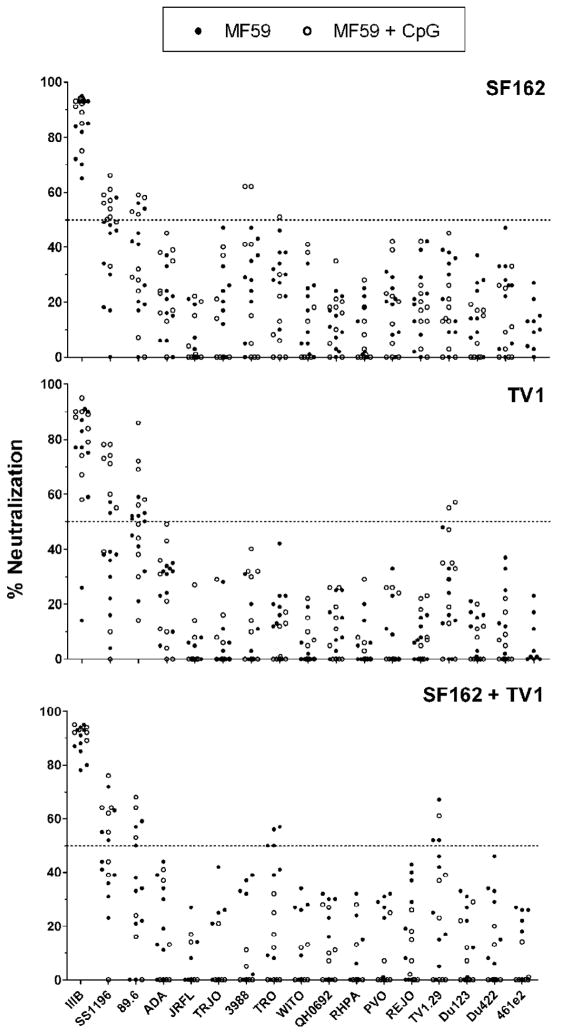
We next assessed the capacity of these sera to neutralize a panel of heterologous viruses. A variety of viruses was selected, including viruses from a range of sensitive and resistant phenotypes, as well as early isolates from the clade B panel (Li et al., 2005). Interestingly, the peak of heterologous neutralization occurred following the third immunization with neutralization breadth appreciably reduced following the fourth injection (data not shown). Therefore, focus was on the p3 time point and the percent virus neutralization for these serum samples is shown (Fig. 4).
Fig. 4.
Neutralization breadth. Values are the percentage of neutralization of viruses by sera from rabbits immunized with SF162 (top panel), TV1 (center panel), or both proteins (bottom panel) in MF59 (●) or MF59 + CpG (○). Neutralization was carried out at a serum dilution of 1:15. Numbers are the average of two experiments performed in triplicate.
Overall, no one particular immunization regimen was found to be vastly superior to another for eliciting a broadly neutralizing antibody response. Against the most sensitive virus tested, IIIB, 73% (41/56) of the sera were able to neutralize at a level of ≥80%. Only two sera (both in the TV1/MF59 group) were unable to neutralize IIIB at the 50% level (Fig. 4, center panel). When specifically comparing the Env component of the immunization, regardless of the adjuvant(s) involved, a total of nine sera from each protein group were able to neutralize SS1196 at ≥50%. Interestingly, neutralization of the dual tropic virus 89.6 was most effective with sera from the subtype C TV1 Env groups (Fig. 4, center panel). Eleven rabbits immunized with TV1 Env were able to neutralize 89.6 at a level ≥50% while only six serum samples from each of the SF162 and bivalent immunization groups neutralized the same virus at this level. However, only a few sera, from the SF162 and bivalent groups, (sera 11, 15, 44, and 48–50) were able to neutralize other clade B viruses (3988 and TRO) above the 50% threshold (Fig. 4, top and bottom panels).
Neutralization of the clade C virus TV1.29 was observed from samples that included the subtype C TV1 Env as part of the immunization regimen (samples 31 and 39 from the TV1 Env groups and sera 44, 46, 48, and 54 from the bivalent Env groups) (Fig. 4, center and bottom panels). The other two clade C viruses tested were not neutralized above the 50% level. No samples were able to neutralize the clade A virus 461e2 at the 50% level.
To determine if we could increase the breadth observed for the most broadly neutralizing sera, IgG from these samples was isolated to increase the antibody concentration above that which could be achieved using the 1:15 sera dilution. However, an IgG concentration of up to 1 mg/ml did not show improvement in neutralization of viruses that were below the 50% level (data not shown).
Discussion
This study was designed to test the effect of the immune potentiator CpG 7909 on antibody responses when used in concert with the delivery system MF59 in a glycoprotein HIV vaccine. In addition, we sought to determine whether a combination of our well-characterized trimeric Envs, SF162 o-gp140ΔV2 and TV1 o-gp140ΔV2, from different subtypes would increase the titers and breadth of antibody responses over a monovalent Env immunization scheme, with specific interest in neutralizing responses. CpG 7909 was chosen as it has previously been shown to act in synergy with MF59 to enhance specific antibody responses to HIV-1 gag protein in mice (O’Hagan et al., 2002). Although all immunization groups elicited high antibody titers to both SF162 and TV1 envelopes, the inclusion of CpG in the monovalent vaccine regimens boosted antibody titers between 2- and 3.8-fold to both SF162 and TV1 proteins. We did not corroborate that this was a genuine CpG ODN effect by including, for example, additional rabbit groups immunized with Env antigens and non-CpG ODN. We reasoned that we could obviate these additional experiments as rabbits have already been shown to be responsive to the CpG 7909 immune stimulatory sequence (2006 equivalent sequence) (Rankin et al., 2001). Interestingly, though, when comparing bivalent immunization regimens, the boosting of antibody titers with CpG was not statistically significant.
Compared to immunization with a single antigen using either MF59 or the combination of adjuvants, bivalent immunization significantly increased antibody titers to the individual heterologous proteins. For example, SF162/TV1/MF59 elicited a greater response to TV1 than did SF162/MF59. This result was not unexpected since we had previously observed a similar effect when employing a DNA prime/protein boost immunization strategy (Lian et al., 2005). The current protein only bivalent immunization in MF59 was also able to significantly increase antibody titers to SF162 Env over immunization with SF162/MF59. The underlying reason for this increase was not readily predicted from peptide mapping studies. Linear and V3 epitopes were not increased relative to the increase seen in overall titers to SF162, nor was antibody avidity significantly increased. However, there was a correlation between the increase in SF162 titers and increases in avidity to SF162 for bivalent immunization groups as compared to those containing TV1 as the single antigen. The CpG-containing monovalent immunization regimens elicited higher SF162 titers than using MF59 alone. In the case of the SF162 monovalent regimen, the addition of CpG significantly increased antibody avidity (P=0.0138, p4).
As determined for SF162 binding titers, the inclusion of CpG in our immunization regimens also significantly increased the neutralization titers against SF162. Similarly, a combination of the two antigens derived from different subtypes in MF59 was able to elicit a greater neutralizing antibody response as compared to using either antigen individually. However, this neutralizing antibody boosting effect observed with bivalent Env immunization was not as obvious when CpG was included in the vaccine formulation. Although bivalent immunization in MF59 resulted in significantly higher neutralizing titers than either monovalent protein in MF59, beginning after the second administration, the addition of CpG boosted neutralizing antibody titers from single antigen immunization to the same levels as that obtained with both proteins with CpG. For reasons unclear at this point, this particular formulation of bivalent immunization along with MF59/CpG does not result in an optimally synergistic response. Not until the fourth immunization did we observe a significant difference between neutralizing antibody titers elicited by SF162/TV1/MF59/CpG and TV1/MF59/CpG (P=0.026).
Although four immunizations boosted SF162 neutralizing titers, neutralizing breadth was greatest following three administrations. Interestingly, as breadth decreased between the third and fourth immunization, so did the ratio of antibodies which recognized conformational versus linear epitopes (Fig. 1B). However, it is unclear at this time whether a bona fide correlation exists between these attributes. Additionally, the neutralizing antibody boosting observed when CpG is included as a component of the vaccine did not translate to an increase in the breadth of the neutralizing response. The lone exception was in the case of the virus SS1196 as SF162/MF59/CpG immunization did increase neutralization (p=0.008). Interestingly, against the tier 2 clade B virus TRO, neutralization was statistically better when CpG was not included in the formulation. When comparing all three regimens, use of the single adjuvant MF59 elicited elevated neutralization levels against TRO (p<0.05, all groups). For all other viruses, no substantial difference was observed between immunizations based on adjuvants. Although the levels are relatively low, evaluation of sera from rabbits immunized with SF162 o-gp140ΔV2 in MF59 showed improved neutralization of a subset of clade B viruses (TRJO, 3988, TRO, WITO, and REJO) as compared to sera from TV1/MF59 rabbits (p<0.02). Overall, relatively few sera (7/58, no p3 sera for two rabbits) were able to neutralize more than three of the viruses tested at the 50% level or above. All seven sera neutralized IIIB, SS1196, and 89.6. Four of these (sera 31, 39, 46, and 54) were also able to neutralize TV1.29. The remaining three sera were able to neutralize five total viruses, including 3988 and TRO (serum 15) or TRO and TV1.29 (sera 44 and 48). Only one of these samples, sera 15, did not include the subtype C Env as part of the immunization regimen.
One argument for eliciting antibodies with greater cross-neutralization potential is to immunize with sequences representative of viruses from differing clades and thus increasing the number of epitopes recognized as opposed to using a lone antigen that may elicit strain-specific antibodies. For example, an examination of V3 loops from JRFL (clade B) and BR025 (clade C) showed that there are intrinsic differences in the V3 loop conformations that are localized to the stem and turn regions. It was predicted that this could lead to subtype-specific neutralizing antibodies that would limit cross-reactivity (Patel et al., 2008). In the current study, viruses from both subtypes B and C were able to be neutralized by some sera from rabbits whose immunization regimen contained the TV1 Env. Although many of these sera are from bivalently immunized rabbits, some are from rabbits immunized only with the TV1 antigen. TV1 contains a V3 loop with a GPGQ motif as opposed to the GPGR sequence found in SF162. Other studies have suggested that clade A viruses which contain the GPGQ V3 sequence are superior for inducing antibodies with greater cross-neutralizing activity than clade B viruses that include the GPGR motif (Gorny et al., 2006; C. Krachmarov et al., 2005). Similarly, using a chimeric SF162 virus expressing different V3 sequences, an analysis of V3 antibodies from clade A and B viruses found that anti-V3A antibodies were able to neutralize more V3 sequences than anti-V3B antibodies (Krachmarov et al., 2006). Additionally, an immunization study using a gp120 DNA/V3 fusion protein boost regimen determined that antibodies elicited by GPGQ non-clade B sequences were able to neutralize viruses with both GPGQ and GPGR motifs (Zolla-Pazner et al., 2008). It was determined that antibodies elicited by GPGR viruses favored GPGR neutralization and suggested that GPGR may be dominant over GPGQ when combined for immunization (Zolla-Pazner et al., 2008). In the current study, immunization with the GPGQ TV1 did lead to better breadth than the GPGR SF162 regimen, but bivalent immunization resulted in the greatest cross-neutralizing activity. However, our study used o-gp140ΔV2 Env while the previous study specifically focused on the V3 epitope by using V3 fusion proteins for boosting. Unfortunately, since the neutralizing breadth we observed was relatively low, determination of the importance of the V3 epitope and its relationship with cross-neutralizing activity was indeterminate.
Peptide mapping was employed to determine the underlying basis for the differences observed in SF162 neutralization between the immunization groups (Table 1). In view of the fact that the highest neutralizing titers were found following the fourth immunization, focus was placed on this time point for analysis. Between the two protein antigens used in this study, TV1 o-gp140ΔV2 resulted in a higher percentage of neutralization that was inhibited by V3 peptides. Depending on the adjuvant(s) used, 73–76% of SF162 neutralization by sera from TV1 immunized rabbits was blocked by the V3 tip peptide as compared to 35% for SF162 immunization. This was accompanied by an increase in V3 tip ELISA titers for TV1/MF59 as compared to SF162/MF59. When CpG was included though, an increase in ELISA titers was not observed. Inclusion of CpG did not effect the SF162 neutralization epitopes for TV1 immunization. Similarly, sera from SF162 immunization with CpG contained a similar level of V3 neutralizing activity as compared to MF59 alone despite the V3 ELISA titers tripling. There was, however, a small amount of neutralization that was inhibited by V1 peptide.
Previous studies in macaques using the SF162 o-gp140ΔV2 construct have reported the elicitation of a high percentage of neutralizing antibodies that recognize the V1 region (Derby et al., 2006). In the current study (conducted in rabbits), V1 neutralizing antibodies were observed at low levels while there was found to be a high level of neutralization due to antibodies of V3-specificity. In contrast, Derby et al. detected only low levels of these V3 neutralizing antibodies. The results in macaques are in agreement with results we have obtained in vaccinated humans where neutralizing antibodies to V1 were detected, but only low levels of V3-specific neutralizing antibodies were found (Barnett, unpublished data). In addition, in recent non-human primate studies, both V1 and V3 binding and neutralizing antibodies were detected (Burke and Barnett, unpublished data). It is important to note that there are several key differences between these studies, most notably, which are the immunization strategies (protein only vs. prime/protein boost), routes of immunization, as well as the subjects (rabbits, macaques, and humans). However, we have also observed differences in the neutralizing antibody epitopes elicited between rabbits and mice when using the same immunogen (Brian Burke, Susan Barnett, Gib Otten, and Hong Liu, unpublished data). Additionally, although we observed that CpG was able to boost the neutralizing antibody response to SS1196 in the SF162 Env immunization regimen, this is in contrast to results observed in a study by Shu et al. where boosting of antibody responses was not observed when using CpG as compared to MF59 alone in the protein boost phase (Shu et al., 2007). However, this study was completed in guinea pigs using an adenovirus or DNA prime/SF162 Env boost scheme. All of these results indicate that further study must be done on the effect of priming and adjuvant combinations as well as which species to use for in vivo studies and how predictive these results are as compared to neutralizing antibody epitopes elicited in humans.
Although the precise mechanisms of adjuvant action for CpG 7909 and MF59 are still subjects of intensive research, ample evidence suggests that CpG 7909/2006 activates B cells and increases production of costimulatory molecules in plasmacytoid dendritic cells while MF59 interacts with macrophages and monocytes and is internalized at the site of intramuscular injection (Dupuis et al., 1998; Kerkmann et al., 2003; Seubert et al., 2008). By acting on the innate immune system via increased endocytosis and antigen uptake as well as enhanced dendritic cell maturation, these adjuvants should lead to a robust priming of the adaptive immune response. It is therefore not surprising that the combination of both adjuvants enhances the immunogenicity of a bivalent HIV vaccine administered intramuscularly. Importantly, both CpG 7909 and MF59 are licensed for human use and have been well tolerated in clinical trials, including hepatitis B vaccine and influenza vaccine trials performed in people living with HIV (Cooper et al., 2004; Cooper et al., 2005; Gabutti et al., 2005; Kahn et al., 1994; Ott et al., 1995). A continuing goal of HIV vaccine development is to achieve high avidity functional neutralizing antibodies following the delivery of Env protein antigens. The present findings indicate that using a multivalent methodology along with the synergistic combination of adjuvants MF59 and CpG can enhance humoral responses against HIV-1. However, optimization of adjuvantation, while necessary for improving neutralizing potency, was not sufficient here for accessing all critical epitopes required for generating the desired neutralization breadth believed to be required for an effective HIV vaccine. As enhancing the quality of antigen-elicited immune responses is critical for the development of future vaccines against HIV and other infectious diseases, these results highlight the continued need for further investigations of not only adjuvants and combinations of adjuvants, but also of novel vaccine regimens, diverse antigens and antigen structures, and combinations thereof.
Materials and methods
Proteins, adjuvants, and immunization of rabbits
Six groups of ten New Zealand White rabbits each were used in this immunogenicity study. Animals in groups 1 and 2 were immunized with subtype B SF162 o-gp140ΔV2, groups 3 and 4 with subtype C TV1 o-gp140ΔV2, and groups 5 and 6 with both subtype B and C proteins. The clade B protein was purified from the CCR5 tropic strain SF162 and contained a 30 amino acid deletion in the V2 loop region as previously described (Srivastava et al., 2003). In a similar manner, the clade C TV1 Env was also prepared and purified as previously described (Lian et al., 2005). Four protein immunizations were administered intramuscularly, in the gluteus, at weeks 0, 4, 12, and 24. Total protein dosage at each immunization was 25 μg. Protein was administered in MF59 (groups 1–6). Groups 2, 4, and 6 also contained 500 μg CpG-7909 (Coley Pharmaceutical Group, Inc., Ottawa, Canada). Serum samples were collected prior to each immunization and two weeks following each immunization.
Envelope–specific antibody titers in rabbit sera
Envelope-specific serum total antibody titers were quantified by a standard ELISA assay as previously described (Srivastava et al., 2002). To determine antibody responses against linear envelope epitopes, ELISA was carried out using SF162 Env protein which was denatured and reduced according to previous methods (Sharma et al., 2006; Srivastava et al., 2002). To determine the antibodies induced against the variable region epitopes of envelope, anti-peptide ELISA was performed. The responses against V3 and V4 of SF162 Env were measured using the peptides V3 tip, V3 cyclic, and V4 cyclic and methods previously described (Sharma et al., 2006; Srivastava et al., 2002).
Antibody avidity measurements
Antibody avidity index determination was performed using an ammonium thiocyanate (NH4SCN) displacement ELISA as described elsewhere (Srivastava et al., 2002).
Assessment of HIV-1 neutralizing antibodies
Neutralization was assessed using molecularly cloned pseudoviruses and a luciferase reporter gene assay in TZM-bl cells (Dr John C. Kappes, Dr Xiaoyun Wu and Tranzyme, Inc.(Durham, NC)) as described previously (Li et al., 2005; Montefiori, 2004). Briefly, a total of 200 TCID50 pseudovirus/well were added to diluted sera samples and incubated at 37 °C for 1 hr. Following incubation, 10,000 cells/well in DEAE-dextran-containing media were added and incubated for 48 hrs at 37 °C. The final concentration of DEAE-dextran was 10 μg/ml. Single round of infection HIV-1 Env pseudoviruses were prepared by co-transfection of 293T cells with an envelope expression plasmid containing a full-length gp160 env gene along with an env-deficient HIV-1 backbone vector (pSG3 env), using TransIT®-LT1 transfection reagent (Mirus Bio Corp., Madison, WI), as previously reported (Lian et al., 2005). After 48 hrs, the cell culture supernatant containing the pseudovirus was filtered through a 0.45 μm filter. Neutralizing activity was measured as reductions in luciferase gene expression. The percent reduction in relative luminescence units (RLU) was calculated relative to the RLU in the presence of pre-immunization serum. Neutralizing antibody titers against SF162 were determined using 3-fold serially diluted sera samples. The breadth of neutralizing antibodies in sera was assessed at a serum dilution of 1:15. The percent neutralization was corrected for non-specific inhibition using the formula described previously with MLV as a control virus (Kothe et al., 2007).
Neutralizing antibody epitope mapping
Mapping of neutralizing epitopes was done using the TZM-bl assay with a few modifications. An additional incubation of peptide with diluted serum samples was carried out for 1 hr at 37 °C prior to the addition of virus. The dilution of serum used was that corresponding to the ID70. The concentration of peptide after addition to serum was 15 μg/ml. Peptides corresponding to regions of the SF162 Env used for epitope mapping were V1 tip (TNLKNATNTKSSNWKEMDRGEIK), V1 cyclic (CTNLKNATNTKSSNWKEMDRGEIKNC), V3 tip (CTRKSITIGPGRAFYC), V3 cyclic (CTRPNNNTRKSITIGPGRAFYATGDIIGDIRQAHC), V4 cyclic (CNSTQLFNSTWNNTIGPNNTNGTITLPC), and V5 (TRDAGKEISNTTEIFR). The control peptide was V3 scramble (TRKSFYATPGRAITIG). Peptides corresponding to the epitopes of the monoclonal antibodies 4E10 (NWFDITNWLWYIKKKK) (Brunel et al., 2006) and b12 (NWPRWWEEFVDKHSSC) (Boots et al., 1997) were also used. All peptides were obtained from Sigma-Genosys (The Woodlands, TX).
Statistical analyses
The Mann Whitney test was used to test for differences between immunization groups. For all comparisons, a two-sided p<0.05 was considered statistically significant. Statistical analyses were performed using the analysis software within the GraphPad Prism package 5.01.
Acknowledgments
This work was supported by the NIH HIVRAD grant #5 P01 AI48225-03 and HIV DDT contract N01-AI-50007. We gratefully acknowledge Jeanne Flandez for help with ELISA assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azizi A, Anderson DE, Torres JV, Ogrel A, Ghorbani M, Soare C, Sandstrom P, Fournier J, Diaz-Mitoma F. Induction of broad cross-subtype-specific HIV-1 immune responses by a novel multivalent HIV-1 peptide vaccine in cynomolgus macaques. J Immunol. 2008;180 (4):2174–2186. doi: 10.4049/jimmunol.180.4.2174. [DOI] [PubMed] [Google Scholar]
- Barnett SW, Lu S, Srivastava I, Cherpelis S, Gettie A, Blanchard J, Wang S, Mboudjeka I, Leung L, Lian Y, Fong A, Buckner C, Ly A, Hilt S, Ulmer J, Wild CT, Mascola JR, Stamatatos L. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J Virol. 2001;75 (12):5526–5540. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots LJ, McKenna PM, Arnold BA, Keller PM, Gorny MK, Zolla-Pazner S, Robinson JE, Conley AJ. Anti-human immunodeficiency virus type 1 human monoclonal antibodies that bind discontinuous epitopes in the viral glycoproteins can identify mimotopes from recombinant phage peptide display libraries. AIDS Res Hum Retroviruses. 1997;13 (18):1549–1559. doi: 10.1089/aid.1997.13.1549. [DOI] [PubMed] [Google Scholar]
- Brunel FM, Zwick MB, Cardoso RM, Nelson JD, Wilson IA, Burton DR, Dawson PE. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J Virol. 2006;80 (4):1680–1687. doi: 10.1128/JVI.80.4.1680-1687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti BK, Ling X, Yang ZY, Montefiori DC, Panet A, Kong WP, Welcher B, Louder MK, Mascola JR, Nabel GJ. Expanded breadth of virus neutralization after immunization with a multiclade envelope HIV vaccine candidate. Vaccine. 2005;23 (26):3434–3445. doi: 10.1016/j.vaccine.2005.01.099. [DOI] [PubMed] [Google Scholar]
- Cherpelis S, Shrivastava I, Gettie A, Jin X, Ho DD, Barnett SW, Stamatatos L. DNA vaccination with the human immunodeficiency virus type 1 SF162deltaV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8(+) T-cell-depleted rhesus macaques. J Virol. 2001;75 (3):1547–1550. doi: 10.1128/JVI.75.3.1547-1550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MW, Kim YB, Lee MK, Gupta KC, Ross W, Plishka R, Buckler-White A, Igarashi T, Theodore T, Byrum R, Kemp C, Montefiori DC, Martin MA. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J Virol. 2001;75 (5):2224–2234. doi: 10.1128/JVI.75.5.2224-2234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Morris ML, Efler SM, Krieg AM, Li Y, Laframboise C, Al Adhami MJ, Khaliq Y, Seguin I, Cameron DW. Safety and immunogenicity of CpG 7909 injection as an adjuvant to fluarix influenza vaccine. Vaccine. 2004;22 (23–24):3136–3143. doi: 10.1016/j.vaccine.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. CpG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19 (14):1473–1479. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- Derby NR, Kraft Z, Kan E, Crooks ET, Barnett SW, Srivastava IK, Binley JM, Stamatatos L. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: Comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162p4 and heterologous HIV-1 infection. J Virol. 2006;80 (17):8745–8762. doi: 10.1128/JVI.00956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186 (1):18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- Dupuis M, Denis-Mize K, LaBarbara A, Peters W, Charo IF, McDonald DM, Ott G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur J Immunol. 2001;31 (10):2910–2918. doi: 10.1002/1521-4141(2001010)31:10<2910::aid-immu2910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gabutti G, Guido M, Durando P, De Donno A, Quattrocchi M, Bacilieri S, Ansaldi F, Cataldini S, Chiriacò PG, De Simone M, Minniti S, Sticchi L, Gasparini R. Safety and immunogenicity of conventional subunit and MF59-adjuvanted influenza vaccines in human immunodeficiency virus-1-seropositive patients. J Int Med Res. 2005;33 (4):406–416. doi: 10.1177/147323000503300406. [DOI] [PubMed] [Google Scholar]
- Gorny MK, Williams C, Volsky B, Revesz K, Wang XH, Burda S, Kimura T, Konings FA, Nádas A, Anyangwe CA, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J Virol. 2006;80 (14):6865–6872. doi: 10.1128/JVI.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K, Langlois AJ, LaRosa GJ, Profy AT, Bolognesi DP, Herlihy WC, Putney SD, Matthews TJ. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990;250 (4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Kahn JO, Sinangil F, Baenziger J, Murcar N, Wynne D, Coleman RL, Steimer KS, Dekker CL, Chernoff D. Clinical and immunologic responses to human immunodeficiency virus (HIV) type 1 SF2 gp120 subunit vaccine combined with MF59 adjuvant with or without muramyl tripeptide dipalmitoyl phosphatidylethanolamine in non-HIV-infected human volunteers. J Infect Dis. 1994;170 (5):1288–1291. doi: 10.1093/infdis/170.5.1288. [DOI] [PubMed] [Google Scholar]
- Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170 (9):4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- Kim JH, Pitisuttithum P, Kamboonruang C, Chuenchitra T, Mascola J, Frankel SS, DeSouza MS, Polonis V, McLinden R, Sambor A, Brown AE, Phonrat B, Rungruengthanakit K, Duliege AM, Robb ML, McNeil J, Birx DL. Specific antibody responses to vaccination with bivalent CM235/SF2 gp120: Detection of homologous and heterologous neutralizing antibody to subtype E (CRF01.AE) HIV type 1. AIDS Res Hum Retroviruses. 2003;19 (9):807–816. doi: 10.1089/088922203769232601. [DOI] [PubMed] [Google Scholar]
- Kothe DL, Decker JM, Li Y, Weng Z, Bibollet-Ruche F, Zammit KP, Salazar MG, Chen Y, Salazar-Gonzalez JF, Moldoveanu Z, Mestecky J, Gao F, Haynes BF, Shaw GM, Muldoon M, Korber BT, Hahn BH. Antigenicity and immunogenicity of HIV-1 consensus subtype B envelope glycoproteins. Virology. 2007;360 (1):218–234. doi: 10.1016/j.virol.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachmarov C, Pinter A, Honnen WJ, Gorny MK, Nyambi PN, Zolla-Pazner S, Kayman SC. Antibodies that are cross-reactive for human immunodeficiency virus type 1 clade A and clade B V3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J Virol. 2005;79 (2):780–790. doi: 10.1128/JVI.79.2.780-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krachmarov CP, Honnen WJ, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Factors determining the breadth and potency of neutralization by V3-specific human monoclonal antibodies derived from subjects infected with clade A or clade B strains of human immunodeficiency virus type 1. J Virol. 2006;80 (14):7127–7135. doi: 10.1128/JVI.02619-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Vödrös D, Kozlowski PA, Montefiori DC, Wilson RL, Akerstrom VL, Chennareddi L, Yu T, Kannanganat S, Ofielu L, Villinger F, Wyatt LS, Moss B, Amara RR, Robinson HL. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369 (1):153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 Env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79 (16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Y, Srivastava I, Gómez-Román VR, Zur Megede J, Sun Y, Kan E, Hilt S, Engelbrecht S, Himathongkham S, Luciw PA, Otten G, Ulmer JB, Donnelly JJ, Rabussay D, Montefiori D, van Rensburg EJ, Barnett SW. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J Virol. 2005;79 (21):13338–13349. doi: 10.1128/JVI.79.21.13338-13349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg K, Rollman E, Eriksson L, Hinkula J, Wahren B. Enhanced immune responses after DNA vaccination with combined envelope genes from different HIV-1 subtypes. Virology. 2002;302 (1):44–57. doi: 10.1006/viro.2002.1547. [DOI] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current protocols in immunology. John Wiley & Sons; New York, NY: 2004. pp. 12.11.11–12.11.15. [DOI] [PubMed] [Google Scholar]
- O’Hagan DT, Singh M, Kazzaz J, Ugozzoli M, Briones M, Donnelly J, Ott G. Synergistic adjuvant activity of immunostimulatory DNA and oil/water emulsions for immunization with HIV p55 Gag antigen. Vaccine. 2002;20 (27–28):3389–3398. doi: 10.1016/s0264-410x(02)00272-4. [DOI] [PubMed] [Google Scholar]
- Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Cristillo A, Mboudjeka I, Shen S, Wu-Chou TH, Montefiori D, Mascola J, Lu S, Markham P. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol. 2005;34 (5–6):226–236. doi: 10.1111/j.1600-0684.2005.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, Hudacik L, Rose N, Mboudjeka I, Shen S, Wu-Chou TH, Montefiori D, Mascola J, Markham P, Lu S. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348 (2):341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11 (4 Suppl):S63–68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- Patel MB, Hoffman NG, Swanstrom R. Subtype-specific conformational differences within the V3 region of subtype B and subtype C human immunodeficiency virus type 1 Env proteins. J Virol. 2008;82 (2):903–916. doi: 10.1128/JVI.01444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001;11 (5):333–340. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Montefiori DC, Villinger F, Robinson JE, Sharma S, Wyatt LS, Earl PL, McClure HM, Moss B, Amara RR. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology. 2006;352 (2):285–294. doi: 10.1016/j.virol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Rollman E, Hinkula J, Arteaga J, Zuber B, Kjerrström A, Liu M, Wahren B, Ljungberg K. Multi-subtype gp160 DNA immunization induces broadly neutralizing anti-HIV antibodies. Gene Ther. 2004;11 (14):1146–1154. doi: 10.1038/sj.gt.3302275. [DOI] [PubMed] [Google Scholar]
- Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79 (5):2956–2963. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MS, Leblanc DF, Grandpre LE, Bartman MT, Montefiori DC, Letvin NL, Mascola JR. Standardized assessment of NAb responses elicited in rhesus monkeys immunized with single- or multi-clade HIV-1 envelope immunogens. Virology. 2007;367 (1):175–186. doi: 10.1016/j.virol.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180 (8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- Sharma VA, Kan E, Sun Y, Lian Y, Cisto J, Frasca V, Hilt S, Stamatatos L, Donnelly JJ, Ulmer JB, Barnett SW, Srivastava IK. Structural characteristics correlate with immune responses induced by HIV envelope glycoprotein vaccines. Virology. 2006;352 (1):131–144. doi: 10.1016/j.virol.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, Barnett SW, Nabel GJ, Mascola JR. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25 (8):1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist CA, Pihlgren M, Tougne C, Efler SM, Morris ML, AlAdhami MJ, Cameron DW, Cooper CL, Heathcote J, Davis HL, Lambert PH. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23 (5):615–622. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Srivastava IK, Stamatatos L, Legg H, Kan E, Fong A, Coates SR, Leung L, Wininger M, Donnelly JJ, Ulmer JB, Barnett SW. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J Virol. 2002;76 (6):2835–2847. doi: 10.1128/JVI.76.6.2835-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Stamatatos L, Kan E, Vajdy M, Lian Y, Hilt S, Martin L, Vita C, Zhu P, Roux KH, Vojtech L, D CM, Donnelly J, Ulmer JB, Barnett SW. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol. 2003;77 (20):11244–11259. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Kan E, Sun Y, Sharma VA, Cisto J, Burke B, Lian Y, Hilt S, Biron Z, Hartog K, Stamatatos L, Cheng RH, Ulmer JB, Barnett SW. Comparative evaluation of trimeric envelope glycoproteins derived from subtype C and B HIV-1 R5 isolates. Virology. 2008;372 (2):273–290. doi: 10.1016/j.virol.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Thongcharoen P, Suriyanon V, Paris RM, Khamboonruang C, de Souza MS, Ratto-Kim S, Karnasuta C, Polonis VR, Baglyos L, Habib RE, Gurunathan S, Barnett S, Brown AE, Birx DL, McNeil JG, Kim JH. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J Acquir Immune Defic Syndr. 2007;46 (1):48–55. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- Wang S, Pal R, Mascola JR, Chou TH, Mboudjeka I, Shen S, Liu Q, Whitney S, Keen T, Nair BC, Kalyanaraman VS, Markham P, Lu S. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350(1):34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, Shen S, Green S, Rothman AL, Ennis FA, Arthos J, Pal R, Markham P, Lu S. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26 (8):1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Martin LN, Slobod KS, Coleclough C, Lockey TD, Brown SA, Stambas J, Bonsignori M, Sealy RE, Blanchard JL, Hurwitz JL. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005;23 (46–47):5306–5320. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cohen SS, Krachmarov C, Wang S, Pinter A, Lu S. Focusing the immune response on the V3 loop, a neutralizing epitope of the HIV-1 gp120 envelope. Virology. 2008;372 (2):233–246. doi: 10.1016/j.virol.2007.09.024. [DOI] [PubMed] [Google Scholar]