Abstract
What makes us become aware? A popular hypothesis is that if cortical neurons fire in synchrony at a certain frequency band (gamma), we become aware of what they are representing. We tested this hypothesis adopting brain-imaging techniques with good spatiotemporal resolution and frequency-specific information. Specifically, we examined the degree to which increases in event-related synchronization (ERS) in the gamma band were associated with awareness of a stimulus (its detectability) and/or the emotional content of the stimulus. We observed increases in gamma band ERS within prefrontal–anterior cingulate, visual, parietal, posterior cingulate, and superior temporal cortices to stimuli available to conscious awareness. However, we also observed increases in gamma band ERS within the amygdala, visual, prefrontal, parietal, and posterior cingulate cortices to emotional relative to neutral stimuli, irrespective of their availability to conscious access. This suggests that increased gamma band ERS is related to, but not sufficient for, consciousness.
Keywords: consciousness, emotion, gamma, MEG, synchronization, visual awareness
Introduction
What makes us become aware of what we see? A popular hypothesis is that if cortical neurons fire in synchrony at a certain frequency band (gamma), we become aware of what they are representing. Neuronal synchronization in the gamma band is considered important for the transient functional integration of neural assemblies across brain areas to achieve various cognitive functions (Crick 1994; Singer 1999; Varela et al. 2001). Thus, Fries (2005) has argued (Communication Through Coherence model) that different neuronal assemblies need to be in phase synchronization to allow the communicative exchange of information. Similarly, it is suggested that visual awareness occurs via an attentional mechanism binding together the neurons representing a visual object and that this is done by generating synchronized oscillations in the gamma band (Crick and Koch 1990; Crick 1994; Engel and Singer 2001). However, this has received relatively little empirical investigation (but see Srinivasan et al. 1999; Meador et al. 2002; Doesburg et al. 2005; Melloni et al. 2007), and the relationship between gamma band synchronization and conscious awareness remains debated (e.g., Gold 1999; Vanderwolf 2000). If gamma band synchronization is related to consciousness, we can make the following predictions. First, gamma band synchronization should be significantly greater for supraliminal relative to subliminal stimuli. Second, regions implicated in conscious awareness in previous work, for example, the prefrontal cortex (PFC), anterior cingulate cortex (ACC), and parietal cortex (Dehaene and Naccache 2001; Rees et al. 2002; Stephan et al. 2002; Tsuchiya and Adolphs 2007) and sensory (visual, in the present study) processing areas (e.g., Fries et al. 1997) should, in particular, show significantly greater gamma band synchronization power for supraliminal relative to subliminal stimuli. Moreover, if gamma band synchronization allows consciousness, subliminal stimuli that do not reach conscious awareness should not be associated with significant gamma synchronization.
Although the current paper focuses on gamma band synchronization, it should be noted that activity in other frequency bands is also likely to be important for different aspects of processing including conscious awareness. For example, Guderian and Duzel (2005) reported that recollection is associated with increased induced theta activity in a distributed network that included prefrontal, mediotemporal, and visual areas. In addition, consciously perceived words have been shown to be associated with enhanced theta oscillations over frontal regions as well as increases in gamma power and phase synchrony (Melloni et al. 2007). Moreover, beta and gamma frequency band phase synchrony has been shown to be enhanced for consciously perceived stimuli (Meador et al. 2002; Gross et al. 2004; Palva et al. 2005) and correlates with conscious perception in binocular rivalry (Fries et al. 1997, 2001; Srinivasan et al. 1999; Doesburg et al. 2005). Indeed, it is worth noting the recent study by Dan Glauser and Scherer (2008) examining differences in gamma and beta band oscillations, using electroencephalogram (EEG), between stimuli that participants reported a subjective feeling toward relative to those that they did not. Stimuli that elicited subjective feelings, relative to stimuli that did not, were associated with widespread reduced beta band activity and reduced gamma band activity within bilateral frontal and prefrontal scalp regions. Other recent work by this group has indicated that the gamma band activity may be particularly important for appraisals relating to goal conduciveness (Grandjean and Scherer 2008).
Emotional processing and consciousness are intimately related. As recently argued, consciousness is critical to aspects of the emotional experience, and structures that potentially regulate the level of consciousness (e.g., the midline cortices) are also implicated in emotional processing (Tsuchiya and Adolphs 2007). Indeed, it has been suggested that 2 main mechanisms are necessary and “sufficient” for the emergence of a conscious feeling (Scherer 2004; Sander et al. 2005; Grandjean et al. 2008). It is necessary for there both to be 1) synchronization of the different subcomponents (peripheral, motor, motivational, monitor, and cognitive systems) of the emotional episode and 2) neuronal synchronization within and across these subcomponents. This is thought to allow the linkage of the different neuronal populations involved in the processing of each component.
In the current study, we are examining a particular form of emotional processing. Specifically, we are examining the processing by which emotional information, via, we assume, interaction between temporal cortex and the amygdala, allows increased representation of the stimulus (and potentially increased gamma band power). We assume that this specific form of emotional processing is likely to influence the participant's conscious access to the percept.
Gamma band oscillatory activity has also been associated with emotional processing (Taylor et al. 2000; Oya et al. 2002; Luo, Holroyd, et al. 2007). Interestingly, recent work has indicated that oscillatory brain activity in the gamma band underlies the emergence of a subjective feeling (Dan Glauser and Scherer 2008). Moreover, the amygdala, an area involved in emotional processing, shows greater gamma band activity in response to emotional stimuli relative to baseline (Oya et al. 2002; Luo, Holroyd, et al. 2007). Two contrasting predictions can be made here. If gamma band synchronization is strictly related to consciousness, gamma band synchronization should be significantly greater for supraliminal emotional relative to subliminal emotional stimuli, but there should be no significant main effect for emotion. Alternatively, it is possible that although gamma band activity may represent an attentional mechanism that binds together the neurons representing a visual object, this activity does not inevitably result in consciousness. In other words, this binding process might occur independently of consciousness and simply relate to degree to which the stimulus is processed. This latter position would predict a main effect of emotion; that is, significantly greater gamma synchronization power for emotional relative to neutral stimuli “irrespective” of awareness. Moreover, this latter position would also predict significant gamma synchronization (event-related synchronization [ERS]) for subliminal emotional stimuli.
There has been little work addressing the above issues. One reason is the technological difficulty associated with its investigation. Methods that have been previously adopted such as EEG, single neuron recording, local field potentials, and functional magnetic resonance imaging (fMRI) have either limited spatial or temporal resolution or are unable to provide frequency-specific information or to yield dynamic spatiotemporal profiles of cognitive processing. However, magnetoencephalography (MEG), particularly when combined with the advanced source analysis technique synthetic aperture magnetometry (SAM) based on the beamformer approach (Vrba and Robinson 2001; Hillebrand et al. 2005; Cornwell et al. 2007; Luo, Holroyd, et al. 2007) and the sliding-window analysis (see Luo, Holroyd, et al. 2007) has considerable advantages. SAM is a spatial filtering technique based on the linear constrained minimum variance beamformer. It uses the second-order covariance between channels rather than single-channel averages and thus is sensitive to spatially correlated activity. In addition, the use of the forward magnetic field solution for a source means that SAM detects dipole sources and therefore is less sensitive to artifacts that do not look like dipoles (Vrba and Robinson 2001). Of course, using SAM, localization is inferred on the basis of source modeling. However, importantly, event-related oscillation, as revealed by SAM, has a demonstrable spatial coincidence with the blood oxygenation level–dependent (BOLD) fMRI response (Crone et al. 1998; Singh et al. 2002; Foucher et al. 2003; Brookes et al. 2005; Hall et al. 2005 see, for a review, Hillebrand et al. 2005; Luo, Holroyd, et al. 2007). In principle, it thus provides not only frequency-specific information but also the dynamic spatiotemporal profiles of event-related oscillations. As such, SAM has become an increasingly popular analytic tool for MEG data (Vrba and Robinson 2001; Singh et al. 2003; Fawcett et al. 2004; Furlong et al. 2004; Brookes et al. 2005; Hall et al. 2005; Hillebrand et al. 2005).
ERS or event-related desynchronization (ERD) reflects localized increase or decrease in oscillatory power (Pfurtscheller and Lopes da Silva 1999). Gamma band ERS is thought to reflect the cooperative behavior of a large number of neurons associated with a task and active information processing allowing rapid coupling between spatially separate cell assemblies (Pfurtscheller and Lopes da Silva 1999).
Briefly, in the present study, we examined visual awareness, emotional processing in relation to and gamma band synchronization using MEG and the sliding-window SAM method within a masking paradigm.
Materials and Methods
Paradigm Design
Twenty-one volunteers, 11 males, between the ages of 22 and 38 participated. All gave written informed consent and were approved by the National Institute of Mental Health Institutional Review Board.
The experiment involved a 2 (supraliminal, subliminal) × 2 (emotional, neutral) design. The stimuli were fear and neutral faces presented for either 30 or 100 ms. Empty ovals (30 or 100 ms) were employed as “filler” trials but not analyzed. Faces/empty ovals were preceded and followed by a 100-ms premask and postmask. The postmask was followed by a blank (200 ms for supraliminal and 270 ms for subliminal faces). The participant judged if a human face appeared after prompted by a 500 ms response cue (Y N). If yes, they pressed the left button with the right index finger; if no, they press the right button with the right middle finger. This was followed by a blank for 600 ms. There were 52 faces (26 male) from 52 individuals in each of the 4 face conditions (each individual's face has both an emotional and a neutral version), selected from Karolinska directed emotional faces (Lundqvist et al. 1998). The same set of emotional/neutral faces were used in the supraliminal and subliminal conditions. To avoid low-level visual effects, the emotional and neutral stimuli were matched for luminance (t51 = 0.683, P = 0.626) Figure 1.
Figure 1.
Stimulus presentation sequence.
Data Acquisition
Both MEG and MRI data were acquired. The MEG data were recorded at 600 Hz using a 275-channel CTF whole head MEG system in a shielded environment. The CTF MEG system is equipped with synthetic third gradient balancing, an active noise cancellation technique that uses a set of reference channels to subtract background interference. The resulting noise floor is in the order of 5–7 fT above 1 Hz. At the beginning and end of each measurement, the participant’s head position was registered with localization coils that were placed at the nasion and the bilateral preauricular points. It was required that head movements did not exceed 0.5 cm. By registration of the head position at these 3 points, the MEG data could be superimposed on the individual anatomical images with an accuracy of a few millimeters.
High-resolution anatomical images were also acquired using a T1-weighted, 3-dimensional, Spoiled GRASS imaging (spgr) sequence (1 × 1 × 1.5 mm3) with a 1.5 Tesla GE scanner.
Data Processing
The VSM/CTF software and software developed at the NIMH MEG core facility together with AFNI (http://afni.nimh.nih.gov/afni/) were used for data processing. Before doing SAM analysis, the data were marked according to the 3 stimulus types. A multisphere head model was created for each participant based on the anatomical image of each participant. The advantage of using a multisphere over a single sphere model is that in the former, each sphere (one per MEG sensor) is fit to a small patch of the head model (directly under the sensor) in order to better model the local return currents. SAM was then used to analyze task-related activation differences in the gamma frequency band (30–50 Hz). SAM estimates source power with high spatial resolution using an optimal linear combination of sensors that suppresses signals from environmental and other brain noise without attenuating power from the target voxel. SAM creates an optimum spatial filter from the covariance between the “active state” and the “control state” to calculate a 3-d source image comparing the source strength for specified time windows for the 2 states in a certain band. It is based on the beamformer technique with the source strength of a beamformer at a voxel being the weighted sum of the signal strength of all channels (Van Veen et al. 1997).
To obtain an image of the dynamic spatiotemporal development of the brain's activity, a sliding-window analysis was used in combination with SAM length of 150 ms and a step of 10 ms. With a window length of 150 ms and a step of 10 ms, we estimated the signal power in each voxel by using dual-state SAM imaging, in which the control state (baseline) was the 150 ms before stimulus onset (or −150 to 0 ms) and the active state was a 150-ms window sliding with a 10 ms step: −150 to 0 ms, −140 to 10 ms, −130 to 20 ms, …, 340–490 ms, 350–500 ms. The dual-state SAM output was the contrast between the active state and the control state. With sliding-window SAM, we could obtain information regarding when significant ERS emerged as well as its peaks and offsets. For example, if an ERS in a region is not seen in the “−110 to 40 ms” window but seen in the “−100 to 50 ms” window, then we could infer that the onset of ERS in this region was between 40 and 50 ms. Fifty dual-state SAM imaging analyses were performed with a spatial resolution of 7 mm. The output results were then concatenated, enabling us to obtain a time course in combination with spatial activation maps across all the time points starting from 150 ms before the stimulus to 500 ms after the stimulus. The time window for button response was not selected, so the analysis was just on face processing. The high-performance computational capabilities of the NIH Biowulf PC/Linux cluster, Bethesda, MD (http://biowulf.nih.gov), were utilized to perform the above computation-intensive tasks.
For group analysis, individual anatomical images were first spatially normalized to the Talairach brain atlas. The SAM results of participants were also normalized (transformed to z score) and registered to their respective anatomical Talairach images. The group analysis for each of the fifty time windows was performed using a random effects 2 × 2 analysis of variance (ANOVA) model in AFNI, which generated the ERS/ERD results. ERS of P < 0.001 (uncorrected) was considered statistically significant.
Results
Behavioral Results and Awareness Assessment
For the behavioral results, a 2 × 2 ANOVA was first performed on the response time (RT) data (see Table 1). No significant main effects were seen for either awareness (F1,20 = 0.253; P > 0.621) or emotion (F1,20 = 0.272; P > 0.608). However, there was a significant awareness by emotion interaction (F1,20 = 5.584; P < 0.05). On the supraliminal trials, the participants were faster to respond that they saw a face if it was fearful rather than neutral, whereas on the subliminal trials, they were slower to deny seeing a face if it was fearful rather than neutral (false negative).
Table 1.
RT and detection rates
| RT (SD) | Detection rate (SD) | |
| SupraE | 288.60 (91.80) ms | 92% (89%) |
| SupraN | 312.00 (111.30) ms | 92% (92%) |
| SubE | 315.10 (90.67) ms | 12% (13%) |
| SubN | 302.50 (103.40) ms | 11% (13%) |
Note: SupraE = Supraliminal emotional, SupraN = Supraliminal neutral, SubE = Subliminal emotional, and SubN = Subliminal neutral. SD, standard deviation.
A nonparametric Mann–Whitney U test was performed on the detection rates (see Table 1). Unsurprisingly, this revealed a highly significant effect of awareness (Mann–Whitney U = 0.0001; P < 0.0001); detection of a face stimulus was considerably higher for supraliminal relative to subliminal trials. There was no significant effect of emotion (Mann–Whitney U = 1921.000; P = 0.756). No valence effect within either supraliminal (P = 0.447; Mann–Whitney U = 191.000) or subliminal trials was found (P = 0.829; (Mann–Whitney U = 212.000), suggesting that the interaction of emotion by awareness was not significant.
Signal detection theory was applied to the detection data to determine the participant's awareness of the subliminal stimuli (Greenwald et al. 1995). Individual discriminability index (d′) was computed for all the participants (median: −0.19311; mean: −3.7 × 10−17; minimum: −1.023384; maximum: 1.45702) based on hit and false alarm rates. This distribution did not differ significantly from a zero-centered Gaussian (Z-test, P = 0.5), suggesting that the participants were not aware of the presence of subliminal stimuli.
Imaging Results: ERS
For the imaging results, a sliding-window SAM analysis in the gamma frequency band was performed. The ANOVAs on the ERS in the gamma band revealed significant effects of both awareness and emotion. For a detailed description of the ERS results for different regions, see Table 2.
Table 2.
Spatiotemporal information for areas showing significant gamma band ERS
| Structure | L/R | Brodmann area | Onset time | Peak time | Offset time | x (at peak) | y (at peak) | z (at peak) | t (at peak) |
| The main effect of awareness | |||||||||
| Posterior region | L–R | 18/31/7 | 30–40 ms | 80–90 ms | 300–310 ms | −2 | −64 | 22 | 5.533 |
| SupraE | L–R | 18/19/17/31/ | a | 110–120 ms | 240–250 ms | −7 | −71 | −1 | 4.424 |
| SupraN | L–R | 18/19//17/7/31 | a | 110–120 ms | 190–200 ms | −14 | −71 | 13 | 4.293 |
| SubE | L–R | 17/18/19//7/31 | a | 100–110 ms | 160–170 ms | −1 | −71 | 13 | 4.199 |
| SubN | L–R | 17/18/19/7 | a | 110–120 ms | 210–220 ms | −14 | −71 | −15 | 4.350 |
| PFC–ACC | L | 9/8/32 | 40–50 ms | 70–80 ms | 120–130 ms | −21 | 34 | 42 | 7.028 |
| SupraE | L | 8/9/32 | a | 50–60 ms | 200–210 ms | −14 | 41 | 42 | 5.934 |
| SupraN | L | 32 | a | 60–70 ms | 90–100 ms | −1 | 34 | 20 | 4.774 |
| STS | R | 22 | 70–80 ms | 150–160 ms | 180–190 ms | 56 | −15 | 6 | 5.805 |
| SupraE | R | 22 | 130–140 ms | 160–170 ms | 170–180 ms | 70 | −8 | 7 | 4.474 |
| SupraN | R | 22 | 140–150 ms | 170–180 ms | 210–220 ms | 42 | −25 | 14 | 6.915 |
| The main effect of emotion | |||||||||
| Amygdala | R | \ | 40–50 ms | 90–100 ms | 260–270 ms | 20 | 1 | −15 | 4.075 |
| SupraE | R | \ | 70–80 ms | 150–160 ms | 300–310 ms | 25 | −7 | −7 | 4.311 |
| SubE | R | \ | 80–90 ms | 100–110 ms | 120–130 ms | 26 | −3 | −17 | 3.316 |
| Posterior region | L–R | 18/31 | 60–70 ms | 80–90 ms | 130–140 ms | −6 | −59 | 20 | 4.474 |
| SupraE | L–R | 17/18/19/7/31 | a | 110–120 ms | 240–250 ms | −7 | −71 | −1 | 4.424 |
| SupraN | L–R | 17/18/19/7/31/ | a | 110–120 ms | 190–200 ms | −14 | −71 | 13 | 4.293 |
| SubE | L–R | 17/18/19//7/31 | a | 100–110 ms | 160–170 ms | −1 | −71 | 13 | 4.199 |
| SubN | L–R | 17/18/19/7 | a | 110–120 ms | 210–220 ms | −14 | −71 | −15 | 4.350 |
| PFC | L | 10/9 | 110–120 ms | 150–160 ms | 180–190 ms | −28 | 55 | 7 | 10.479 |
| SupraE | L | 10/9 | 90–100 ms | 110–120 ms | 210–220 ms | −33 | 43 | 11 | 4.908 |
| SubE | L | 10 | 110–120 ms | 160–170 ms | 190–200 ms | −28 | 48 | 7 | 5.164 |
Note: The bolded lines indicate the main effects; the following unbolded lines indicate the ERS of individual conditions for areas showing the main effects. The onset, offset, and peak times were all relative to the start of face presentation.
Due to processing associated with the premask, onset time for face processing in some areas was not obtainable.
The ERS results in different brain areas were described in terms of ERS onset, peak, and offset. ERS onset/offset means that, at a certain time, ERS became statistically significant/insignificant versus the control period. ERS peak means that, at a certain time, the activity reaches the highest level. We focused on the ERS after the face rather than after the premask presentation, so the onset, offset, and peak times were all relative to the start of face presentation, for example, ERS onset at 50 ms means that ERS started to be significant at 50 ms after face presentation (although it was 150 ms after the premask presentation).
The Main Effect of Awareness
The ANOVAs of ERS revealed a significant main effect of awareness in a large region of posterior cortex covering bilateral visual (BA18), parietal (BA7), and posterior cingulate cortex (BA 31). In addition, there was significantly greater ERS to supraliminal relative to subliminal stimuli within the right superior temporal sulcus (STS) (BA 22), left PFC–ACC (BA 9 but extending into BA 8 and 32), and right medial frontal cortex (BA 6).
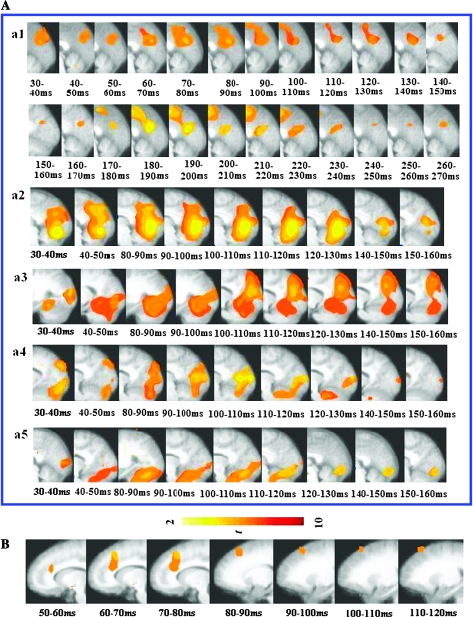
Within the large region of posterior cortex (including bilateral visual [BA18], parietal [BA7], and posterior cingulate cortex [BA 31]), the greater ERS power to supraliminal relative to subliminal trials became significant at 30–40 ms following face stimulus onset, differed most significantly at 80–90 ms, and was no longer significant by 300–310 ms. In the right STS, ERS became significant at 70–80 ms following face stimulus onset, differed most significantly at 150–160 ms, and was no longer significant by 190–200 ms. In the left PFC–ACC, ERS became significant at 40–50 ms following face stimulus onset, differed most significantly at 70–80 ms, and was no longer significant by 210–220 ms. In the right medial frontal cortex, ERS became significant at 60–70 ms following face stimulus onset, differed most significantly at 120–130 ms, and was no longer significant by 170–180 ms after face onset Figure 2.
Figure 2.
Significant awareness effect (A) shows the gamma band ERS profiles in the posterior region (BA 18, 31, and 7): (a1) shows the main awareness effect in the posterior region (BA 18, 31, and 7); (a2–a5) show significant ERS in SuprE, SuprN, SubE, and SubN in the in the posterior region (BA 18, 31, and 7), respectively. (B) Shows the significant main effect of awareness in PFC–ACC (BA 9 and 32). The onset, offset, and peak times here are all relative to the start of face presentation.
The Main Effect of Emotion
The ANOVAs of ERS revealed a significant main effect of emotion in the right amygdala, a large region of posterior cortex covering bilateral visual (BA18), parietal (BA7), and posterior cingulate cortex (BA 31) (P < 0.005), and PFC (BA 10 but extending into BA 9). In all cases, there was significantly greater ERS to emotional relative to neutral trials. Within the amygdala, this significant difference emerged at 40–50 ms, was most significant at 90–100 ms, and was no longer significant at 260–270 ms. For an illustration of the amygdala response, see Figure 3.
Figure 3.
Significant emotion effect (A) shows the ERS profiles in the right amygdala: (a1) shows the significant main effect of emotion in the right amygdala; (a2 and a3) indicate significant ERS in SuprE and SubE in the right amygdala. (B) Shows the significant main effect of emotion in the posterior cortex (BA 18 and 31). (C) Shows the significant main effect of emotion in the PFC (BA 10 and 9). R refers to the right and L to the left hemisphere. The onset, offset, and peak times here are all relative to the start of face presentation.
Within the large region of posterior cortex covering visual (BA 18), parietal (BA 7), and posterior cingulate (BA 31), the significant main effect of emotion emerged at 60–70 ms, being most significant at 80–90 ms, and being no longer significant at 130–140 ms. In PFC, the significant main effect of emotion emerged at 110–120 ms, being most significant at 360–370 ms, and was still significant by 390–400 ms (the offset was not observed before the response window).
Examining Gamma ERS for Subliminal Stimuli
Our first follow-up analysis examined whether there was significant gamma ERS for subliminal stimuli. The main effect for our initial ANOVA had shown significantly greater gamma ERS for supraliminal relative to subliminal stimuli within posterior cortex (BA18, 7, and 31), STS (BA 22), and left PFC–ACC (BA 9 and extending to 8 and 32). However, this did not identify whether there were any indications of significant gamma ERS to the subliminal stimuli; that is, relative to baseline. We thus examined ERS for subliminal emotional and neutral stimuli relative to baseline. In both cases, there was significant gamma ERS within posterior cortex (visual, parietal, and posterior cingulate cortex; P < 0.005; see Fig. 2, a1–a5). Subliminal emotional stimuli were also associated with significant gamma ERS in the right amygdala (P < 0.005; see Fig. 3, a2 and a3) and medial frontal cortex (BA 10). No significant gamma ERS was found for subliminal stimuli in either STS or within the region of PFC–ACC identified through the main effect of awareness.
The Impact of Stimulus Duration
In the present study, supraliminal and subliminal trials differed in timing. It could thus be argued that the awareness effect (greater gamma power for supraliminal rather than subliminal trials) related to this difference in timing, rather than the difference in awareness. To test such possibility, we analyzed the awareness effect involving the filler trials—empty ovals with the same supraliminal and subliminal presentation durations. We were interested to see if by subtracting the empty ovals from faces, the awareness effect could still be obtained. First, the contrasts between supraliminal faces versus subliminal ovals (contrast result: SupraPure) as well as subliminal faces versus subliminal ovals (contrast result: SubPure) were computed. The resultant contrasts SupraPure and SubPure were then entered for t-tests for each sliding window. The threshold was the same as that for the ANOVA: P < 0.001 except for visual cortex (P < 0.005).
The results indicated that a significant awareness effect was still obtained in the left PFC, the right STS, and bilateral posterior cortex (visual, parietal, and posterior cingulate cortices) for the same time period shown in the ANOVA analysis. However, a significant awareness effect was not observed for medial frontal cortex. This suggests that the awareness effect in the left PFC, the right STS, and bilateral posterior cortex was not due to the physical difference between supra- and subliminal trials.
Discussion
In this study, we investigated whether increases in awareness and emotionality were associated with increased gamma band synchronization. Our results revealed that first, there was significantly greater gamma band ERS for supraliminal relative to subliminal stimuli. Second, regions showing this effect included PFC–ACC, superior temporal cortex, and visual, parietal, and posterior cingulate cortex. Third, the large region of posterior cortex that included visual, parietal, and posterior cingulate cortex, but not PFC–ACC and STC, showed significant gamma band ERS to subliminal emotional and neutral stimuli as well, though to a lesser extent than, supraliminal stimuli. Fourth, a main effect of emotion was seen for gamma band ERS in several regions including PFC, amygdala, and visual, parietal, and posterior cingulate cortices.
Behavioral Performance
The detection rates indicated that the participants were unsurprisingly successful in detecting the presence of faces in supraliminal trials but poor in detecting the presence of faces in subliminal trials. Indeed, the individual discriminability index (d′) indicated that the participants were unaware of subliminal faces.
Although there was no significant impact of emotion on detection rate, emotion did significantly influence RT when making detection judgments. Participants were faster to respond to (detect) emotional rather than neutral faces at the supraliminal level, but they were slower to respond to (deny) emotional than neutral faces at the subliminal level. In visual search paradigms, faster detection of emotional stimuli is suggested to be associated with enhanced visual attentional processing and has been reported when the emotional stimuli are supraliminal (Fox et al. 2000; Öhman et al. 2001). Our study reveals that participants were slower to deny the existence of a subliminally presented fearful face than a neutral one (false negative). This suggests that emotion stimuli, even when subliminal, still have a significant effect on perceptual decision-making and emotional stimuli probably have a lower awareness threshold than neutral ones.
Gamma Band Synchronization and Emotion
In the present study, we observed that emotional relative to neutral stimuli were associated with significantly greater gamma band synchronization. This is consistent with our previous MEG–SAM study of gamma band ERS (Luo, Holroyd, et al. 2007) and broadly previous reports of emotional modulation of gamma band activity (Müller et al. 1999; Taylor et al. 2000; Oya et al. 2002). In particular, the present study showed emotional modulation of ERS within PFC (BA 10 and 9), amygdala, and visual, parietal, and posterior cingulate cortices.
The significant main effect of emotion in the right amygdala is consistent with our previous study (Luo, Holroyd, et al. 2007) and a report using intracranial recordings (Oya et al. 2002). Notably, we observed a significant main effect of emotion and significant gamma band ERS for subliminal emotional stimuli within the right amygdala. These gamma band ERS data are thus consistent with previous fMRI data examining the BOLD response showing significant amygdala activity to both supraliminal and subliminal emotional expressions (e.g., Whalen et al. 1998, 2004; Morris et al. 1999). These data also further support the ability of MEG to detect signal from deep sources such as the amygdala (Ioannides et al. 1995; Streit et al. 2003; Cornwell et al. 2007; Luo, Holroyd, et al. 2007).
A main effect for emotion for gamma band ERS was also seen within a large region of posterior cortex that included visual, posterior parietal, and posterior cingulate cortex (albeit P < 0.005 rather than P < 0.001) and a more lateral, anterior, and inferior region of PFC (BA 10, 9) than that was also partly observed for the awareness main effect (BA9, 8, 32) (see below). Interestingly, subliminal emotional stimuli showed significant gamma ERS within this PFC region (BA 10) although to a lesser degree than supraliminal emotional stimuli did. In short, significant gamma band ERS can be seen to some (emotional) subliminal stimuli within frontal cortex.
Gamma Band Synchronization and Visual Awareness
Our primary goal in this study was to investigate the hypothesis that visual awareness is realized through an attentional mechanism that binds together the neurons representing a visual object and that this is done by generating synchronized oscillations in the gamma band (cf. Crick and Koch 1990; Crick 1994). This hypothesis generated several hypotheses: in particular, gamma band ERS should be significantly greater for supraliminal relative to subliminal stimuli particularly in regions previously implicated in conscious awareness, for example, the midline frontal and parietal cortices (Dehaene and Naccache 2001; Rees et al. 2002; Stephan et al. 2002) and visual processing area (e.g., Fries et al. 1997). In line with these predictions, we did observe significantly increased gamma band synchronization in response to supraliminal relative to subliminal trials in the PFC–ACC, STS, and a large region of posterior cortex that included visual, parietal, and posterior cingulate cortices.
Previous work, using intracranial recordings, EEG or MEG (without source modeling), has reported enhanced gamma band synchrony in response to perceived but not to unperceived stimuli (Fries et al. 1997; Rodriguez et al. 1999; Srinivasan et al. 1999; Meador et al. 2002; Doesburg et al. 2005; Melloni et al. 2007). Our results, taken together with the earlier work, are consistent with the suggestion that neuronal synchronization in the gamma band is important for consciousness (Crick and Koch 1990; Crick 1994; Engel and Singer 2001; Varela et al. 2001). However, there are critical caveats to that conclusion.
First, it is important to note that the current study also identified significant gamma band ERS for subliminal stimuli within the identified region of posterior cortex (visual cortex, extending into parietal cortex and posterior cingulate cortex)—though ERS within this region was significantly greater for supraliminal stimuli. This suggests that significant gamma band ERS is not a sufficient condition for conscious awareness. It remains possible that the degree of rather than the existence of gamma band synchronization reflects the level of conscious awareness (cf. Grandjean et al. 2008).
Second, there was significantly greater gamma band ERS to emotional relative to neutral stimuli, irrespective of the awareness level of the stimuli, within the amygdala and a region of PFC that was lateral, anterior, and inferior of the PFC–ACC region revealed by the main effect of awareness. These data strongly suggest that significant gamma band ERS is not a sufficient condition for conscious awareness.
Third, the increased gamma band ERS seen for supraliminal relative to subliminal stimuli was relatively region specific. Thus, we observed significantly greater gamma band ERS for supraliminal relative to subliminal stimuli within PFC–ACC, superior temporal cortex, and a large posterior region that included visual cortex. Previous work (Fries et al. 1997, 2001) has reported increased gamma band synchronization in association with visual awareness in visual cortex. Our findings were thus consistent with this. However, as noted above, there was significant gamma band ERS for subliminal stimuli within this region. Moreover, there was also significantly greater gamma band ERS to emotional relative to neutral stimuli, irrespective of the awareness level of the stimuli, within this region (see Table 2). Thus, the increased gamma band ERS observed in this region while correlated with awareness does not necessarily result in awareness.
We also observed significantly greater gamma band ERS for supraliminal relative to subliminal stimuli within PFC–ACC. Considerable previous work has implicated PFC and ACC in awareness (see Dehaene and Naccache 2001; Tsuchiya and Adolphs 2007). Interestingly, the region revealed by the awareness main effect here had a peak within relatively medial BA 9 that extended into dorsal ACC (dACC) (BA 32) and into relatively lateral regions of BA 9 and 8 (see Fig. 2). Significant ERS within this region was only seen for supraliminal stimuli, irrespective of emotionality. Moreover, although emotionality was associated with significant ERS within frontal cortex, the region implicated was BA 10 (though this ERS did extend into some lateral regions of BA 9), and no significant emotionality effect was seen for dACC. These data suggest that (gamma band) activity within dACC and associated midline cortex may be critical for consciousness.
One caveat that should be considered relates to the recent concern raised by Yuval-Greenberg et al. (2008) that gamma band activity only reflects eye saccades in EEG. The concern applies to MEG data also as muscle artifacts do contribute broadband noise to the sensors. However, the problem is diminished when using the SAM analysis technique. This is because SAM effectively filters the data through forward dipole models that are located in the brain. That is, the broadband noise originating from the eyes creates fields at the sensor that do not appear to come from the brain.
A second caveat that should be considered is the index of consciousness used here; that is, the “detectability” of the stimulus. It is possible that data concerning consciousness on the basis of detectability judgments may not generalize, for example, to judgments regarding whether a stimulus is associated with a consciously accessible feeling state or not (cf. Grandjean and Scherer 2008).
Conclusions
Our results revealed that gamma band ERS did distinguish supraliminal and subliminal processing. However, significant gamma band ERS was obtained not only for the supraliminal but also for the subliminal conditions in areas including visual, parietal, posterior cingulate cortex, and the amygdala (amygdala: just for emotional stimuli) and a region within PFC (BA 10). In short, these data do not support the suggestion that gamma band synchronization is a sufficient condition for visual awareness.
It remains possible, however, that an important component of cortical processing involves binding together the neurons representing a visual object and that this is done by generating synchronized oscillations in the gamma band (cf. Crick and Koch 1990; Crick 1994). Certainly, gamma band ERS was observed to supraliminal and subliminal emotional stimuli within regions implicated in emotional processing through fMRI (e.g., Luo, Mitchell, et al. 2007).
The current study observed that although gamma ERS was greater for supraliminal relative to subliminal stimuli within visual cortex, it was still significantly shown for subliminal stimuli. This suggests that the binding process may occur within visual cortex if the object is processed. The binding may propagate through the system as a function of stimulus parameters; in the current study, appearing in emotion relevant regions such as the amygdala and BA 10 even for subliminal stimuli. However, the current data suggest that the individual will only become aware of this stimulus if the binding propagates to relatively dorsal regions of medial frontal cortex (medial BA 9 and particularly 32).
Funding
NIMH Intramural Research Program. Funding to pay the Open Access publication charges of this article was provided by the NIMH Intramural Research Program.
Acknowledgments
Conflict of Interest: None declared.
References
- Brookes MJ, Gibson AM, Hall SD, Furlong PL, Barnes GR, Hillebrand A, Singh KD, Holliday IE, Francis ST, Morris PG. GLM-beamformer method demonstrates stationary field, alpha ERD and gamma ERS colocalisation with fMRI BOLD response in visual cortex. Neuroimage. 2005;26:302–308. doi: 10.1016/j.neuroimage.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Baas JM, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. The astonishing hypothesis. New York: Scribner's; 1994. [Google Scholar]
- Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;1121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Dan Glauser ES, Scherer KR. Neuronal processes involved in subjective feeling emergence: oscillatory activity during an emotional monitoring task. Brain Topogr. 2008;20(4):224–231. doi: 10.1007/s10548-008-0048-3. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–38. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Doesburg S, Kitajo K, Ward L. Increased gamma-band synchrony precedes switching of conscious perceptual objects in binocular rivalry. Neuroreport. 2005;16:1139–1142. doi: 10.1097/00001756-200508010-00001. [DOI] [PubMed] [Google Scholar]
- Engel A, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Fawcett IP, Barnes GR, Hillebrand A, Singh KD. The temporal frequency tuning of human visual cortex investigated using synthetic aperture magnetometry. Neuroimage. 2004;21:1542–1553. doi: 10.1016/j.neuroimage.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D. The BOLD response and the gamma oscillations respond differently than evoked potentials: an interleaved EEG-fMRI study. BMC Neurosci. 2003;4:22. doi: 10.1186/1471-2202-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: are angry faces detected more efficiently? Cogn Emot. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Roelfsema P, Engel A, Konig P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proc Natl Acad Sci USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, Thompson DG, Hamdy S. Dissociating the spatiotemporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–1455. doi: 10.1016/j.neuroimage.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Gold I. Does 40-Hz oscillation play a role in visual consciousness? Conscious Cogn. 1999;8:186–195. doi: 10.1006/ccog.1999.0399. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Scherer KR. Conscious emotional experience emerges as a function of multilevel, appraisal–driven response synchronization. Conscious Cogn. 2008;17(2):484–495. doi: 10.1016/j.concog.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Scherer KR. Unpacking the cognitive architecture of emotion processes. Emotion. 2008;8(3):341–351. doi: 10.1037/1528-3542.8.3.341. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Klinger MR, Schuh ES. Activation by marginally perceptible (“subliminal”) stimuli: dissociation of unconscious from conscious cognition. J Exp Psychol Gen. 1995;124:22–42. doi: 10.1037//0096-3445.124.1.22. [DOI] [PubMed] [Google Scholar]
- Gross J, Schmitz I, Schnitzler K, Kessler K, Shapiro B, Hommel A, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guderian S, Duzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15(7):901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Hall SD, Holliday IE, Hillebrand A, Singh KD, Furlong PL, Hadjipapas A, Barnes GR. The missing link: concurrent human and primate cortical gamma oscillations. Neuroimage. 2005;15:13–17. doi: 10.1016/j.neuroimage.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Furlong PL, Holliday IE, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides AA, Liu MJ, Liu LC, Bamidis PD, Hellstrand E, Stephan KM. Magnetic field tomography of cortical and deep processes: examples of “real-time mapping” of averaged and single trial MEG signals. Int J Psychophysiol. 1995;20:161–175. doi: 10.1016/0167-8760(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska directed emotional faces. Stockholm (Sweden): Karolinska Institute; 1998. [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, Blair RJ. Common regions of dorsal anterior cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. Neuroimage. 2007;38:631–639. doi: 10.1016/j.neuroimage.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K, Ray P, Echauz J, Loring D, Vachtsevanos G. Gamma coherence and conscious perception. Neurology. 2002;59:847–854. doi: 10.1212/wnl.59.6.847. [DOI] [PubMed] [Google Scholar]
- Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating ‘unseen’ fear. Proc Natl Acad Sci USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Keil A, Gruber T, Elbert T. Processing of affective pictures modulates right-hemispheric gamma band EEG activity. Clin Neurophysiol. 1999;110:1913–1920. doi: 10.1016/s1388-2457(99)00151-0. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, III, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Linkenkaer-Hansen K, Naatanen R, Palva M. Early neural correlates of conscious somatosensory perception. J Neurosci. 2005;25:5248–5258. doi: 10.1523/JNEUROSCI.0141-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Netw. 2005;18:317–352. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Feelings integrate the central representation of appraisal-driven response organization in emotion. In: Manstead ASR, Frijda NH, Fischer AH, editors. Feelings and emotions: the Amsterdam symposium. Cambridge (UK): Cambridge University Press; 2004. pp. 136–157. [Google Scholar]
- Singer W. Neurobiology. Striving for coherence. Nature. 1999;397(6718):391–393. doi: 10.1038/17021. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A. Group imaging of taskrelated changes in cortical synchronisation using non-parametric permutation testing. Neuroimage. 2003;19:1589–1601. doi: 10.1016/s1053-8119(03)00249-0. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A, Forde EM, Williams AL. Task-related changes in cortical synchronization are spatially coincident with the hemodynamic response. Neuroimage. 2002;16:103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Russell D, Edelman G, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999;19:5435–5448. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KM, Thaut MH, Wunderlich G, Schicks W, Tian B, Tellmann L, Schmitz T, Herzog H, McIntosh C, Seitz RJ, et al. Conscious and subconscious sensorimotor synchronization—prefrontal cortex and the influence of awareness. Neuroimage. 2002;15:345–352. doi: 10.1006/nimg.2001.0929. [DOI] [PubMed] [Google Scholar]
- Streit M, Dammers J, Simsek-Kraues S, Brinkmeyer J, Wölwer W, Ioannides A. Time course of regional brain activations during facial emotion recognition in humans. Neurosci Lett. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Adolphs R. Emotion and consciousness. Trends Cogn Sci. 2007;11:158–167. doi: 10.1016/j.tics.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C. Are neocortical gamma waves related to consciousness? Brain Res. 2000;855:217–224. doi: 10.1016/s0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brain web: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MB. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]