Abstract
Projection neurons and interneurons populate the cerebral cortex in a layer-specific manner. Here, we studied the role of Cyclin-dependent kinase 5 (Cdk5) and its activator p35 in cortical interneuron migration and disposition in the cortex. We found that mice lacking p35 (p35−/−) show accumulation of interneurons in the upper part of the cortex. We also observed an inverted distribution of both early- and late-born interneurons, with the former showing a preference for the upper and the latter for the lower aspects of the cortex. We investigated the causes of the altered laminar organization of interneurons in p35−/− mice and found a cell-autonomous delay in their tangential migration that may prevent them from reaching correct positions. Incomplete splitting of the preplate in p35−/− mice, which causes accumulation of cells in the superficial layer and defects in the “inward” and “outward” components of their radial movement, may also account for the altered final arrangement of interneurons. We, therefore, propose that p35/Cdk5 plays a key role in guiding cortical interneurons to their final positions in the cortex.
Keywords: cortex, development, layering, preplate layer, tangential migration
Introduction
Cortical interneurons are vital for the proper function of the cerebral cortex. Abnormalities in their development could lead to serious disorders such as schizophrenia, epilepsy, and mental retardation (Nakajima 2007). Cortical interneurons originate in the ventral telencephalon and populate the cortex in a layer-specific manner (Marin and Rubenstein 2003; Metin et al. 2006). Initially, they migrate tangentially into the developing cortex in defined streams through the preplate (PPL) and intermediate zone (IZ) and, after the splitting of the PPL, through layer I (LI), subplate (SP), and subventricular zone (SVZ).
Little is known about the way interneurons become incorporated into the developing cortical plate (CP). They first spread tangentially throughout the cortex, moving in all directions within their streams (Ang et al. 2003; Tanaka et al. 2006). Once they reach the area in which they are going to reside, they migrate radially, inward and outward, from the tangential streams toward the CP in search of the correct layer (Polleux et al. 2002; Ang et al. 2003; Tanaka et al. 2003, 2006; Hevner et al. 2004). Most interneurons settle in the developing cortex in an “inside-out” manner and coexist in a given layer with projection neurons born at the same time (Miller 1985; Fairen et al. 1986; Valcanis and Tan 2003). However, it has not yet been fully established when these cells receive laminar position information: is it at the time of birth, during their tangential migratory journey, or when moving radially toward their final positions in the CP? Furthermore, the signals that lead these cells to settle in the “correct” layer remain unknown.
Two signaling pathways are thought to control the positioning of neurons and thus affect the formation of cortical layers: Reelin and Cdk5. Reelin is an extracellular matrix protein secreted mainly by Cajal–Retzius neurons in PPL/LI (D'Arcangelo et al. 1995; Ogawa et al. 1995). Mutation in the reelin gene results in the reeler phenotype, which is characterized by lack of PPL splitting and abnormal cortical lamination (Caviness 1982; Tissir and Goffinet 2003). Cdk5, a proline-directed serine/threonine kinase, phosphorylates proteins associated with the cytoskeleton and thus plays a role in neuronal migration and morphogenesis (Dhavan and Tsai 2001). p35 Is the primary Cdk5 activator expressed during corticogenesis (Tsai et al. 1994), and p35−/− and Cdk5−/− mice display improper PPL splitting and aberrant cortical lamination (Ohshima et al. 1996; Chae et al. 1997; Gilmore et al. 1998; Rakic et al. 2006).
Recent studies have proposed a Reelin- (Hevner et al. 2004; Pla et al. 2006) and p35/Cdk5-independent (Gilmore and Herrup 2001; Patzke et al. 2003; Hammond et al. 2004, 2006) mechanism for the laminar organization of cortical interneurons. However, the migration, morphology, and distribution of interneurons in embryonic p35−/− and Cdk5−/− mice have not been examined extensively. We present here new evidence indicating that Cdk5 signaling plays a key role in interneuron migration.
Materials and Methods
Animals
p35- (provided by Dr Li-Huei Tsai, MIT; Chae et al. 1997) and Cdk5-knockout mice (The Jackson Laboratory; Ohshima et al. 1996), as well as Glutamic acid decarboxylase 67-green fluorescence protein (GAD67-GFP) (Δneo) mice (Tamamaki et al. 2003), maintained in C57/Black6 background, were used in this study. Brains from reeler litters (Orleans allele), maintained in CD1 background, were kindly provided by Dr Andre Goffinet (University of Louvain, Belgium). Midday of the day of vaginal plug formation was considered as embryonic (E) day 0.5 and the day of birth as postnatal (P) day 0. Pregnant dams were culled by cervical dislocation. Embryos were dissected in cold artificial cerebral spinal fluid. Brains were removed and either fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 2–6 h or used for preparation of tissue cultures and protein isolation. Postnatal mice were anesthetized either by hypothermia (P0 and P2) or by using halothane (P12) and transcardially perfused with PFA, followed by a 6-h postfixation in PFA. All procedures were performed under license and in accordance to the regulations of the UK Home Office and the University College London Animal Ethics Committee.
Bromodeoxyuridine Injections
Bromodeoxyuridine (BrdU), a marker of dividing cells during S phase, was administered intraperitoneally (50 μg/g of body weight; Sigma, Gillingham, UK) into timed-pregnant mice at E13.5 and E15.5 or at E12.5, E13.5, and E15.5, and the distribution of BrdU+ cells was examined at P12 or E18.5, respectively.
Dissociated Cell Cultures
The cortex and medial ganglionic eminence (MGE) were dissected from embryonic mouse brains and incubated in 0.05% trypsin with 100 μg/mL DNaseI in Neurobasal medium (Invitrogen, Paisley, UK) at 37 °C for 15 min. Trypsinization was quenched by addition of Neurobasal medium containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen) at 37 °C for 5 min and the cells resuspended in growth media (Neurobasal medium, 2 mM L-glutamine, 50 units/mL penicillin, and 50 μg/mL streptomycin, with 1:50 dilution of B27; Invitrogen). Cells were plated out on 13-mm poly-L-lysine:laminin (10:10 μg/mL; Sigma) coverslips at a density of 1–2 × 106 cells per mL and incubated at 37 °C with 5% CO2. After 1 or 3 days in vitro, cells were fixed in PFA for 20 min.
Immunohistochemistry
Embryonic, P0, and P2 brains were cryoprotected in 30% sucrose in PBS, frozen in Tissue-Tek® O.C.T™ Compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), and sectioned using a cryostat (20 μm; Bright Instruments, Huntingdon, UK). P12 brains were snap frozen on dry ice and sectioned by freezing microtome (40 μm). Sections were treated with 0.3% H2O2 in PBS, for 20 min at room temperature (RT), followed by 3 washes in PBS, blocked in PBS containing 5% normal goat serum (Vector Laboratories, Burlingame, CA) and 0.5% Triton X-100 (Sigma), for 1 h at RT, and subsequently incubated with primary antibodies, diluted in blocking solution at RT overnight. Sections were subsequently processed with immunoperoxidase or immunofluorescence methods. For the immunoperoxidase method, sections were washed in PBS and incubated with biotinylated secondary antibodies at RT for 30 min. They were processed using the avidin-biotin-horseradish peroxidase complex (ABC–HRP kit, 1/100 dilution; Vector Laboratories) at RT for 1 h. Peroxidase enzyme activity was revealed and sections visualized using 0.005% 3,3′-diaminobenzidine tetrachloride (DAB; Sigma) and 0.005% H2O2 as substrate. Sections were rinsed, dehydrated, and mounted in Di-n-butylphthalate in Xylene (DPX; VWR International, Lutterworth, UK). For immunofluorescence, incubation with fluorescence-conjugated secondary antibodies was carried out for 2 h at RT, after which the samples were washed in PBS, and mounted in CytoFluor solution (Agar, Essex, UK) containing 50% glycerol in PBS.
For BrdU staining, sections were treated with 2 M HCl for 45 min, followed by 30-min treatment with 0.1 M boric acid, pH 8.5, prior to standard immunohistochemical procedures.
Antibodies
The following primary antibodies were used: mouse monoclonal anti-BrdU (1:100, clone BU5.1; Progen, Heidelberg, Germany), rabbit polyclonal anti-calbindin (CB; 1:2000; Swant, Bellinzona, Switzerland), rabbit polyclonal anti-calretinin (CalR; 1:1000; Swant), rabbit polyclonal anti-γ-aminobutyric acid (GABA) (1:1000; Sigma), mouse monoclonal parvalbumin (PV; 1:250; Swant), rabbit polyclonal anti-somatostatin (Sst; 1:100; Chemicon, Temecula, CA), rabbit polyclonal anti-p35 (1:100, C-19; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal anti-Cdk5 (1:10; clone DC27; provided by Dr Li-Huei Tsai, MIT), and rabbit polyclonal anti-LHX6 (1:100; provided by Dr Vassilis Pachnis, National Institute for Medical Research, UK). The following secondary antibodies were used: biotinylated anti-rabbit or anti-mouse immunoglobulin G (IgG) (1:200; Vector Laboratories), Alexa 488–conjugated anti-rabbit or anti-mouse IgG (1:400; Invitrogen), Alexa 568–conjugated anti-rabbit or anti-mouse IgG (1:400; Invitrogen).
Nuclear Staining
Sections were stained either with 0.025% thionin solution (Nissl staining; Sigma) or with 2.5 μg/mL bisbenzimide (Sigma).
Quantification
To estimate the proportion of Cdk5+ and GABA+ cells in primary neuronal cultures, the images of 3 fields from each coverslip were collected using a confocal microscope. Four coverslips per genotype from 3 experiments were analyzed. The results were expressed as mean percentage ± standard error of the mean (SEM). For quantification of GFP+ and immunopositive cells in postnatal mice, a radial column of somatosensory cortex, 100 μm wide, was divided into 10 equal bins, and labeled cells were counted in each bin. In embryonic mice, immunopositive cells were counted in each of the developing layers of dorsal cortex (radial column, 100 μm wide). The relative number of cells was expressed as mean percentage ± SEM of the total number of labeled cells in all bins or layers. For measurements of cortical thickness, Nissl-stained sections of developing dorsal cortex (radial column, 100 μm wide) were used. Layer thickness was calculated as a percentage of the entire cortical thickness and expressed as mean percentage ± SEM. At least 6 sections from a minimum of 3 brains from each genotype were used for quantitative analyses, and littermates were examined whenever possible. Statistical significance of the results was calculated by the Chi-square (χ2) test and the Student's t-test (Microsoft Excel).
Protein Isolation
Mouse ganglionic eminence (GE) and cortices were lysed in prechilled lysis buffer (25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, pH 8.0, 1% Triton X-100, and 10% glycerol; Sigma) containing protease (phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin; Sigma) and phosphatase inhibitors (sodium orthovanadate and sodium fluoride; Sigma). The cell suspension was centrifuged at 13 000 rpm at 4 °C for 8 min, and the supernatant was collected for kinase assay.
In Vitro Kinase Assay
Cdk5 was isolated from cell lysates by immunoprecipitation (Tsai et al. 1994). In brief, 10 μL of rabbit anti-Cdk5 antibody (C-8; Santa Cruz Biotechnology) was incubated with 200 μL of lysates at 4 °C for 1 h. Protein G sepharose beads (100 μL) (GE Healthcare, Chalfont St Giles, UK), prepared as a 10% solution in lysis buffer, were added, and the suspension was incubated at 4 °C for another hour. Immunoprecipitates were washed with lysis buffer and kinase buffer (30 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES], pH 7.2, 10 mM MgCl2, 5 mM MnCl2, 1 mM dithiothreitol; Sigma). Reactions were performed in kinase buffer containing 2 μg of histone H1 (Roche Diagnostics, Burgess Hill, UK) and 1 μCi(32P)γATP (GE Healthcare) for 30 min at RT, stopped with 2× sample buffer, and processed for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and autoradiography.
Chemomigration Assay
The MGEs were removed from embryos at E13.5 and mechanically dissociated as described above. The cells were pelleted by centrifugation at 1000 × g for 3 min and resuspended in growth medium without antibiotics. Migration of MGE-derived cells was studied using a 48-well Boyden chamber as described by Cariboni et al. (2006). Poly-L-lysine/laminin (10:10 μg/mL) precoated filters containing 10-μm pores were used to separate the upper from the lower compartments of the chamber. For chemotaxis, Neurobasal medium with 10% FBS was added in the lower compartment, and a 2 × 106 cells per mL suspension was placed into the upper compartment of each well. After overnight incubation (up to 16 h) at 37 °C in a 5% CO2-humidified incubator, the cells that had migrated through the filter pores were fixed in PFA (for 5 min) and stained using the Diff-Quick stain kit (Biomap, Milan, Italy). Three fields were imaged per well and the number of cells that had migrated counted in each field. Results from 4 experiments were expressed as mean number of cells per well ± SEM, and significance was calculated by the Student's t-test.
Electroporation of Forebrain Slices
Forebrain slice cultures, obtained from E13.5 mice, were prepared as described previously (Alifragis et al. 2004). Coronal slices (300 μm) were mounted onto nitrocellulose filters (0.45 μm; Millipore, Watford, UK) and used for electroporation experiments. The following plasmids were applied: pCAG-IRES-EGFP (a gift from Dr Mikio Hoshino, Kyoto University, Japan, and Dr Jun-ichi Miyazaki, Osaka University Medical School, Japan) and pCMVneo-p35 (a gift from Dr Li-Huei Tsai, MIT). DNA (50 nL of 2 μg/μL plasmid) was injected focally into the MGE using a microinjector (Picospritzerr II, IntraCel, Frederick, MD) through a glass micropipette (Drummond Scientific, Broomall, PA). Square pulses were generated using an electroporator (Electrosquare porator; ECM 830 BTX, San Diego, CA). The following electroporation conditions were used: voltage pulse of 70 V, 3 pulses with duration of 5 ms, and 100-ms interval between pulses. Sections were subsequently incubated in growth medium at 37 °C in a 5% CO2-humidified incubator for at least 48 h. The medium comprised Dulbecco's modified Eagle medium (DMEM-F12 containing HEPES, NaHCO3, and phenol-red; Sigma), 5% FBS (Invitrogen), 1× N2 supplement (Invitrogen), 100 μM L-glutamine (Invitrogen), 2.4 g/L D-glucose (Sigma), 50 units penicillin per mL, and 50 μg/mL streptomycin (Invitrogen). Slices were fixed in PFA at 4 °C overnight.
Quantification of Cell Branching
CB+ neurons, with clearly visible neurites from immunostained 20-μm-thick forebrain sections, were drawn using a camera lucida attached to a microscope. GFP-expressing neurons, from electroporated forebrain slices, were photographed with a confocal microscope. The number of branch points of cells that had migrated into the cortex was counted using MetaMorph software (Universal Imaging Corporation, West Chester, PA) and results expressed either as a mean number of branch points per cell ± SEM (CB+) or as a percentage of the control experiment (GFP+).
Digital Image Capture and Analysis
DAB- and Nissl-stained samples were imaged using a Leica DM microscope and Leica DC 500 digital camera. Immunofluorescent samples were photographed using a Leica DM microscope or a Leica TCS SP2 confocal laser scanning microscope, where appropriate. A sequential series of images was compared with those from a single optical plane for the assessment of antigen colocalization. The confocal images were reconstructed using MetaMorph software (Universal Imaging Corporation). All images were finally processed using Photoshop CS2 software (Adobe).
Results
Abnormal Number and Laminar Distribution of Interneurons in p35−/− Mice
To confirm that p35 plays a role in cortical neuronal lamination, we injected BrdU into pregnant p35+/+ and p35−/− mice at E13.5 and E15.5 in order to mark early- and late-born cortical neurons, respectively. Control sections at P12 confirmed the well-established pattern of corticogenesis, with early-born neurons present in the lower layers and late-born cells distributed superficially (Supplementary Fig. 1A,C). In contrast, and as shown previously, early-born cells in p35−/− mice populated mostly the upper part of the cortex, whereas late-born neurons were present predominantly in the lower aspects of the cortex and in a thin band located superficially within and below LI (Supplementary Fig. 1B,D) (Chae et al. 1997; Rakic et al. 2006).
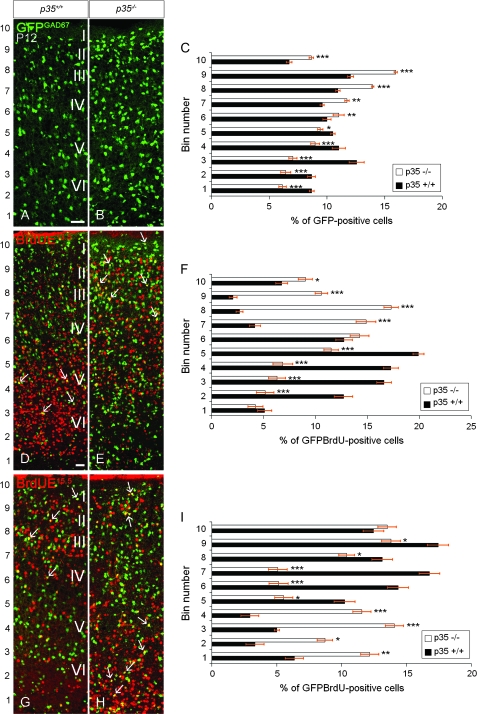
In order to establish whether lack of p35 affects the laminar distribution of interneurons, we utilized GAD67-GFP mice (GAD67GFP/+GAD67GFP/+ mice), where GFP expression is driven by the endogenous GAD67 promoter and consequently labels most interneurons (Tanaka et al. 2006). At P12, when all interneurons should have ceased migrating (Ang et al. 2003; Hevner et al. 2004), GFP+ cells in p35+/+GAD67GFP/+ mice were found uniformly distributed in the cortex with a slight predominance in layers II–III and V (Fig. 1A; bins 8, 9 and 3, 4, Fig. 1C). In contrast, in age-matched p35−/−GAD67GFP/+ mice, increased accumulation of GFP+ cells was observed in the upper portion of the cortex (Fig. 1B; bins 6–10, Fig. 1C).
Figure 1.
The laminar arrangement of cortical interneurons is altered in p35−/− mice. Coronal sections of P12 somatosensory cortex, showing expression of GFP (under GAD67 promoter; A, B), and BrdU labeling. (C, F, I) Binned quantification of the distribution of GFP+ and GFP+/BrdU+ cells (mean percentage ± SEM); bin1 closest to the lateral ventricle. In both genotypes, GFP+ cells are distributed throughout the cortex, but in p35−/− mice, they are more dense in the upper cortex with increased accumulation in LI compared with controls (A, B). The relative distribution of GFP+ cells in the cortex is shown in (C). In p35+/+ mice, early-born (E13.5) interneurons occupy the lower layers (D, arrows), whereas they acquire mostly superficial positions, including LI, in p35−/− mice (E, arrows). The relative distribution of GFP+/BrdU+ (E13.5) cells in the cortex is shown in (F). In p35+/+ mice, late-born (E15.5) interneurons are confined to the upper cortical layers (G, arrows). In contrast, in p35−/− mice, these cells are found mostly in lower aspect of the cortex. Some cells accumulated superficially within and below LI (H, arrows). The relative distribution of GFP+/BrdU+ (E15.5) cells in the cortex is shown in (I). Error bars represent SEM. A significant difference in layer distribution of GFP+ (P < 0.005, χ2 test) and GFP+/BrdU+ (E13.5, P < 0.001; E15.5, P < 0.001; χ2 test) cells in the cortex is observed between p35+/+ and p35−/− mice. Bin comparison is done by Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.005). Roman numerals designate mature neocortical layers. Scale bar, 100 μm (A, B, D, E, G, H).
It is widely thought that cortical interneurons are generated in an inside-out spatiotemporal gradient (Miller 1985; Fairen et al. 1986; Valcanis and Tan 2003). To confirm these findings, we performed birthdating experiments by injecting BrdU at E13.5 and E15.5 in GAD67-GFP mice. We found that in p35+/+GAD67GFP/+ animals, at P12, most early-born (E13.5) GFP+/BrdU+ cells populated the lower layers of the cortex (Fig. 1D; bins 2–6, Fig. 1F). In contrast, early-born neurons in p35−/−GAD67GFP/+ mice were enriched in the upper part of the cortex (Fig. 1E; bins 6–10, Fig. 1F). In normal p35+/+GAD67GFP/+ mice, late-born (E15.5) GFP+/BrdU+ cells settled superficially in the cortex (Fig. 1G; bins 5–10, Fig. 1I) in contrast to interneurons in age-matched p35−/−GAD67GFP/+ mice that mostly populated the lower aspect of the cortex (Fig. 1H; bins 1–4, Fig. 1I). A substantial accumulation of late-born cells was also observed within and below LI (Fig. 1H; bins 8–10, Fig. 1I). These results suggest that the position of cortical interneurons is inverted, following the loss of p35 expression.
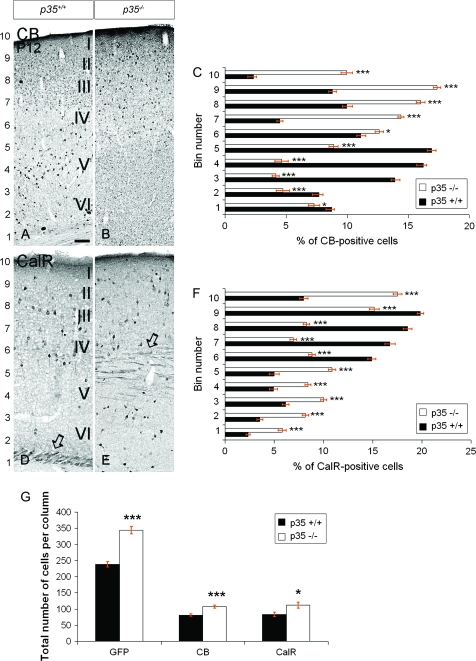
To test this hypothesis, we examined subpopulations of interneurons that can be identified by specific neurochemical markers. In postnatal animals, CB is expressed in a subpopulation of cortical interneurons and some pyramidal cells. CB immunohistochemistry at P12 showed that, in p35+/+ mice, the majority of labeled cells populated the lower cortical layers, particularly layer V (Fig. 2A; bins 3–5, Fig. 2C), whereas they resided mostly superficially in p35−/− mice (Fig. 2B; bins 6–10, Fig. 2C). CalR-containing interneurons are born mainly late in corticogenesis and arise for the most part in the caudal GE (Xu et al. 2004). In p35+/+ mice, at P12, we found the majority of CalR+ cells in the upper cortical layers (layers II–IV; Fig. 2D; bins 6–9, Fig. 2F) and CalR+ axons within the subcortical white matter (Fig. 2D). However, in p35−/− mice, CalR+ neurons were most prominent in the lower and upper cortices (Fig. 2E; bins 3–5 and 9–10; Fig. 2F), above and below dispersed CalR+ axons (Fig. 2E). Analysis of PV- and Sst-labeling also revealed a defect in the laminar distribution of distinct interneuron populations. PV-positive cells were preferentially found in layers IV and V of the cortex in control mice but were abnormally spread within the whole depth of the cortex in p35−/− mice (Supplementary Fig. 2A,B). Sst cells, typically found in deep layers of the cortex in control mice, were observed superficially in the cortex in p35−/− mice (Supplementary Fig. 2C,D). Importantly, we noticed a significant increase in the proportion of GFP+, GFP+/BrdU(E13.5)+, CB+, and CalR+ cells that had accumulated in LI of p35−/− mice compared with controls (bin 10 in Figs 1C,F and 2C,F).
Figure 2.
The laminar arrangement and density of cortical interneurons are altered in p35−/− mice. Coronal sections of P12 somatosensory cortex showing expression of CB (A, B) and CalR (D, E). (C, F) Binned quantifications of the distribution of CB+ and CalR+ cells (mean percentage ± SEM); bin1 closest to the lateral ventricle. (G) Quantification of the number of GFP+, CB+, and CalR+ cells (mean number ± SEM). In p35+/+ mice, CB+ cells mostly populate lower layers, whereas in p35−/− mice, these cells tend to reside in the superficial cortex and LI (A, B). The relative distribution of CB+ cells in the cortex is shown in (C). In p35+/+ mice, CalR+ cells are observed predominately in the upper half of the cortex, whereas in the p35−/− animals, they populate the whole depth; note accumulation of cells in LI (D, E). CalR+ thalamocortical fibers are located below the cortex in p35+/+ (D, open arrow) and within the cortex in p35−/− mice (E, open arrow). The relative distribution of CalR+ cells in the cortex is shown in (F). Loss of p35 gene significantly increases the number of GFP+, CB+, and CalR+ cells in postnatal cortex (G). Error bars represent SEM. A significant difference in layer distribution of CB+ (P < 0.001, χ2 test) CalR+ (P < 0.001, χ2 test) cells in the cortex is observed between p35+/+ and p35−/− mice. Bin comparison is done by Student's t-test (*P < 0.05, ***P < 0.005). Roman numerals designate mature neocortical layers. Scale bar, 150 μm (A, B, D, E).
The overall number of GFP+, CB+, and CalR+ cells was also altered in p35−/− mice at P12. There was a significant increase (ca., 30%) in the number of GFP+ cells (344 ± 4.9 vs. 238.5 ± 8.5 cells per radial column), CB+ cells (117 ± 5.9 vs. 91.1 ± 3.7 cells), and CalR+ cells (83.6 ± 6.7 vs. 68 ± 6.7 cells) in p35−/− mice compared with control littermates (Fig. 2G). Taken together, these results indicate that the laminar distribution and density of interneurons are altered in the p35−/− cortex.
Cdk5 Expression in Interneurons and Its Activity in the Developing Forebrain
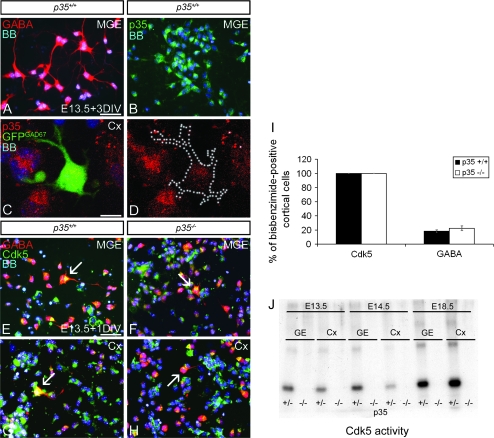
To confirm the importance of Cdk5, we investigated its expression in interneurons, both at their origin in the MGE and in the cortical mantle, at the time of intense migration into the cortex (E13.5). Dissociated cell cultures, prepared from MGE and cortex, were examined using immunofluorescence with anti-p35, GABA, and Cdk5 antibodies. MGE cells derived from control mice contained mostly GABA-labeled neurons, which also expressed p35 (Fig. 3A,B); no p35 expression was detected in cultures prepared from p35−/− mice (data not shown). p35 Immunocytochemistry of cortical cultures obtained from GAD67GFP/+ mice revealed the presence of p35 in GFP+ cortical interneurons (Fig. 3C,D). Cdk5 was detected in MGE and cortical GABA-containing neurons in both control and p35−/− mice (Fig. 3E–H). Quantitative analysis revealed that all cultured cortical cells expressed Cdk5, regardless of genotype, and that the percentage of cells expressing GABA was similar in p35−/− and control littermates (22.4 ± 3.3 vs. 18.3 ± 2.0; Fig. 3I), suggesting that p35/Cdk5 is not necessary for the expression of GABA.
Figure 3.
Cdk5 is expressed, but not active, in p35-deficient interneurons. Cultured neurons from E13.5 MGE (A, B, E, F) or Cx (C, D, G, H), immunoreacted for GABA (A), p35 (B–D), or GABA/Cdk5 (E–H), and stained with bisbenzimide (BB) to show nuclei. The majority of p35+/+ MGE cells express GABA (A) and p35 (B). p35 Is also present in GFP+ cortical interneurons obtained from GAD67GFP/+ mice (C, D). Cdk5 is expressed in GABAergic MGE (E, F) and Cx (G, H) neurons (arrows) regardless of genotype. (I) Graph shows the proportion of cortical cells expressing Cdk5+ and GABA+ in dissociated cell cultures. No significant difference in GABA expression is observed between control and p35−/− mice. (J) Cdk5 was isolated from cell lysates of GE and Cx by immunoprecipitation and its activity measured by an in vitro kinase assay in the presence of histone H1. Cdk5 activity is undetectable in embryonic GE and Cx of p35−/− compared with p35+/− mice. M, medial; Cx, cortex; DIV, day in vitro. Error bars represent SEM. Scale bar, 50 μm (A, B, E–H), 10 μm (C, D).
To study the temporal profile of Cdk5 activity in control and p35−/− mice, Cdk5 was isolated from embryonic (E13.5, 14.5, and 18.5) GE and cortex and its activity assessed using in vitro kinase assays. In controls, Cdk5 activity was developmentally regulated and increased with time (Fig. 3J). Interestingly, the GE had slightly higher levels of activity when compared with the cortex at the time of most intense tangential migration of interneurons toward the cortex (E13.5–14.5), while the opposite was the case just before birth, when active radial interneuron migration takes place (E18.5). No Cdk5 activity was detectable in the GE or cortex of p35−/− animals (Fig. 3J). These results suggest highly reduced or absent Cdk5 activity in cortical interneurons of embryonic p35−/− mice.
Altered Initial Migration and Morphology of Interneurons in p35−/− Mice
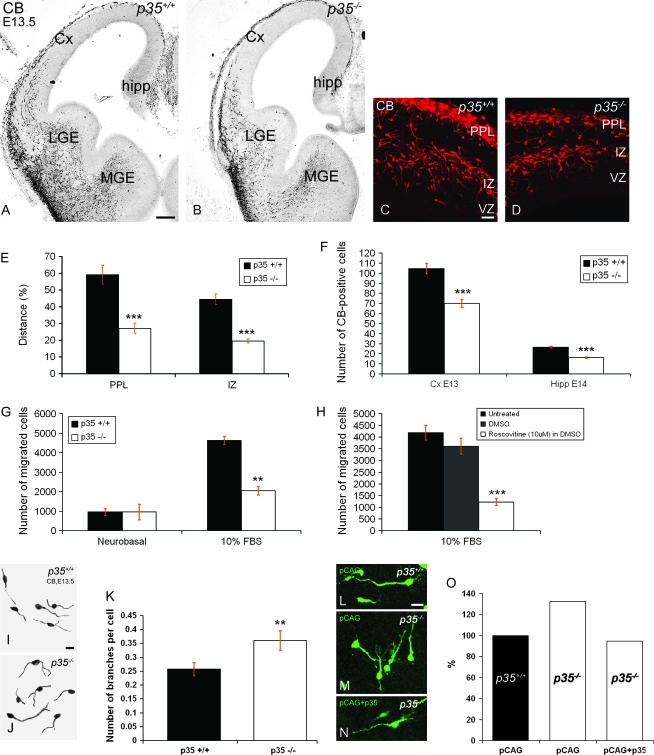
We asked whether lack of Cdk5 activity altered tangential migration of cortical interneurons that had arisen in the GE. CB immunohistochemistry is an established method used to label migrating interneurons in the developing mouse forebrain (Anderson et al. 1997; Ang et al. 2003). At E13.5, we observed that fewer CB+ interneurons in p35−/− mice had entered the cortex from the MGE than that seen in control littermates (Fig. 4A–D). To quantify our observations, the distance traveled by CB+ cells in the PPL and IZ migratory streams at E13.5 was established as the proportion of the distance between the corticostriatal notch and the hippocampal curvature. Importantly, CB+ neurons in p35−/− mice had migrated a significantly shorter distance into the cortex in both streams (PPL, 27.2 ± 3.0%; IZ, 19.6 ± 1.3%; Fig. 4E) compared with littermate controls (PPL, 59.1 ± 5.6%; IZ, 44.6 ± 3.1%; Fig. 4E). We also counted the total number of CB+ neurons in the cortex that had passed the corticostriatal notch at E13.5 and found significantly fewer cells in p35−/− mice (70.0 ± 4.0) compared with controls (104.5 ± 5.2; Fig. 4F). Similarly, we found that markedly fewer CB+ cells had migrated into the developing hippocampus a day later, at E14.5, in p35−/− mice (16.1 ± 0.7) compared with controls (26.5 ± 0.9; Fig. 4F).
Figure 4.
p35-deficient interneurons demonstrate impaired motility and morphology. (A–D) Coronal sections of E13.5 forebrain labeled for CB. CB+ interneurons migrate in clearly defined streams from the GE toward cortex, in both p35+/+ (A) and p35−/− (B) mice. However, fewer interneurons are observed in each stream (PPL and IZ) in p35−/− mice (D) compared with controls (C). Delay in CB+ interneuron migration as indicated by a shorter distance (E; mean percentage ± SEM) and fewer cells evident in the Cx (at E13.5; F) and hippocampus (at E14.5; F) of p35−/− mice compared with controls. Total number of CB+ cells is presented as mean number ± SEM. (G, H) Results of chemomigratory assay using the Boyden chamber. The chemotactic response of MGE-derived cells (E13.5) from p35+/+ or p35−/− mice toward a control (Neurobasal medium) or 10% FBS (G) or following treatment of p35+/+ MGE cells with DMSO or 10 μM Roscovitine and exposure to 10% FBS (H). A significant impairment in chemotactic response to FBS is observed upon downregulation of p35/Cdk5 (mean number of migrated cells ± SEM). (I, J) Morphology of E13.5 CB+ cells in p35+/+ (I) and p35−/− (J) mice. (K) Branch measurements of E13.5 CB+ cells shown as mean number of branches per cell ± SEM. A significant increase in the number of branches is observed in p35−/− mice (n = 305 cells) compared with controls (n = 420 cells). (L–N) Forebrains from p35+/+ and p35−/− mice, injected and electroporated into the MGE with pCAG (control green vector; injected into p35+/+, L, and injected into p35−/−, M) or pCAG together with p35 plasmid (injected into p35−/−, N). Abnormal branching of migrating cells observed in the p35−/− mice (23 forebrain slices) is rescued by expression of p35 (34 forebrain slices) (O). The number of branches per cell calculated as a percentage of the pCAG-transfected control (p35+/+ mice; 23 forebrain slices). Cx, cortex; LGE, lateral ganglionic eminence; VZ, ventricular zone. Error bars represent SEM. **P < 0.01 and ***P < 0.005; Student's t-test. Scale bars, 250 μm (A–B); 100 μm (C–D); 25 μm (I, J, L–N).
To evaluate whether loss of p35/Cdk5 activity can directly affect the motility of interneurons, we studied the migratory properties of cortical interneurons lacking Cdk5 activity in vitro. To determine whether Cdk5 mediates the migration of interneurons toward stimulatory guidance cues, such as FBS (Maggi et al. 2000), an in vitro migratory assay was performed using a Boyden chamber. We compared E13.5 cells dissociated from MGE of control and p35−/− mice or MGE from control animals treated with 10 μM Roscovitine, a specific Cdk5 inhibitor. Migration toward a negative control (Neurobasal medium) did not differ between genotypes (control, 961 ± 168; p35−/−, 954 ± 204; Fig. 4G). However, the chemotactic response to 10% FBS was robust in controls but significantly reduced in cells derived from p35-deficient mice (control, 4629 ± 408; p35−/−, 2045 ± 221; Fig. 4G). Similarly, pretreatment of control MGE-derived cells with 10 μM of Roscovitine (dissolved in dimethyl sulfoxide—DMSO), 30 min prior to the assay, significantly decreased their migration toward 10% FBS compared with untreated or DMSO-treated controls (1223 ± 141.5 vs. 4189 ± 314.5 or 3610.5 ± 344.5; Fig. 4H). These findings suggest that Cdk5 activity can have a direct role on interneuronal migration.
To explain the delayed arrival of CB+ interneurons in the cortex of p35−/− mice in vivo, we analyzed their morphology at E13.5 revealing significantly increased branching of mutant cells (29.8%; Fig. 4J,K) compared with controls (Fig. 4I,K). We further analyzed the phenotype by electroporating an EGFP-expressing vector into the MGE of control (Fig. 4L) or p35−/− (Fig. 4M) forebrain slices. After a 48-h incubation, we observed a 31.25% increase in the number of branch points of migrating GFP-positive cortical cells in the slices taken from p35−/− mice compared with controls (Fig. 4O). This increased branching was rescued by coexpression of p35 (90.9% of the control; Fig. 4N,O). Taken together, our results indicate that lack of p35/Cdk5 activity impairs tangential migration and alters the morphology of cortical interneurons in a cell-autonomous manner.
The PPL Splitting Defines the Positions of Interneuron Migratory Streams
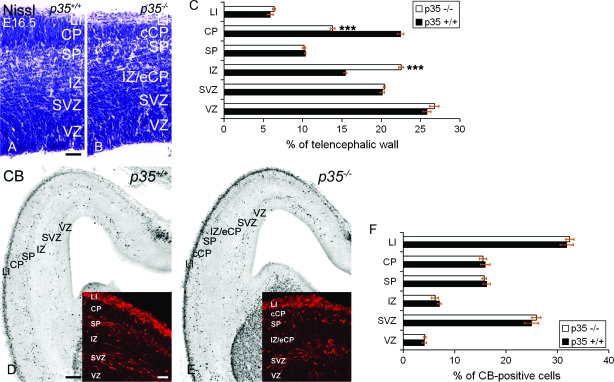
A marked difference between the cytoarchitecture of control and p35−/− mice is highly apparent from E16.5 (Fig. 5A,B), as we previously reported (Rakic et al. 2006). This includes the presence of a compact CP (cCP) and an ectopic CP (eCP) located in the IZ in p35−/− mice (Fig. 5B). At E16.5, the cCP appeared thinner (13.8%) and the IZ thicker (22.5%) in p35−/− mice compared with controls (CP, 22.5%, and IZ, 15.4%) (Fig. 5C). This was due to the accumulation of neurons that did not reach the cCP but, instead, became trapped in the IZ. The emerging CP splits the PPL into the superficial LI and the deep SP in both genotypes, although some disorganization was evident in p35−/− mice, as reported previously (Rakic et al. 2006). Significantly, in mice lacking p35, the SP is located more superficially due to the aberrant migration of late-born projection neurons (Chae et al. 1997). We observed that interneurons followed the compartmentalization of the cortex determined by the arrival of the CP neurons. They appeared in 3 tangential streams regardless of genotype (Fig. 5D,E), although the SP stream was situated more superficially in p35−/− mice most likely due to the displacement of this layer (Fig. 5E). Notably, at E16.5, there was no difference in the distribution of CB+ cells between control and p35−/− mice (Fig. 5F), and the total number of CB+ neurons was similar in the 2 genotypes (control, 118.1 ± 3.4; p35−/−, 123.8 ± 2.9). These results indicate that the appearance of the 2 migratory streams in LI and SP is due to the splitting of the PPL by the migration of cortical projection neurons that form the CP.
Figure 5.
PPL splitting defines the positions of the tangential interneuron streams. (A, B) Cortical layers in E16.5 Nissl-stained sections. (C) The relative layer thickness in the developing cortex (mean percentage ± SEM). The CP is significantly thinner and the IZ thicker in p35−/− mice compared with controls. (D–E) Sections of the same age, labeled for CB revealing 3 (LI, SP, and SVZ) distinct migratory streams in p35+/+ (D, inset) and p35−/− (E, inset) mice. In p35−/− cortex, the position of the SP stream is more superficial (due to the thinner CP; E, inset). (F) The relative distribution of CB+ cells in the developing cortex (mean percentage ± SEM). No difference in layer distribution of CB+ cells in the cortex is observed between p35+/+ and p35−/− mice. VZ, ventricular zone. Error bars represent SEM. ***P < 0.005; Student's t-test. Scale bars, 50 μm (A, B); 250 μm (D, E); 100 μm (D, E, inset).
Embryonic p35−/− Interneurons Accumulate in LI and the IZ
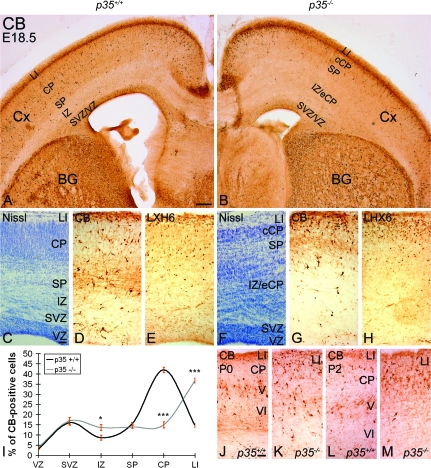
At E18.5, as observed 2 days earlier, the CP was thinner and the IZ thicker in p35−/− mice compared with control littermates (Fig. 6C,F; Rakic et al. 2006). The majority of CB+ cells were present in the LI/CP/SP zones in both genotypes (control, 72.3 ± 2.1%; p35−/−, 65.6 ± 2.9%; Fig. 6A,B,D,G,I). The distribution of labeled cells in the SVZ was not affected in the p35−/− animals (control, 16.0 ± 1.2%; p35−/−, 16.9 ± 1.7%; Fig. 6A,B,D,G,I). A slight but significant increase in the number of CB+ cells was observed in the IZ of p35−/− mice (compared with control), where most of the eCP cells resided as determined by Nissl staining (control, 8.7 ± 1.2%; p35−/−, 13.7 ± 1.5%; Fig. 6A–D,F,G,I). The most prominent cytoarchitectonic feature in the p35−/− mice was a markedly thicker and cell-packed LI compared with controls (Fig. 6C,F; Rakic et al. 2006). Interestingly, CB+ cells accumulated extensively in LI of the p35−/− cortex, which was significantly greater than that seen in controls (36.7 ± 0.9% vs. 14.7 ± 1.2%; Fig. 6D,G,I). This finding was confirmed with immunohistochemistry using an anti-LHX6 antibody (Fig. 6E,H), a marker of migrating and differentiated cortical interneurons (Lavdas et al. 1999; Cobos et al. 2006). An increase of CB+ neurons in the superficial portion of the p35−/− cortex (compare Fig. 6K,M with Fig. 6J,L) was also noted in the early postnatal period (P0 and P2). In conclusion, loss of p35 expression primarily causes the excessive accumulation of interneurons in developing LI as well as their enrichment in the IZ.
Figure 6.
Abnormal accumulation of cortical interneurons in LI and IZ of p35−/−mice. (A, B) E18.5 coronal forebrain sections labeled for CB. CB+ cells are mainly found in the upper part of the cortex, in zones LI/CP/SP, and only rarely in the IZ, in both p35+/+ (A) and p35−/− (B) mice. (C) Cortical layers are clearly delineated in Nissl-stained sections of p35+/+ mice. (D, E) CB+ and LHX6+ cells are found evenly distributed within LI, CP, and SP layers. (F) In Nissl-stained p35−/− cortex, CP is thinner and the IZ as well as LI appear thicker and cell dense compared with controls. (G, H) CB+ and LHX6+ cells, although present in all developing layers, are mostly accumulated in LI. Normal position and cell content of the SVZ interneuron stream is found in both genotypes. (I) Relative distribution of CB+ cells in the cortex (mean percentage ± SEM). Error bars represent SEM. A significant difference in layer distribution of CB+ (P < 0.05, χ2 test) cells in the cortex is observed between p35+/+ and p35−/− mice. Bin comparison is done by Student's t-test (*P < 0.05, ***P < 0.005). (J–M) The positions of CB+ cells in the early postnatal (P0 and P2) cortex differ between the genotypes. In controls, they are mostly in layer V (J, L), contrasting with the upper cortex, including LI, in p35−/− mice (K, M). Cx, cortex; BG, basal ganglia; VZ, ventricular zone. Scale bars, 500 μm (A, B); 100 μm (C–H); 50 μm (J–M).
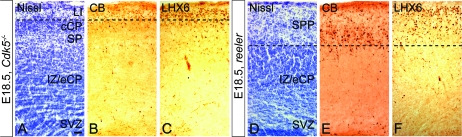
To further test whether the abnormal accumulation of interneurons in the altered LI of p35−/− mice is due to the incomplete splitting of the PPL, we examined their distribution in Cdk5−/− and in reeler mice. Significantly, the PPL splits only partially in Cdk5−/− (Fig. 7A) and remains undivided in reeler mice, where it is called the superplate (SPP; Fig. 7D). CB and LHX6 immunohistochemistry showed abnormal accumulation of interneurons in LI of Cdk5−/− mice (Fig. 7B,C) and in the SPP in reeler (Fig. 7E,F). Together, these results suggest that molecules originally present in the PPL can positively affect the position of migrating interneurons.
Figure 7.
Abnormal accumulation of interneurons in LI and SPP in Cdk5−/− and reeler mice, respectively. E18.5 coronal sections, of Cdk5−/− and reeler forebrain, stained with Nissl and labeled with antibodies against CB and LHX6. In the Cdk5−/− cortex, LI is wide and cell dense (A) and packed with numerous CB+ (B) and LHX6+ (C) cells. In reeler mice, SPP, containing undivided (or mixed) LI and SP cells, is a thick cortical layer (D) containing an abundance of CB+ (E) and LHX6+ (F) cells. Dashed lines represent the border between LI or SPP and the rest of the developing cortical layers. Scale bar, 100 μm.
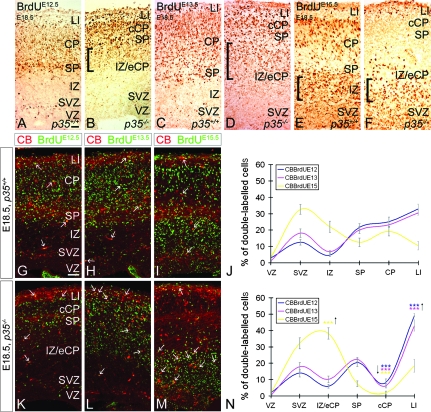
To compare the relationship between migrating projection neurons and interneurons, we first examined the exact positions of early- and late-born cortical neurons in embryonic p35−/− mice by applying BrdU birthdating analyses. Cells born at E12.5, E13.5, and E15.5 were targeted by a single injection of BrdU and observed at E18.5. Heavily labeled BrdU+ cells, born at E12.5, were found in LI and SP, layers that originate from the PPL, in both control and p35−/− mice (Fig. 8A,B). Weakly labeled BrdU+ cells were found within the control CP (Fig. 8A). However, in p35−/− mice, these cells were also located below the SP, indicating that some of them had not migrated into the cCP (Fig. 8B). BrdU+ cells, born at E13.5, were found in LI/CP/SP in both genotypes (Fig. 8C,D). In p35−/− mice, as seen for neurons born at E12.5, there were also numerous BrdU+ cells in the IZ, immediately below the SP (Fig. 8D). In control brains, cells born at E15.5 resided in the upper part of the CP (Fig. 8E). Surprisingly, many BrdU+ cells were also seen below the CP, in the IZ, and in the proliferative zones. In p35−/− mice, nearly all late-born cells were observed in the ventricular zone/SVZ/IZ layers, apart from some cells found in LI (Fig. 8F). Together, our birthdating results reveal that, contrary to previous reports (Kwon and Tsai 1998; Gilmore and Herrup, 2001), not all early-born neurons in p35−/− mice are confined to the cCP but are also found underneath the SP, accompanied by late-born cortical cells (Hoerder-Suabedissen et al., in press).
Figure 8.
Abnormal distribution of early- and late-born pyramidal cells and interneurons in embryonic p35−/− mice. Pregnant dams received a single BrdU injection at E12.5, E13.5, or E15.5 to label early or late-born cortical cells. Coronal sections of E18.5 cortices labeled for BrdU and BrdU/CB. In both p35+/+ and p35−/− mice, early-born cells are mostly present in the LI/CP/SP layers (A–D). In p35−/− mice, these cells are also found trapped in the IZ (square brackets; B, D). Late-born cells, in control mice, migrate into the CP but fail to position themselves in the cCP in p35−/− mice; note BrdU+ cells in the IZ of both genotypes (square brackets; E, F). (G–I, K–M) Early- and late-born CB+ cells are present in all layers of both genotypes (arrows). The relative distribution of CB+/BrdU+ cells in the cortical layers of p35+/+ (J) and p35−/− (N) is presented as mean percentage ± SEM. In control mice, early- and late-born CB+ cells enter the CP and are distributed evenly (J). Many late-born CB+ cells also populate the SVZ (J). In p35−/−, few CB+/BrdU+ cells are present in the cCP and are arrested in LI (mostly early born) and in the IZ (mainly late born) compared with controls (N). Error bars represent SEM. A significant difference in layer distribution of CB+/BrdU+ (E12.5, P < 0.05; E13.5, P < 0.025; E15.5, P < 0.001; χ2 test) cells in the cortex is observed between p35+/+ and p35−/− mice. Bin comparison is done by Student's t-test (***P < 0.005). VZ, ventricular zone. Scale bar, 100 μm.
To specifically distinguish subpopulations of BrdU-labeled interneurons, we examined the distribution of cells at E18.5 that had been labeled with BrdU at E12.5, E13.5, and E15.5 and were also CB positive. In the control cortex, early- (E12.5 or E13.5) and late-born (E15.5) CB+ cells distributed evenly in the LI/CP/SP layers (Fig. 8G–J), suggesting that they had reached the CP simultaneously. However, most of the late-born CB+ cells still resided in the SVZ (Fig. 8I,J). In contrast, in p35−/− mice, few cells made it to the cCP (Fig. 8K–N). Instead, the majority, particularly those born early, were found in LI (Fig. 8K,L,N). An increase in the presence of late-born CB+ neurons was also observed in this layer compared with controls (Fig. 8M,N). Finally, a significant accumulation of late-born, but not early-born, cells was found in the IZ (Fig. 8M,N). These experiments clearly demonstrate that many early- and late-born interneurons in p35−/− mice are found trapped in cortical LI and IZ, correspondingly. In addition, the finding that most early- and late-born cortical neurons, but proportionally not as many interneurons, in p35−/− mice are in the IZ suggests that embryonic interneurons can travel separately from their pyramidal counterparts during early phases of their intracortical movements.
Discussion
Interneurons follow complex paths as they migrate from the ventral forebrain to the correct layers of the cortex. Investigators have in recent years examined the role of Cdk5 and its activator p35 in this process, but a consensus has yet to emerge. Here, we propose that Cdk5 plays a role in the migration of interneurons.
Previous examination of p35 (Hammond et al. 2004) or Cdk5 (Gilmore and Herrup 2001) mutant mouse chimeras led the authors to suggest a Cdk5-independent mechanism of cortical interneuron migration and laminar organization. Interneurons of either mutant genotype appeared capable of migrating into the chimeric cortices; however, detailed examination of the laminar fate of interneurons was not performed in either study. Further, Hammond et al. (2006) proposed that only the positions of early-born interneurons were affected in p35−/− mice, but in a p35/Cdk5-independent manner.
The present study has shown abnormal laminar organization of interneurons in postnatal p35−/− animals, particularly the accumulation of these cells in the outer cortex. Specifically, early-born p35−/− interneurons favored upper whereas late-born interneurons lower aspects of the cortex. Our findings are not in complete accord with the study of Hammond et al. (2006), who suggested that late-born p35−/− interneurons took up normal positions. This may be accounted for by differences in the experimental protocols used. In brief, we studied interneurons in GAD67-GFP mice, whereas Hammond et al. (2006) visualized interneurons with an anti-GABA antibody. The advantage of our approach is the use of an established and reliable model for specific visualization of the majority of interneurons, which is not dependent on the affinity and variability of a single immunological reagent.
We report here that cortical interneurons in p35−/− mice display a delayed entry into the cortex. Our observations suggest that this is not due to a directional defect, as these cells enter the cortex using conventional streams but, rather, due to a change in their motility as confirmed by in vitro experiments. Thus, we found that the chemotactic response of MGE-derived cells to multiple stimulatory guidance cues present in FBS was impaired in p35−/− mice or following inhibition of Cdk5 with Roscovitine. Whereas Gilmore and Herrup (2001) suggested that Cdk5-deficient cerebellar neurons do not display general locomotive impairment, cortical interneurons and cerebellar cells may not share the same migratory or signaling properties, at least those mediated by Cdk5. We also observed a significant increase in branching of migrating interneurons in p35−/− mice. This is in agreement with Gupta et al. (2003) who, using time-lapse imaging, observed a branched migratory morphology of p35−/− pyramidal neurons. They, too, concluded that this was a cell-autonomous feature of migrating neurons as the branching defect was rescued by p35 expression. We suggest that the migratory morphology of interneurons, and consequently their movement, depend directly on the activation of Cdk5 by p35. The observed defect in motility and morphology of p35−/− interneurons may also be due to their reduced sensitivity to environmental cues. Significantly, the delayed migration of interneurons could affect their ability to reach their correct destinations at the normal developmental time points.
Several explanations could account for the accumulation of p35−/− interneurons in LI. One hypothesis is a key role for the PPL in directing the migration of interneurons. In support, our study clearly showed that the arrival of CP cells splits the PPL and the interneurons migrating within it, into 2 streams: LI and SP (see Fig. 2 in Metin et al. 2006). In p35−/− and Cdk5−/− mice, the distance between these 2 streams is reduced due to a thinner cCP. Furthermore, reeler mice display only one superficial migratory stream, SPP, as a result of an undivided PPL. It is, therefore, quite likely that the PPL positively directs the migration of cortical interneurons. The substrate or molecules involved in guiding interneurons toward the PPL are not known, although likely candidates include PPL cells and early corticofugal fibers or chondroitin sulfate proteoglycans. How emerging CP cells divide the PPL and the PPL stream of interneurons also remains a mystery. Further confirmation for the importance of the PPL in guiding interneurons was gained from the analysis of p35−/− mice that exhibited a markedly thicker, cell-packed LI and misplaced SP neurons (Rakic et al. 2006). One explanation for the initial accumulation of interneurons in most upper parts of the developing cortex is the shift in distribution of SP cells toward the cCP and LI.
Several recent studies have suggested that interneurons seek their correct positions in the CP around the time (E18.5) when we clearly see the abnormal accumulation of interneurons in LI and IZ in p35−/− mice. Hence, defects in the “inward” and “outward” radial movements of interneurons may also account for their altered final distribution. Specifically, the accumulation of early interneurons in LI may result from their delayed downward migration to the cCP. Similarly, late-born interneurons, found caught in the IZ/eCP, might have defective upward migration toward LI. The fact that we also found a cell-autonomous delay in tangential migration of interneurons from the ventral telencephalon further confirms this hypothesis. Consequently, we propose that the inward and outward migration of cortical interneurons is a Cdk5-dependent event. In light of this hypothesis, it would be interesting to follow the inward/outward interneuron migration from LI and IZ into the CP, in both control and p35 mutant GAD67-GFP mice, using real-time imaging.
It has been proposed that interneurons wait until the settlement of pyramidal neurons before entering the CP (Pla et al. 2006; Tanaka et al. 2006; López-Bendito et al. 2008) and that the final laminar arrangement of interneurons may be determined by the position of their pyramidal counterparts born on the same day. However, pyramidal cells are neither required for tangential migration of cortical interneurons from the subpallium to the pallium nor directly involved in their initial radial movements, such as migration between the developing cortical layers (interzonal movements). This hypothesis is confirmed in our p35 mutant model. Our birthdating results revealed that most early- and late-born cortical neurons (predominantly pyramidal neurons) reside in the IZ at E18.5. In contrast, at the same developmental time point, most interneurons accumulate in LI, which is largely devoid of pyramidal neurons. Nevertheless, we cannot rule out the possibility that at later developmental stages, pyramidal neurons provide laminar cues for migrating interneurons. Namely, in p35 mutants, early-born interneurons remain in close proximity to the early-born pyramidal cells mostly found in the cCP. Likewise, late-born interneurons stay in the IZ/eCP, where they may be attracted by their pyramidal counterparts born on the same day.
In conclusion, we used p35−/− mice to assess the role of p35/Cdk5 in the disposition of interneurons in the cortex. We found cell-autonomous delayed tangential migration that may affect correct layer compositions. We noted an abnormal accumulation of interneurons in the upper aspects of the cortex, especially in LI, and suggest that this is due to p35/Cdk5-dependent incomplete splitting of the PPL. Defects in the morphology and consequently inward and outward radial movements of interneurons may also account for their altered final distribution. These results support a complex, direct, and indirect role for p35/Cdk5 in the migration and distribution of cortical interneurons.
Supplementary Material
Supplementary materials can be found at http://www.cercor.oxfordjournals.org/.
Funding
Wellcome Trust Project Grant (number 069441) to MN; Wellcome Trust Programme Grant to JGP (number 074549); Grant-in-Aids for Scientific Research from the MEXT to YY.
Acknowledgments
The authors are grateful to Dr Li-Huei Tsai for p35 mutant mice, DC27 anti-Cdk5 antibody, and pCMVneo-p35 plasmid; Dr Andre Goffinet for reeler brains; Dr Vassilis Pachnis for anti-LHX6 antibody; Drs Mikio Hoshino and Jun-ichi Miyazaki for pCAG-IRES-EGFP vector; Drs Navin Parasram and Frédéric Causeret for assistance with statistical analysis; Mason Yeh for help with morphometric analysis; Dr Anna Cariboni for assistance with chemotactic assay; and Drs Gaëlle Friocourt, William Andrews, and David Hunt for constructive comments on the manuscript. Conflict of Interest: None declared.
References
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–5648. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr., Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariboni A, Rakic S, Liapi A, Maggi R, Goffinet A, Parnavelas JG. Reelin provides an inhibitory signal in the migration of gonadotropin-releasing hormone neurons. Development. 2006;132:4709–4718. doi: 10.1242/dev.02033. [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–i88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Fairen A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Gilmore EC, Herrup K. Neocortical cell migration: GABAergic neurons and cells in layers I and VI move in a cyclin-dependent kinase 5-independent manner. J Neurosci. 2001;21:9690–9700. doi: 10.1523/JNEUROSCI.21-24-09690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–1291. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Hammond V, So E, Gunnersen J, Valcanis H, Kalloniatis M, Tan SS. Layer positioning of late-born cortical interneurons is dependent on Reelin but not p35 signaling. J Neurosci. 2006;26:1646–1655. doi: 10.1523/JNEUROSCI.3651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond V, Tsai LH, Tan SS. Control of cortical neuron migration and layering: cell and non cell-autonomous effects of p35. J Neurosci. 2004;24:576–587. doi: 10.1523/JNEUROSCI.4529-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A. Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience. 2004;124:605–618. doi: 10.1016/j.neuroscience.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakić S, Parnavelas JG, Reim K, Paulsen O, Molnár Z. doi: 10.1093/cercor/bhn195. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cerebral Cortex (in press) [DOI] [PubMed] [Google Scholar]
- Kwon YT, Tsai LH. A novel disruption of cortical development in p35(-/-) mice distinct from reeler. J Comp Neurol. 1998;395:510–522. doi: 10.1002/(sici)1096-9861(19980615)395:4<510::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Sánchez-Alcañiz JA, Pla R, Borrell V, Picó E, Valdeolmillos M, Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi R, Pimpinelli F, Molteni L, Milani M, Martini L, Piva F. Immortalized luteinizing hormone-releasing hormone neurons show a different migratory activity in vitro. Endocrinology. 2000;141(6):2105–2112. doi: 10.1210/endo.141.6.7494. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Metin C, Baudoin JP, Rakic S, Parnavelas JG. Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci. 2006;23:894–900. doi: 10.1111/j.1460-9568.2006.04630.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Nakajima K. Control of tangential/non-radial migration of neurons in the developing cerebral cortex. Neurochem Int. 2007;51:121–131. doi: 10.1016/j.neuint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzke H, Maddineni U, Ayala R, Morabito M, Volker J, Dikkes P, Ahlijanian MK, Tsai LH. Partial rescue of the p35-/- brain phenotype by low expression of a neuronal-specific enolase p25 transgene. J Neurosci. 2003;23:2769–2778. doi: 10.1523/JNEUROSCI.23-07-02769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla R, Borrell V, Flames N, Marin O. Layer acquisition by cortical GABAergic interneurons is independent of Reelin signaling. J Neurosci. 2006;26:6924–6934. doi: 10.1523/JNEUROSCI.0245-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Rakic S, Davis C, Molnar Z, Nikolic M, Parnavelas JG. Role of p35/Cdk5 in preplate splitting in the developing cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i35–i45. doi: 10.1093/cercor/bhj172. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2006;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2003;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle I, Caviness VS, Jr., Chae T, Harlow E. p35 Is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Valcanis H, Tan SS. Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci. 2003;23:5113–5122. doi: 10.1523/JNEUROSCI.23-12-05113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La CE, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.