Abstract
Using the zebrafish, we previously identified a central function for perlecan during angiogenic blood vessel development. Here, we explored the nature of perlecan function during developmental angiogenesis. A close examination of individual endothelial cell behavior revealed that perlecan is required for proper endothelial cell migration and proliferation. Because these events are largely mediated by VEGF-VEGFR2 signaling, we investigated the relationship between perlecan and the VEGF pathway. We discovered that perlecan knockdown caused an abnormal increase and redistribution of total VEGF-A protein suggesting perlecan is required for the appropriate localization of VEGF-A. Importantly, we linked perlecan function to the VEGF pathway by efficiently rescuing the perlecan morphant phenotype by microinjecting VEGF-A165 protein or mRNA. Combining the strategic localization of perlecan throughout the vascular basement membrane along with its growth factor-binding ability, we hypothesized a major role for perlecan during the establishment of the VEGF gradient which provides the instructive cues to endothelial cells during angiogenesis. In support of this hypothesis we demonstrated that human perlecan bound in a heparan sulfate-dependent fashion to VEGF-A165. Moreover, perlecan enhanced VEGF mediated VEGFR2 activation of human endothelial cells. Collectively, our results indicate that perlecan coordinates developmental angiogenesis through modulation of VEGF-VEGFR2 signaling events. The identification of angiogenic factors, such as perlecan, and their role in vertebrate development will not only enhance overall understanding of the molecular basis of angiogenesis, but may also provide new insight into angiogenesis-based therapeutic approaches.
Keywords: Perlecan, Heparan sulfate proteoglycan, VEGF, VEGF receptor, Angiogenesis, Endothelial cell, Zebrafish
1. Introduction
The vascular basement membrane encompassing various collagens, laminins and perlecan serves not only a structural (Yurchenco et al., 2004) but also a signaling (Ramirez and Rifkin, 2003) function. A perlecan-mediated growth factor response spans the contexts of development and disease (Wight et al., 1992; Iozzo, 1994; Iozzo et al., 1994; Hassell et al., 2003; Iozzo, 2005; Whitelock et al., 2008). Mutations in the C. elegans unc-52 perlecan ortholog disrupt muscle organization (Rogalski et al., 1993; Mullen et al., 1999) and disturb distal tip cell migration via influence on FGF1, TGFβ and Wnt-like signaling (Merz et al., 2003). Drosophila perlecan, encoded by trol, regulates neuroblast proliferation through the FGF and Hh pathways (Voigt et al., 2002; Park et al., 2003). Recently trol has been linked to other growth factor signaling pathways functioning either during brain development by TGF and Wnt, or perhaps regulating plasmatocyte proliferation by VEGF/PDGF (Lindner et al., 2007). The perlecan-null mice exhibit a complex phenotype characterized at one level by reduced chondrocyte proliferation, most likely the result of altered growth factor signaling (Costell et al., 1999; Arikawa-Hirasawa et al., 1999). Recent evidence also links murine perlecan to regulating floor plate Shh and forebrain development (Girós et al., 2007). Beyond embryonic development, perlecan-mediated growth factor modulation has been associated with human prostate cancer (Datta et al., 2006b). Essentially perlecan binding to Shh promotes Shh downstream signaling and supports prostate cancer cell growth (Datta et al., 2006a).
The widespread developmental expression (Carson et al., 1993; Handler et al., 1997) and its complex modular structure (Iozzo and Murdoch, 1996; Iozzo, 1998; Farach-Carson and Carson, 2007) suggest that perlecan is involved in a number of physiological and pathological events. Indeed, perlecan is implicated in lipid catabolism (Fuki et al., 2000), epidermal formation (Sher et al., 2006), chondrogenesis (SundarRaj et al., 1995; French et al., 2002), vascular injury and thrombosis (Nugent et al., 2000; Kinsella et al., 2003), atherosclerosis (Tran-Lundmark et al., 2008), and cancer growth and invasion (Cohen et al., 1994; Iozzo et al., 1997; Mathiak et al., 1997). Perlecan-growth factor interactions are mediated through the perlecan protein core or the attached heparan sulfate chains (Iozzo and San Antonio, 2001; Smith et al., 2007; Whitelock et al., 2008). The heparan sulfate chains are required for HS-binding growth factor signaling and influence the distribution or movement of growth factors (Nakato and Kimata, 2002). The pivotal role of the perlecan HS chains to growth factor response has been evidenced by the HS-deficient perlecan mice. These transgenic animals (Hspg2Δ3/Δ3) harbor a partial deletion of perlecan domain I (exon 3), thereby loosing the HS attachment sites (Rossi et al., 2003). HS deficiency was linked to decreased matrix binding of FGF-2, which correlated with increased smooth muscle cell proliferation in vitro and in vivo (Tran et al., 2004). The perlecan HS-deficient mice also exhibited delayed wound healing as well as impaired tumor growth and angiogenesis induced by FGF-2 (Zhou et al., 2004). Antisense strategies targeting the expression of perlecan protein core cause a marked suppression of tumor growth, angiogenesis, and an attenuated response to FGF-2 in several cell systems (Sharma et al., 1998; Aviezer et al., 1997). Perlecan knockdown in prostate cancer cells has also been shown to disrupt in vitro responses to VEGF-A and FGF-2, both HS-binding growth factors (Savoré et al., 2005).
Analysis of the HS-modifying enzymes in zebrafish also supports the relationship between HS and growth factor modulation (Cadwallader and Yost, 2006b; Cadwallader and Yost, 2006a; Cadwallader and Yost, 2007). Investigation of zebrafish heparan sulfate 6-O sulfotransferase revealed that HS6ST was required for muscle and angiogenic vascular development (Bink et al., 2003; Chen et al., 2005). Interestingly HS6ST function, through an interaction with VEGF, is essential for branching morphogenesis of the developing caudal vein (Chen et al., 2005). The heparin-binding VEGF-A actually serves as a ligand for 6-O sulfated heparan sulfate (Ono et al., 1999). Accordingly, the binding is essential for vessel branch establishment and for influencing the spatial restriction of VEGF which regulates branch pattern (Ruhrberg et al., 2002). Perlecan binding to growth factors may establish a morphogen gradient supporting key events such as those alluded to during vessel guidance (Siekmann and Lawson, 2007; Hellstrom et al., 2007; Leslie et al., 2007). Perlecan can also serve as a sink for various growth factors which, in a context-dependent manner, could favor or disfavor receptor activation and downstream events (Aviezer et al., 1994). Along these same lines heparanase or proteolytic cleavage of the core could generate functional growth factor complexes (Whitelock et al., 1996). A similar concept was presented in hepatoblastoma xenografts which exhibited initial tumor regression and angiogenesis as a result of VEGF therapy, but eventual recurrence associated with vessel recovery and an increase in both perlecan and heparanase expression (Kadenhe-Chiweshe et al., 2008). Essentially perlecan was sequestering and heparanase was releasing VEGF in the tumor vessel microenvironment which ultimately favored VEGFR2 activation and vessel survival (Kadenhe-Chiweshe et al., 2008).
The purpose of the current study was to further explore perlecan function within the context of angiogenesis and growth factor biology. Given the nature of perlecan function, we hypothesized perlecan may modulate VEGF-VEGFR2 activation as a means to coordinate developmental angiogenesis.
2. Results
2.1. A closer examination: perlecan is required for angiogenic blood vessel development
Perlecan knockdown significantly inhibits angiogenic blood vessel development throughout the trunk and tail (Fig. S1). Angiogenic sprouts, the intersegmental vessels (ISVs), emerge from the dorsal aorta but fail to extend past the region of the notochord to form a complete dorsal longitudinal anastomotic vessel (DLAV) along the dorsal side of the embryo (Zoeller et al., 2008). Live DIC video microscopy revealed these vessel sprouts are largely non-lumenized, non-functional vessels unable to carry flow.
Normal angiogenic blood vessel development of the ISVs in the trunk and tail involves a number of coordinated cell behaviors, among four to six cells, that include sprouting, migration, proliferation, establishment of cell-cell junctions and lumen formation in order to form a complete and functional circulatory network (Isogai et al., 2001; Childs et al., 2002; Isogai et al., 2003; Blum et al., 2008). Our observations of perlecan morphant vasculature indicate that angiogenic sprouting is initiated and occurring at proper intervals, but the fact that the sprouts fail to continue suggests perlecan knockdown may interfere with the subsequent angiogenic cellular events described above.
To determine the role of perlecan in endothelial cell behavior, we examined the consequences of perlecan knockdown on vascular development in Tg(fli1a:nuclearEGFP)y7 zebrafish embryos (Siekmann and Lawson, 2007). Previous experiments utilized the Tg(fli1:EGFP)y1 zebrafish line which express cytoplasmic GFP under the control of fli1 a vascular specific marker- fluorescently labeling the entire zebrafish vascular network and permitting in vivo analysis of vascular development during real time (Lawson and Weinstein, 2002). The Tg(fli1a:nEGFP)y7 were engineered in nearly the same manner but harbor nuclear localized GFP expression, permitting analysis of individual endothelial cell behavior in vivo and over real time (Siekmann and Lawson, 2007). Analysis of perlecan knockdown in the Tg(fli1a:nEGFP)y7 was capable of further defining the nature of abnormal ISV development at the individual cell level, which includes assessing sprout cell number, migratory behavior and cell division in the absence of perlecan.
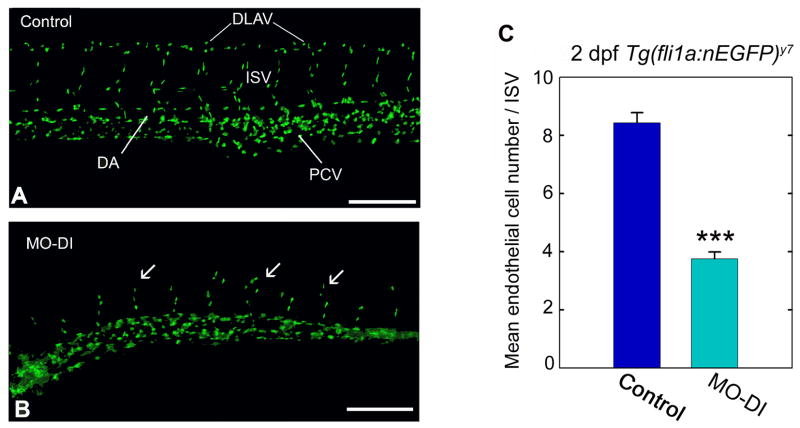
We found perlecan morphant ISVs contained a significantly less number of endothelial cells throughout the ISV region when compared to matched control embryos (Fig. 1). On average, the 2 dpf perlecan morphants displayed four endothelial cell nuclei per ISV region (Fig. 1B) versus eight endothelial cell nuclei per ISV region in controls (Fig. 1A). Given the abnormal nature of ISV cell behavior in the morphant embryos, we hypothesized that perlecan supports the migratory and or proliferative events necessary for proper angiogenic blood vessel development. Since such proangiogenic events are controlled by the vascular endothelial cell growth factor and its receptor, we investigated a link between perlecan and VEGF-VEGFR2.
Fig. 1.
Perlecan knockdown influences endothelial cell number. Vascular analysis of control (A) or perlecan morphant (B) Tg(fli1a:nEGFP)y7 zebrafish embryos. Notice the abnormal formation of ISVs (arrows) and the lack of a DLAV in the perlecan morphants. (C) Endothelial cell number was assessed by counting the endothelial cell nuclei present per intersegmental vessel region in 2 dpf control and morphant embryos. Control embryos typically displayed eight endothelial cells/ISV region compared to perlecan morphants which typically displayed four cells in the comparable area. Data summarize the endothelial cell counts from a total of 40 ISV regions for controls and a comparable 40 ISV regions for morphants. The forty counts were derived from four separate embryos, with 10 ISV regions assessed in each (***P<0.001). Scale bar, 250 μm.
2.2. Perlecan is required for the proper localization of VEGF-A
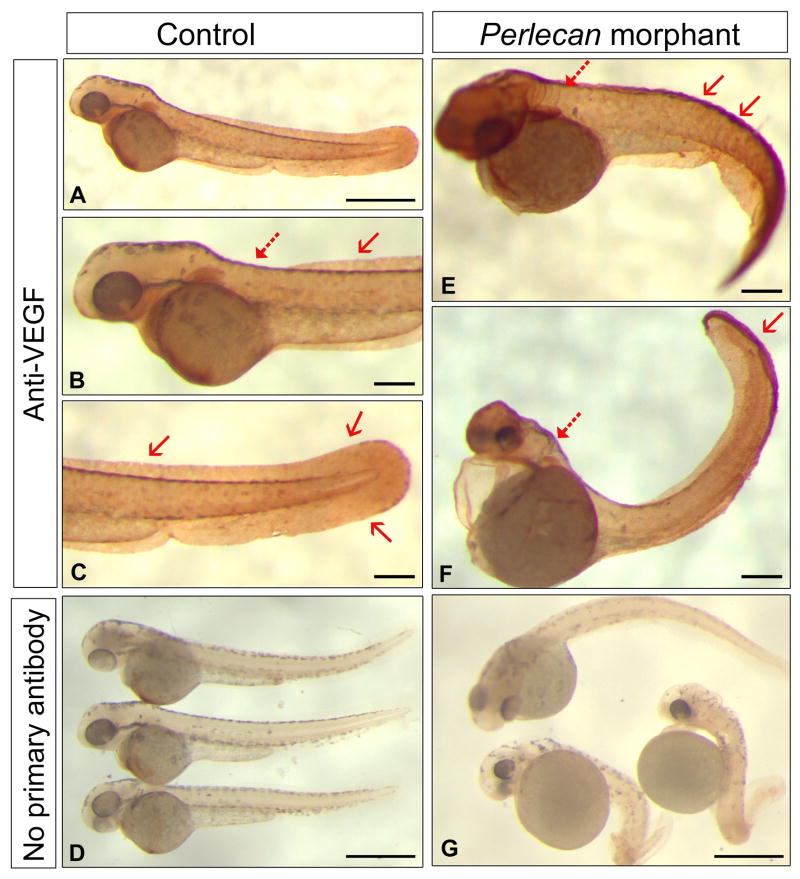
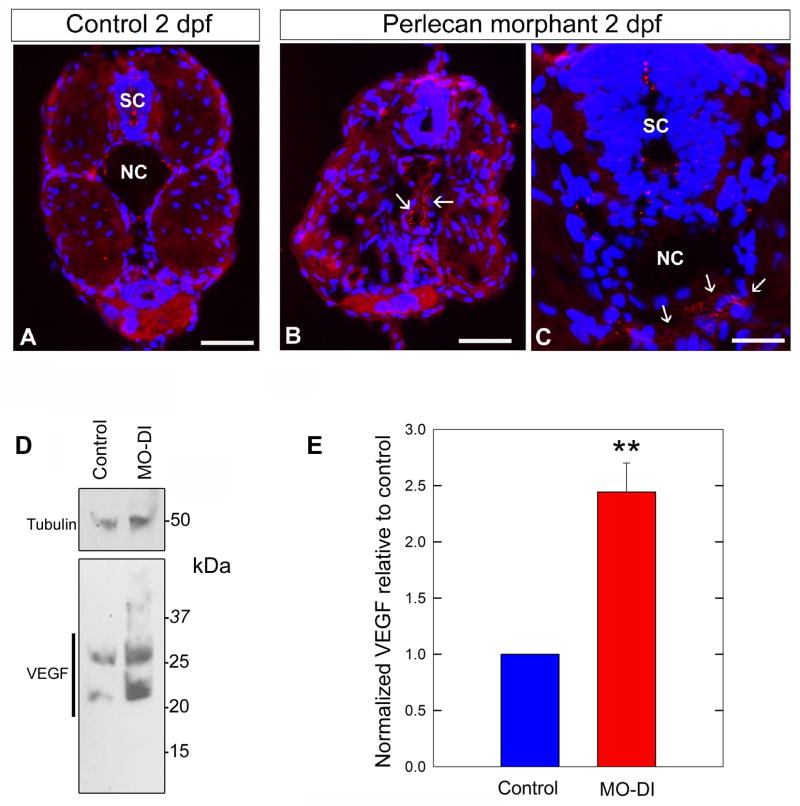
We hypothesized that perlecan would sequester VEGF, thereby regulating VEGF positional distribution or availability and functional activity through VEGFR during angiogenesis. Whole-mount immunohistochemistry with anti-VEGF-A, showed that VEGF was primarily expressed in the fin and neck region in 2–3 dpf control embryos (Fig. 2A–C). To our surprise, the amount of VEGF was markedly increased and abnormally distributed in the perlecan morphants (Fig. 2E,F). Specifically, in the perlecan morphants VEGF accumulated diffusely in the dorsal and ventral regions of the future caudal fin (Fig. 2E–F, arrows) and in the hindbrain (Fig. 2E–F, dotted arrows). Given the nature of the perlecan morphant angiogenic phenotype we suggest that the VEGF deposits are largely nonfunctional in the absence of perlecan. We further explored this finding by performing additional immunostaining using cross-sections through the trunk (Fig. 3). We observed VEGF as significant punctuate deposits around regions of the developing notochord and spinal cord in the perlecan morphants (Fig. 3B,C) compared to matched control embryos (Fig. 3A). These data support the original observations at the whole organism level and provide new insight regarding abnormal VEGF localization to key regions.
Fig. 2.
Perlecan knockdown results in the abnormal redistribution and accumulation of VEGF protein in morphant embryos. Compare the VEGF whole-mount immunostaining between 2–3dpf control (A–D) and MO-DI (E–G) embryos. Notice the accumulation and abnormal localization of VEGF epitopes in the dorsal and ventral soft tissues of the future caudal fin (red arrows) and an increase in the hindbrain region (dotted red arrows). Panels (D) and (G) are staining with secondary antibody alone. All images are left-side views with dorsal up and anterior to the left. Scale bars, 600 μm (A, D, G) and 305μm (B, C, E, F).
Fig. 3.
Perlecan knockdown in zebrafish results in an increase in VEGF protein levels. Cross-section analysis revealed regions of abnormal VEGF deposits in perlecan morphant embryos. Immunostaining identified abnormal VEGF aggregates surrounding the notochord (NC, arrows in B and C) and within the region of the spinal cord (SC) in 2dpf perlecan morphant embryos (B and C). Panel A represents a frozen cross-section from a matched 2 dpf control embryo. Immunostaining was performed with anti-human VEGF-Aand visualized by anti-rabbit-Rhodamine (red) and counterstained with DAPI (blue). Scale bar, 90 μm. (D) Protein extracted from 2 dpf control (n = 26) and MO-DI (n = 26) embryos was assayed for VEGF expression levels by immunoblotting with anti-VEGF antibody and anti-acetylated tubulin as a load control. Notice the significant increase in total VEGF in the perlecan morphants. The various immunoreactive bands correspond to monomers, dimers and higher-order complexes of VEGF. (E) Numerical estimation of the data presented in D, n = 3 sample runs (**P<0.01). Blots were quantified using the ImageJ 1.41o software package (NIH).
2.3. Perlecan influences VEGF-A protein levels
To biochemically assess our VEGF-A immunostaining data, we performed immunoblotting on protein samples derived from pooled 2 dpf control or morphant embryos (n = 26 each, Fig. S2). Immunoblotting with anti-VEGF revealed that perlecan morphants exhibited elevated levels of total VEGF-A when compared to control embryos (Fig. 3D, bottom). The multiple band patterns represent multiple VEGF-A isoforms and or dimer formation. Immunoblotting with anti-acetylated tubulin served as a load control (Fig. 3D, top). Our biochemical analysis of the perlecan morphants indicates that the abnormal topographical distribution of VEGF-A (Figs. 2 and 3A–C) was associated with an abnormal increase in VEGF-A protein levels (Fig. 3E).
2.4. VEGF-A can partially rescue the perlecan morphant phenotype
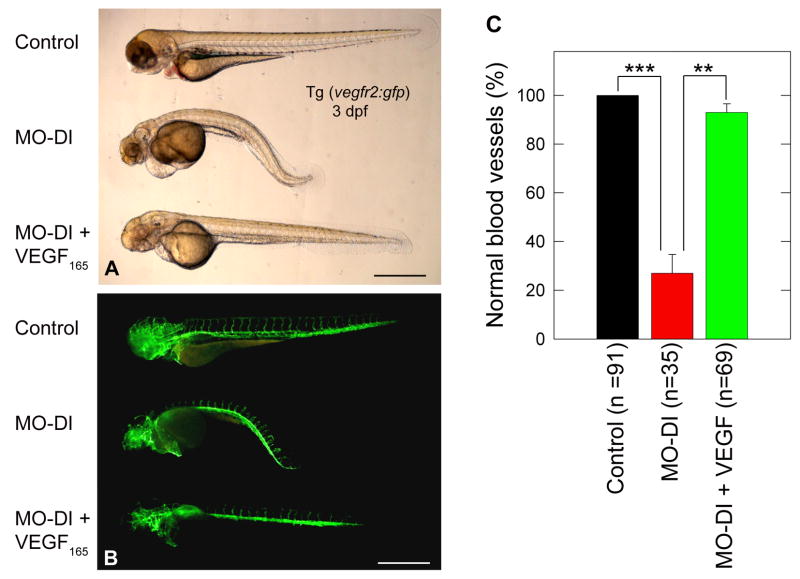
Next, we tested whether microinjection of either VEGF-A165 protein or mRNA could rescue the phenotype evoked by knockdown of endogenous perlecan. To this end, embryos were injected with MO-DI alone or in combination with VEGF-A165 (1.25 ng/embryo). In three independent experiments, VEGF was capable of rescuing the curved-body phenotype as well as the structure and organization of the ISV/DLAV (Fig. 4). We obtained a similar rescue of the vascular phenotype using zebrafish VEGF-A165 mRNA, although at a 40% rescue response (Fig. S3). Microinjection of VEGF-A165 alone did not induce any observable phenotype (data not shown). Thus, perlecan morphants can be partially rescued by VEGF-A, indicating that perlecan acts upstream of the VEGF-VEGFR2 signaling axis.
Fig. 4.
VEGF-A165 protein can rescue the morphant phenotype induced by MO-DI perlecan knockdown. Panels (A,B) are live bright field and corresponding GFP composite images of a representative control (top), MO-DI (middle) and rescue (MO-DI + VEGF-A165, bottom) sample. Note the clear rescue of both the muscle and vascular phenotype by VEGF-A165, as compared to MO-DI alone. Microinjection of VEGF-A165 alone did not result in any observable phenotype (data not shown). All images are left-side views of 3dpf Tg(vegfr2:g-rcfp) embryos with dorsal up and anterior to the left. Scale bar, 500 μm. The graph displayed in (C) represents the mean ± S.E.M. of three individual experiments, with rescue screening based upon embryos exhibiting normal vascular development (**P<0.01; ***P<0.001).
2.5. Perlecan binds VEGF-A via the HS side chains
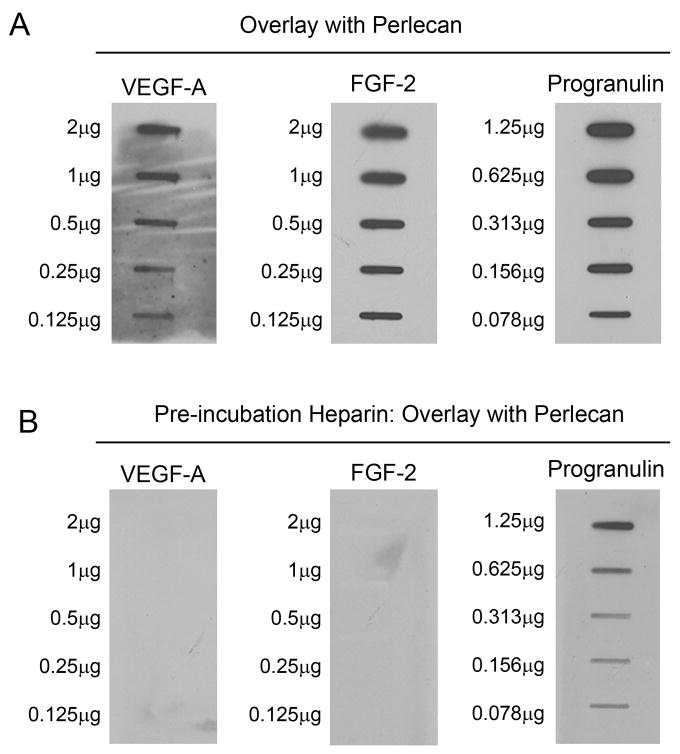
Our VEGF rescue experiments indicated that perlecan acts upstream, at the level of VEGF-VEGFR, within the VEGF signaling pathway. We hypothesized that perlecan directly regulates VEGF during angiogenic blood vessel development. To gain further insight and to support a direct mechanism of action, we examined perlecan-VEGF protein-protein interaction and the relationship between perlecan and VEGF function. Using overlay assay we tested human VEGF-A and FGF-2, two established HS-binding proteins, and as positive control for the protein core we used recombinant progranulin, a secreted growth factor which specifically binds to domain V/endorepellin of human perlecan (Gonzalez et al., 2003). The different proteins were absorbed onto nitrocellulose membranes using scalar dilutions, followed by overlay with perlecan derived from human coronary artery endothelial cells. Immunoblotting with anti-perlecan/domain V revealed that perlecan bound VEGF-A, FGF-2 and progranulin (Fig. 5A). We found that pre-incubation with excess heparin, prior to overlay with perlecan, inhibited perlecan binding to VEGF-A and FGF-2 but not to progranulin (Fig. 5B). Our results support a perlecan protein core mediated interaction with progranulin and indicate that perlecan binds both FGF-2 and VEGF-A via its HS side chains. We propose that the perlecan-VEGF interaction is required for VEGF function.
Fig. 5.
Perlecan binds VEGF-A in overlay assays. (A) Human coronary artery endothelial cell derived perlecan binds VEGF-A, FGF-2 and progranulin. Proteins were slot-blotted onto nitrocellulose membranes at the indicated amounts, blocked for 30 min in 5% milk, followed by ~4 h overlay with perlecan (125 ng/ml). Perlecan binding was revealed by immunodetection with anti-human perlecan domain V. (B) Heparin competition inhibits perlecan binding to VEGF-A and FGF-2 but not progranulin. The blots presented in (A) were stripped, examined for residual signal by ECL, blocked in 5% milk for 30 min, followed by pre-incubation with heparin (10 μg/ml) for ~1 h and subsequent overlay with perlecan (125 ng/ml) for 2 h. Perlecan binding was examined as described in A.
2.6. Perlecan enhances VEGFR2 activation by VEGF-A
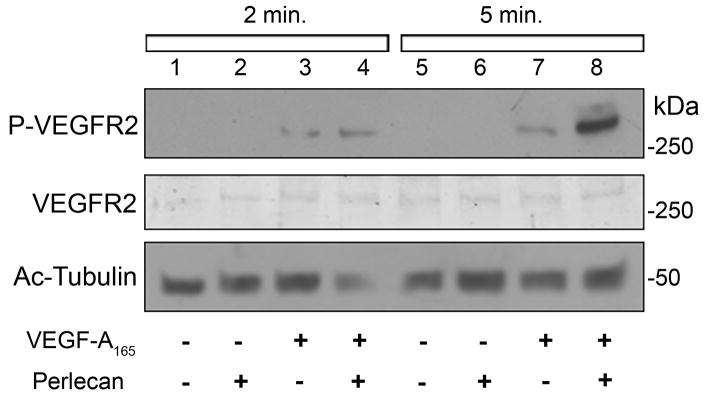
Next, we investigated the influence of a perlecan-VEGF interaction on endothelial cell function vis-à-vis activation of VEGFR2, the major signaling receptor tyrosine kinase which mediates multiple events during the angiogenic cascade in response to VEGF-A ligands (Olsson et al., 2006; Dai and Rabie, 2007). By immunoblotting, we analyzed human endothelial cell VEGFR2 phosphorylation status in response to a 2 min or 5 min stimulation with human perlecan or VEGF-A alone, versus a combination of the two. Interestingly, we found that a pre-incubation of perlecan plus VEGF-A followed by a short application to the endothelial cells evoked stronger VEGFR2 phosphorylation compared to either used alone (Fig. 6). As shown in Fig. 6, a 5-min stimulation using perlecan plus VEGF induced a seven fold increase in VEGFR2 phosphorylation in relation to VEGF stimulation alone. Our results suggest that the interaction between perlecan and VEGF-A promotes VEGFR2 signaling during angiogenesis.
Fig. 6.
Perlecan enhances VEGF-A induced phosphorylation of VEGFR2. Human endothelial cells were exposed to human perlecan, human VEGF-A165 or a combination of both for a 2 min or 5 min stimulation. Total protein lysates were immunoblotted for phospho-VEGFR2 (Y951), total VEGFR2 and acetylated tubulin as a load control. The combination of perlecan plus VEGF-A was capable of enhancing VEGFR2 activation status (lanes 4 and 8) compared to VEGF-A (lanes 3 and 7) or perlecan (lanes 2 and 6) alone. Lanes 1 and 5 represent endothelial cell cultures incubated with only media.
3. Discussion
Our previous work applied the zebrafish animal model to dissect perlecan function. These studies identified a central role for perlecan during muscle and vascular development (Zoeller et al., 2008). Perlecan knockdown largely inhibited angiogenic blood vessel development of the intersegmental vessels, dorsal longitudinal anastomotic vessel and sub-intestinal vessels, while unaffecting vasculogenesis of the axial vessels (dorsal aorta and posterior cardinal vein). The vascular phenotype characterized by perlecan knockdown strikingly resembles knockdown of key components of the VEGF signaling pathway. Morpholino-mediated knockdown of zebrafish VEGF-A (Nasevicius et al., 2000), the major VEGF-A receptor VEGFR2 (Habeck et al., 2002; Covassin et al., 2006), and PLCγ1, the major downstream target of VEGF-VEGFR angiogenic signaling (Lawson et al., 2003), all phenocopy the perlecan morphant vascular phenotype. These observations also suggested perlecan knockdown may interfere with the VEGF-VEGFR signaling cascade during angiogenic blood vessel development. A clear link between perlecan and the VEGF signaling pathway during zebrafish vascular development was established via rescue of the perlecan morphant vascular phenotype with VEGF-A165. Utilizing protein or mRNA, we found VEGF-A165 could partially rescue the abnormal angiogenic sprouting in the perlecan morphant trunk and tail—supporting a link to perlecan function through VEGF.
Our rescue with VEGF-A suggested perlecan functions upstream of VEGFR2. Accordingly, we predicted the heparan sulfate proteoglycan perlecan was capable of interacting with VEGF-A, a known heparin-binding growth factor. We hypothesized that perlecan binding sequesters VEGF and thereby regulates VEGF positional distribution, availability and functional activity through VEGFR during angiogenesis. Using whole-mount immunohistochemistry we found that perlecan and VEGF exhibited largely overlapping expression profiles. Interestingly, we have found that lack of endogenous perlecan resulted in the abnormal accumulation and redistribution of VEGF, supporting a perlecan-VEGF positional role. We predict that these VEGF deposits are largely non-functional, non-utilized growth factors. Interestingly, the abnormal increase in VEGF may also represent a compensatory effect whereby the embryo would try to overcome inhibition of angiogenesis by upregulating a key component of this biological process. Accordingly, hypoxic conditions induce VEGF to promote angiogenesis (Nomura et al., 1995). Along these same lines, we could predict that the embryo alone cannot compensate but an excess of VEGF-A165 mRNA or protein, supplied via rescue, could be possibly sufficient to evoke VEGFR2 activation and ultimately rescue of the phenotype. Intriguingly, the upregulation of VEGF might actually represent a means to induce perlecan synthesis in the perlecan morphant embryos. VEGF-A165 has been identified to increase perlecan expression in human brain microvascular endothelial cells (Kaji et al., 2006).
We confirmed the binding between perlecan and VEGF-A, and defined the interaction as largely mediated via the heparan sulfate side chains. The functional consequences of perlecan-VEGF complexes were characterized as enhancing VEGFR2 phosphorylation status. The role of perlecan HS is supported by heparin-mediated enhanced VEGFR2 phosphorylation as well (Ashikari-Handa et al., 2005). VEGFR2 activation in response to perlecan-VEGF would favor downstream signaling events. Accordingly, endothelial cell migration and proliferation would proceed in support of the angiogenic cascade. Combined these results suggest HSPG perlecan serves as a crucial growth factor-interacting partner which can influence growth factor receptor signaling.
Our results establish perlecan function through the coordinated modulation of VEGFA-VEGFR2 signaling. Combined our in vivo and in vitro data indicate that perlecan binds and localizes VEGF-A in a tissue-specific manner. Furthermore, perlecan/VEGF-A interaction enhances VEGFR2 activity thereby promoting endothelial cell migration and proliferation during vascular development. Perlecan function within this context represents one aspect, out of the multiple and complex events, contributing to developmental angiogenesis.
4. Experimental procedures
4.1. Generation and analysis of the perlecan morphants
Perlecan knockdown was achieved by utilizing a translation blocking morpholino (MO-DI; Gene Tools, LLC) as previously described (Zoeller et al., 2008). All wild-type and vascular transgenic Tg(fli1:egfp)y1 (Lawson and Weinstein, 2002), Tg(fli1a:negfp)y7 (Siekmann and Lawson, 2007), Tg(vegfr2:g-rcfp) (Cross et al., 2003) embryos, were housed in the zebrafish facility of Thomas Jefferson University, cared for according to standard practice and imaged on the platforms previously described (Zoeller et al., 2008).
4.2. Analysis of zebrafish VEGF-A protein levels
For immunoblotting: Total protein was extracted in RIPA buffer from pooled 2 dpf perlecan morphant and matched control embryos. A portion of the lysate was subjected to standard SDS-PAGE and transfer to nitrocellulose membranes. Immunoblotting was performed using anti-VEGF (A-20:sc-152, Santa Cruz) or anti-acetylated tubulin (T7451, Sigma), followed by donkey anti-rabbit HRP (GE Healthcare) or goat anti-mouse HRP (Pierce) and detection by ECL (Pierce). For immunohistochemistry: Whole-mount immunostaining was performed on groups of morphant and matched control 2–3 dpf embryos using anti-VEGF (A-20:sc-152, Santa Cruz) as described previously (Zoeller et al., 2008). Cross sections were prepared by standard cryosection, immunostaining was performed by blocking in 5% FBS, followed by incubation with anti-VEGF (A-20:sc-152, Santa Cruz) and detection by anti-rabbit Rhodamine, both in 1% FBS.
4.3. Rescue with VEGF-A165 protein or RNA
Vascular transgenic 1-cell stage embryos were microinjected with either MO-DI or VEGF-A165 alone, or a combination of the two in a dose equal to the injection of either component alone. Recombinant human VEGF-A165 (293-VE/CF, R&D Systems) was used similar to previous applications (Ma et al., 2007; Serbedzija et al., 2000). Zebrafish VEGF-A165 mRNA (pCS2:vegf165 kindly provided by N. Lawson, (Lawson et al., 2002)) was prepared by in vitro transcription using SP6 mMESSAGE mMACHINE (Ambion). Rescue was assessed at 2 dpf by analyzing the embryos’ gross and vascular phenotype.
4.4. Protein-protein interaction by perlecan overlay assay
Human perlecan was immunoaffinity purified from the secretions of human coronary arterial endothelial cells using an affinity column containing a monoclonal antibody against domain III of perlecan protein core (Murdoch et al., 1994) using protocols described before (Whitelock et al., 1999; Whitelock and Iozzo, 2002). Recombinant human VEGF-A165 (293-VE/CF, R&D Systems), recombinant human FGF-2 or progranulin (Gonzalez et al., 2003) were spotted by slot blot onto nitrocellulose membranes. Membranes were briefly washed in PBS followed by blocking for 30 min. in 5% milk. Membranes were overlaid with human coronary artery endothelial cell (HCAEC) derived perlecan (Whitelock and Iozzo, 2002; Whitelock et al., 1999) for ~4 h in PBS at room temperature. Membranes were briefly washed in PBS prior to detection of a positive binding interaction by standard immunoblotting using anti-perlecan/domain V (Bix et al., 2004), donkey anti-rabbit HRP (GE Healthcare) and ECL (Pierce). For heparin competition experiments, blots were pre-incubated with excess heparin (10 μg/ml) for 1 h prior to similar overlay (~2 h) and detection as described above.
4.5. Analysis of endothelial cell VEGFR2 activation
Early passage HUVEC were grown to confluency in complete media according to standard protocol. Monolayers were stimulated for 2 min. or 5 min. with either HCAEC perlecan (~1 μg/ml) or human recombinant VEGF-A165 (10 ng/ml; 293-VE/CF, R&D Systems) alone or a combination of the two (~1 μg/ml perlecan plus 10 ng/ml VEGF-A165, pre-incubated for 1 h at room temperature). Total cell lysates were extracted in RIPA buffer and subjected to standard SDS-PAGE and transfer to nitrocellulose membranes. Immunoblotting was performed using phospho-VEGFR2 (Y951) antibody (2471, Cell Signaling), anti-FLK-1/VEGFR2 (C-1158:sc-504, Santa Cruz) or anti-acetylated tubulin (T7451, Sigma), followed by donkey anti-rabbit HRP (GE Healthcare), anti-rabbit IRDye 800CW (Li-COR) or goat anti-mouse HRP (Pierce) respectively.
4.6 Statistical analysis
Experiments were run in triplicates and were statistically analyzed by Student’s t test using Sigma Stat 10.0 (SPSS). Differences were considered significant at P <0.05.
Supplementary Material
Fig. S1. Perlecan knockdown inhibits developmental angiogenesis. Vascular analysis of the perlecan morphant phenotype at 1.5 (A) and 2 (B) dpf in Tg(vegfr2:g-rcfp) embryos. (B′) and (B″) represent higher magnification views of the boxed regions in (B). Notice the significant inhibition of ISV (intersegmental vessel) and DLAV (dorsal longitudinal anastomotic vessel) formation. Scale bars, 350 μm (A, B) and 150 μm (B′, B″).
Fig. S2. Protein extracted from 2dpf control (A, n = 26) and MO-DI (B, n = 26) embryos was assayed for VEGF expression levels by immunoblotting with anti-VEGF antibody and anti-acetylated tubulin as a load control (Fig. 3D). Scale bars, 1.5 mm.
Fig. S3. Zebrafish VEGF-A165 mRNA can rescue the morphant phenotype induced by MO-DI perlecan knockdown. (A) Representative agarose gel electrophoresis analysis of a zebrafish VEGF-A165 mRNA preparation. RNA was prepared by in vitro transcription using SP6 and the pCS2:vegfa165 plasmid. Lane 1, 1-kb ladder; lane 2, NotI linearized plasmid; lane 3, plasmid DNA plus transcribed RNA; lane 4, plus DNase treatment; lane 5, LiCl concentrated RNA; lane 6, 100-bp ladder. (B) Summary of two separate rescue experiments using VEGF-A165 mRNA (n = 10 MO-DI and n = 22 Rescue embryos per group; *P<0.05). (C) Vascular analysis of MO-DI injected embryos revealed partial sprouting of the ISVs throughout the trunk and tail. (D) Microinjection of zebrafish VEGF-A165 mRNA was capable of rescuing the partial sprouting of the ISVs. Scale bars, 1.35 mm.
Acknowledgments
We thank N. Lawson for providing valuable reagents and S-Y. Ho for expert advice. This work was supported in part by NIH grants RO1 CA39481, RO1 CA47282 and RO1 CA120975 (to R.V. Iozzo), and by a grant from the Mizutani Foundation for Glycoscience (to R.V. Iozzo). J.J. Zoeller was supported by National Research Service Award Training Grant T32 AA07463. This work is a part fulfillment for a doctoral thesis in Cell and Developmental Biology for J.J. Zoeller.
Footnotes
Abbreviations: FGF, fibroblast growth factor; TGF, transforming growth factor; Hh, hedgehog; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; Shh, sonic hedgehog; GAG, glycosaminoglycan; HS, heparan sulfate; HS6ST, heparan sulfate 6-O sulfotransferase; HSPG, heparan sulfate proteoglycan; VEGFR2, vascular endothelial growth factor receptor 2; ISV, intersegmental vessel; DLAV, dorsal longitudinal anastomotic vessel; SIV, sub-intestinal vessel; DA, dorsal aorta; PCV, posterior caudal vein; DIC, differential interference contrast; PLCγ1, phospholipase C gamma-1; HCAEC, human coronary artery endothelial cell; HUVEC, human umbilical vein endothelial cell.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arikawa-Hirasawa E, Watanabe E, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nature Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Ashikari-Handa S, Habuchi H, Kariya Y, Kimata K. Heparin regulates vascular endothelial growth factor 165-dependent mitogenic activity, tube formation, and its receptor phopshorylation of human endothelial cells. J Biol Chem. 2005;280:31508–31515. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Aviezer D, Iozzo RV, Noonan DM, Yayon A. Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol Cell Biol. 1997;17:1938–1946. doi: 10.1128/mcb.17.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bink RJ, Habuchi H, Lele Z, Dolk E, Joore J, Rauch GJ, Geisler R, Wilson SW, Den Hertog J, Kimata K, Zivkovic D. Heparan sulfate 6-O-sulfotransferase is essential for muscle development in zebrafish. J Biol Chem. 2003;278:31118–31127. doi: 10.1074/jbc.M213124200. [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Höök M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Cadwallader AB, Yost HJ. Combinatorial expression patterns of heparan sulfate sulfotransferases in zebrafish: II. The 6-0-sulfotransferase family. Develop Dyn. 2006a;235:3432–3437. doi: 10.1002/dvdy.20990. [DOI] [PubMed] [Google Scholar]
- Cadwallader AB, Yost HJ. Combinatorial expression patterns of heparan sulfate sulfotransferases in zebrafish: I. The3-0-sulfotransferase family. Develop Dyn. 2006b;235:3423–3431. doi: 10.1002/dvdy.20991. [DOI] [PubMed] [Google Scholar]
- Cadwallader AB, Yost HJ. Combinatorial expression patterns of heparan sulfate sulfotransferases in zebrafish: III.2-0-sulfotransferase and C5-epimerases. Develop Dyn. 2007;236:581–586. doi: 10.1002/dvdy.21051. [DOI] [PubMed] [Google Scholar]
- Carson DD, Tang JP, Julian J. Heparan sulfate proteoglycan (perlecan) expression by mouse embryos during acquisition of attachment competence. Dev Biol. 1993;155:97–106. doi: 10.1006/dbio.1993.1010. [DOI] [PubMed] [Google Scholar]
- Chen E, Stringer SE, Rusch MA, Selleck SB, Ekker SC. A unique role for 6-O sulfation modification in zebrafish vascular development. Dev Biol. 2005;284:364–367. doi: 10.1016/j.ydbio.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Cohen IR, Murdoch AD, Naso MF, Marchetti D, Berd D, Iozzo RV. Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 1994;54:5771–5774. [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross LM, Cook MA, Lin S, Chen JN, Rubinstein AL. Rapid analysis of angiogenic drugs in a live fluorescent zebrafish assay. Arterioscler Thromb Vasc Biol. 2003;23:911–912. doi: 10.1161/01.ATV.0000068685.72914.7E. [DOI] [PubMed] [Google Scholar]
- Dai J, Rabie ABM. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, DeGueme AM, Dhir R, Shah RB, Farach-Carson C, Barrett A, Datta S. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol Cancer. 2006a;5:9. doi: 10.1186/1476-4598-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Pierce M, Datta MW. Perlecan signaling: Helping hedgehog stimulate prostate cancer growth. Int J Biochem Cell Biol. 2006b;38:1855–1861. doi: 10.1016/j.biocel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC, Carson DD. Perlecan - a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- French MM, Gomes RR, Jr, Timpl R, Höök M, Czymmek K, Farach-Carson MC, Carson DD. Chondrogenic activity of the human heparan sulfate proteoglycan perlecan maps to the N-terminal domain I. J Bone Miner Res. 2002;17:48–55. doi: 10.1359/jbmr.2002.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki I, Iozzo RV, Williams KJ. Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J Biol Chem. 2000;275:25742–25750. doi: 10.1074/jbc.M909173199. [DOI] [PubMed] [Google Scholar]
- Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol. 2007;7:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: Potential effects on tumor growth. J Biol Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- Habeck H, Odenthal J, Walderich B, Maischein HM, Schulte-Merker S. Analysis of zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12:1405–1412. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210:130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Yamada Y, Arikawa-Hirasawa E. Role of perlecan in skeletal development and diseases. Glycoconj J. 2003;19:263–267. doi: 10.1023/A:1025340215261. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe M, Kalén M, Gerhardt H, Betsholtz C. DII4 signalling through notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Perlecan: a gem of a proteoglycan. Matrix Biol. 1994;14:203–208. doi: 10.1016/0945-053x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nature Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grässel S, Murdoch AD. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302:625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Murdoch AD. Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. FASEB J. 1996;10:598–614. [PubMed] [Google Scholar]
- Iozzo RV, Pillarisetti J, Sharma B, Murdoch AD, Danielson KG, Uitto J, Mauviel A. Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming factor-β via a nuclear factor 1-binding element. J Biol Chem. 1997;272:5219–5228. doi: 10.1074/jbc.272.8.5219. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae JO, Huang J, Fisher J, Lefkowitch JH, Feirt N, Rudge J, Holash J, Yancopoulos GD, Kandel JJ, Yamashiro DJ. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- Kaji T, Yamamoto C, Oh-i M, Fujiwara Y, Yamazaki Y, Morita T, Plaas AH, Wight TN. The vascular endothelial growth factor VEGF165 induces perlecan synthesis via VEGF receptor-2 in cultured human brain microvascular endothelial cells. Biochem Biophys Acta. 2006;1760:1465–1474. doi: 10.1016/j.bbagen.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Tran PK, Weiser-Evens MCM, Reidy M, Majack RA, Wight TN. Changes in perlecan expression during vascular injury. Role in the inhibition of smooth muscle cell proliferation in the late lesion. Arterioscler Thromb Vasc Biol. 2003;23:608–614. doi: 10.1161/01.ATV.0000063109.94810.EE. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascualr endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the notch ligand delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Hillman PR, Barrett AL, Jackson MC, Perry TL, Park Y, Datta S. The drosophila perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev Biol. 2007;7:121. doi: 10.1186/1471-213X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ACH, Lin R, Chan PK, Leung JCK, Chan LYY, Meng A, Verfaillie CM, Liang R, Leung AYH. The role of survivin in angiogenesis during zebrafish embryonic development. BMC Dev Biol. 2007;7:50. doi: 10.1186/1471-213X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak M, Yenisey C, Grant DS, Sharma B, Iozzo RV. A role for perlecan in the suppression of growth and invasion in fibrosarcoma cells. Cancer Res. 1997;57:2130–2136. [PubMed] [Google Scholar]
- Merz DC, Alves G, Kawano T, Zheng H, Culotti JG. UNC-52/perlecan affects gonadal leader cell migrations in C. elegans hermaphrodites through alterations in growth factor signaling. Dev Biol. 2003;256:173–186. doi: 10.1016/s0012-1606(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Mullen GP, Rogalski TM, Bush JA, Gorji PR, Moerman DG. Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol Biol Cell. 1999;10:3205–3221. doi: 10.1091/mbc.10.10.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- Nakato H, Kimata K. Heparan sulfate fine structure and specificity of proteoglycan functions. Biochim Biophys Acta. 2002;1573:312–318. doi: 10.1016/s0304-4165(02)00398-7. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270:28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claeson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Ono K, Hattori H, Takeshita S, Kurita A, Ishihara M. Structural features in heparin that interact with VEGF165 and modulate its biological activity. Glycobiology. 1999;9:705–711. doi: 10.1093/glycob/9.7.705. [DOI] [PubMed] [Google Scholar]
- Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, Welsh CJR, McDermott S, Datta S. Drosophila perlecan modulates FGF and Hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Cell signaling events: a view from the matrix. Matrix Biol. 2003;22:101–107. doi: 10.1016/s0945-053x(03)00002-7. [DOI] [PubMed] [Google Scholar]
- Rogalski TM, Williams BD, Mullen GP, Moerman DG. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 1993;7:1471–1484. doi: 10.1101/gad.7.8.1471. [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoré C, Zhang C, Muir C, Liu R, Wyrwa J, Shu J, Zhau HE, Chung LW, Carson DD, Farach-Carson MC. Perlecan knockdown in metastatic prostate cancer cells reduces heparin-binding growth factor responses in vitro and tumor growth in vivo. Clin Exp Metastasis. 2005;22:377–390. doi: 10.1007/s10585-005-2339-3. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Flynn E, Willett CE. Zebrafish angiogenesis: a new model for drug screening. Angiogenesis. 2000;3:353–359. doi: 10.1023/a:1026598300052. [DOI] [PubMed] [Google Scholar]
- Sharma B, Handler M, Eichstetter I, Whitelock J, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102:1599–1608. doi: 10.1172/JCI3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, Ron D. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem. 2006;281:5178–5187. doi: 10.1074/jbc.M509500200. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Smith SML, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol. 2007;26:175–184. doi: 10.1016/j.matbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci. 1995;108:2663–2672. doi: 10.1242/jcs.108.7.2663. [DOI] [PubMed] [Google Scholar]
- Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ Res. 2004;94:550–558. doi: 10.1161/01.RES.0000117772.86853.34. [DOI] [PubMed] [Google Scholar]
- Tran-Lundmark K, Tran PK, Paulsson-Berne G, Fridén V, Soinen R, Tryggvason K, Wight TN, Kinsella MG, Borén J, Hedin U. Heparan sulfate in perlecan promotes mouse atherosclerosis. Roles of lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res. 2008;103:43–52. doi: 10.1161/CIRCRESAHA.108.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Iozzo RV. Isolation and purification of proteoglycans. In: Adams JC, editor. Methods in Cell-Matrix Adhesion. San Diego: Academic Press; 2002. pp. 53–67. [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J Biol Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- Wight TN, Kinsella MG, Qwarnström EA. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol. 1992;4:793–801. doi: 10.1016/0955-0674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- Zoeller JJ, McQuillan A, Whitelock J, Ho SY, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Perlecan knockdown inhibits developmental angiogenesis. Vascular analysis of the perlecan morphant phenotype at 1.5 (A) and 2 (B) dpf in Tg(vegfr2:g-rcfp) embryos. (B′) and (B″) represent higher magnification views of the boxed regions in (B). Notice the significant inhibition of ISV (intersegmental vessel) and DLAV (dorsal longitudinal anastomotic vessel) formation. Scale bars, 350 μm (A, B) and 150 μm (B′, B″).
Fig. S2. Protein extracted from 2dpf control (A, n = 26) and MO-DI (B, n = 26) embryos was assayed for VEGF expression levels by immunoblotting with anti-VEGF antibody and anti-acetylated tubulin as a load control (Fig. 3D). Scale bars, 1.5 mm.
Fig. S3. Zebrafish VEGF-A165 mRNA can rescue the morphant phenotype induced by MO-DI perlecan knockdown. (A) Representative agarose gel electrophoresis analysis of a zebrafish VEGF-A165 mRNA preparation. RNA was prepared by in vitro transcription using SP6 and the pCS2:vegfa165 plasmid. Lane 1, 1-kb ladder; lane 2, NotI linearized plasmid; lane 3, plasmid DNA plus transcribed RNA; lane 4, plus DNase treatment; lane 5, LiCl concentrated RNA; lane 6, 100-bp ladder. (B) Summary of two separate rescue experiments using VEGF-A165 mRNA (n = 10 MO-DI and n = 22 Rescue embryos per group; *P<0.05). (C) Vascular analysis of MO-DI injected embryos revealed partial sprouting of the ISVs throughout the trunk and tail. (D) Microinjection of zebrafish VEGF-A165 mRNA was capable of rescuing the partial sprouting of the ISVs. Scale bars, 1.35 mm.