Abstract
Single words and sentences referring to bodily actions activate the motor cortex. However, this semantic grounding of concrete language does not address the critical question whether the sensory–motor system contributes to the processing of abstract meaning and thought. We examined functional magnetic resonance imaging activation to idioms and literal sentences including arm- and leg-related action words. A common left fronto-temporal network was engaged in sentence reading, with idioms yielding relatively stronger activity in (pre)frontal and middle temporal cortex. Crucially, somatotopic activation along the motor strip, in central and precentral cortex, was elicited by idiomatic and literal sentences, reflecting the body part reference of the words embedded in the sentences. Semantic somatotopy was most pronounced after sentence ending, thus reflecting sentence-level processing rather than that of single words. These results indicate that semantic representations grounded in the sensory–motor system play a role in the composition of sentence-level meaning, even in the case of idioms.
Keywords: action words, fMRI, idioms, motor cortex, semantic somatotopy
Introduction
Semantic information conveyed by language is reflected in the brain response (Barsalou 1999, 2007; Pulvermüller 2001, 2005; Martin 2007). Specific brain activation patterns reveal fine-grained differences between semantic categories such as actions and objects (Pulvermüller et al. 1999; Martin and Chao 2001), tools and animals (Martin et al. 1996; Beauchamp and Martin 2007), as well as color and form (Moscoso del Prado Martin et al. 2006; Pulvermüller and Hauk 2006). Functional magnetic resonance imaging (fMRI) activation along the motor strip distinguishes between words and sentences that refer to actions involving the face, arms, or legs (Pulvermüller et al. 2001; Hauk et al. 2004; Tettamanti et al. 2005; Aziz-Zadeh et al. 2006; Kemmerer et al. 2007). This “semantic somatotopy” has provided a major argument supporting the idea that semantic mechanisms are grounded in action–perception systems of the brain (Pulvermüller 2005; Barsalou 2007; Glenberg 2007). A range of behavioral, transcranial magnetic stimulation (TMS), and neuropsychological studies also supports this view (Glenberg and Kaschak 2002; Neininger and Pulvermüller 2003; Buccino et al. 2005; Pulvermüller, Hauk, et al. 2005; Bak et al. 2006; Boulenger et al. 2006; Zwaan and Taylor 2006; Boulenger, Mechtouff, et al. 2008; Boulenger, Silber, et al. 2008; Nazir et al. 2008).
Previous research on the grounding of semantics in action–perception circuits has, however, suffered from a major shortcoming. Only concrete meaning of single words (“kick” vs. “pick”) and sentences (“she kicks the ball” vs. “she picks the pen”) has appropriately been examined. Here we ask whether semantic somatotopy in the motor system persists during processing of idiomatic sentence meaning (e.g., “she kicks the habit”). In a previous study, Aziz-Zadeh et al. (2006) examined the pattern of brain activation during reading of metaphorical sentences including action words (e.g., “biting off more that you can chew”) compared with action-related literal sentences (e.g. “biting the peach”). Although their results showed somatotopic activity in the premotor cortex for literal stimuli, they failed to reveal any significant motor activation for metaphorical sentences. This absence of effects may however be explained by methodological issues such as the limited number of stimuli (5 sentences per condition, repeated 8 times each). The aim of the present study was to more suitably test and clarify whether the motor system comes into play during comprehension of figurative action-related language. If the grounding of semantics in sensory–motor processes is a universal feature of the human cognitive system (Glenberg 1997; Lakoff and Johnson 1999; Glenberg and Kaschak 2002; Jeannerod 2006), the prediction is that action–perception information should influence semantic brain activation to sentences, even if their meaning is highly abstract. To test this, we chose to look at idioms that include words referring to actions performed using the arm and leg (e.g., “He grasped the idea” and “He kicked the habit,” respectively) and examined fMRI activation in the motor areas related to upper and lower limbs as the dependent variable.
The study also allowed us to address more general issues in cognitive science. According to compositional theories of semantics (Davidson 1967; Cacciari and Tabossi 1988; Titone and Connine 1999), the meaning of abstract sentences is computed from the meaning of words included in these sentences and from combinatorial information. Similar to a semantic grounding perspective (Glenberg 1997; Barsalou 2007), compositional theories would therefore receive support from semantic somatotopy to idioms that include action-related words. In this case, it would be argued that the meaning of the constituent words influences fMRI activation patterns at the sentence level (Gibbs and O'Brien 1990). Alternatively, abstract idioms could be stored separate from their constituent words as whole units and could be retrieved similar to the way long words are accessed in long-term memory (Bobrow and Bell 1973; Gibbs 1980). In such a “lexicalist” approach to idioms, no action word-related semantic activation and therefore no semantic somatotopy should emerge in the fMRI signature of idioms.
In language comprehension, early lexico-semantic processing of single words (Pulvermüller 2007 for a review) may be followed, after a delay, by the understanding of sentence meaning (Barber and Kutas 2006). In support of this view, metabolic activity related to semantic integration at the sentence level was found to be maximal at about 6–8 s after sentence completion (Humphries et al. 2007) or even later (Simmons et al. 2008). In the present study, we chose to monitor brain activation in 2 time-windows that might reflect different temporal steps during comprehension of idioms. Cortical activity was examined at the onset of the critical word of the sentences (“He grasped the IDEA”), which disambiguated the sentences as either idiomatic or literal (early analysis window), and 3 s after its end (late analysis window). The prediction was that (1) differences in cortical activation between literal and idiomatic sentences would emerge in both time-windows and that (2) semantic contribution of action words would become evident as semantic somatotopy at the sentence level, that is, in the late time-window.
Materials and Methods
Participants
Eighteen healthy right-handed native English speakers (8 females) participated in the study. They had normal or corrected-to-normal vision and no history of neurological or psychiatric disorder. The mean age of the volunteers was 24.3 years (SD = 6.3). They were paid for their participation. Ethical approval was obtained from the Cambridge Local Research Ethics Committee.
Materials
Seventy-six pairs of idiomatic and literal English sentences were used in this experiment. In each condition, half of the sentences included an arm-related action word (e.g., “John grasped the idea” and “John grasped the object”) and the other half contained a leg-related action word (e.g., “Pablo kicked the habit,” which means “to stop doing something that is difficult to stop doing,” and “Pablo kicked the ball”). Four experimental conditions were thus compared: arm-related idiomatic sentences (n = 38), arm-related literal sentences (n = 38), leg-related idiomatic sentences (n = 38), and leg-related literal sentences (n = 38). Sentence length varied from 3 to 7 words. The critical words of the sentences (e.g. “idea” and “object”), which disambiguated the sentences as either idiomatic or literal, were matched using the CELEX lexical database for relevant psycholinguistic variables, including word frequency, lemma frequency, length in letters, number of syllables, bigram frequency, trigram frequency, and number of orthographic neighbors. Arm- and leg-related action words were matched along the same variables (see Table 1). The 2 types of sentences were also matched for syntactic structure (i.e., only the critical words differed between idiomatic and literal conditions) and cloze probability. The latter parameter was defined as the number of occurrences, on http://www.google.co.uk, of the critical verb phrase of the sentences (e.g., “grasped the idea”; 53 917 ± 12 571 for idioms vs. 37 892 ± 13 856 for literal sentences, P > 0.05).
Table 1.
Mean values of word frequency, lemma frequency, length in letters, number of syllables, bigram frequency, trigram frequency, and number of orthographic neighbors are reported for idiomatic and literal critical words of the sentences, and for arm- and leg-related action verbs
| Critical words |
Action words |
|||||
| Idiomatic | Literal | ANOVA (by items) | Arm | Leg | ANOVA (by items) | |
| WORD FQ | 83.8 | 80.8 | P = 0.835 | 15.25 | 22.58 | P = 0.391 |
| LEMMA FQ | 143.7 | 117.9 | P = 0.251 | 93.75 | 113.33 | P = 0.683 |
| LETT | 5.55 | 5.51 | P = 0.891 | 4.67 | 4.17 | P = 0.193 |
| SYLL | 1.59 | 1.53 | P = 0.579 | 1 | 1 | P = 0.1 |
| BIGR | 39 137 | 40 133 | P = 0.616 | 32 984 | 23 052 | P = 0.176 |
| TRIG | 4811 | 5126 | P = 0.554 | 4859 | 1892 | P = 0.122 |
| ORTH NEIGH | 5.34 | 5.35 | P = 0.989 | 7.08 | 7.67 | P = 0.783 |
Note: P values for ANOVAs (by items) are reported. WORD FQ = word frequency (per million); LEMMA FQ = lemma frequency (p/m); LETT = length in letters; SYLL = number of syllables; BIGR = bigram frequency (p/m); TRIG = trigram frequency (p/m); ORTH NEIGH = number of orthographic neighbors.
Seventy-six baseline stimuli, consisting of strings of meaningless hash-marks matched in length with the sentences (e.g., “## ####### ### ####”), were also constructed (see Hauk et al. 2004 for similar methods). Finally, 6 literal sentences (different from and not related to the experimental stimuli; e.g., “John opened the door”) were used as probe sentences in a simple motor response task.
Procedure
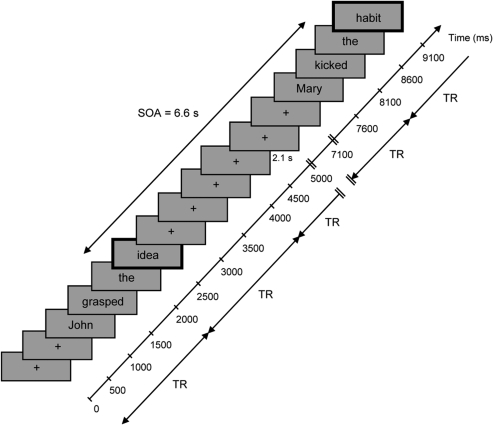
The main experiment was run in 2 blocks, each block consisting of 117 trials (76 experimental trials, 38 baseline trials and 3 probe trials). Sentences were presented word by word, each for 500 ms (stimulus onset asynchrony, SOA = 500 ms), in lower-case letters at the center of a computer screen. The SOA between critical words of 2 consecutive sentences was fixed (6.6 s) and the intersentence interval (ISI) (i.e., time interval between the offset of a sentence and the onset of the next sentence), during which a fixation cross remained on the screen, varied between 2.6 and 5.1 s (mean = 4.04 s, SE = 0.05; Fig. 1). Participants were given the following instructions: “After display of a fixation cross at the center of the screen, sentences will be presented word by word. Please read words silently but attentively. Sequences of symbols will also be displayed, please look at them attentively.” They were told to attend to the meaning of each sentence and to be prepared to respond to test questions probing their comprehension. To this end, they had to answer simple yes/no questions about probe sentences, interspersed between critical sentences, by pressing a button on a 2-button response box either with their left index or middle finger. For instance, after reading “John opened the door”, they had to answer “no” to the question “Did John open the fridge?”. Note that subjects did not know which sentences were probes, that is, they had to expect questions after any sentence. Stimuli were presented in a randomized order by means of E-Prime software (Psychology Software Tools, 2001) and viewed via a back-projection screen located in front of the scanner and a mirror placed on the head coil.
Figure 1.
Design of the experiment. Each trial was composed of 10 displays/screens, here represented by gray boxes, where the consecutive stimuli—fixation cross “+” and words making sentences—appeared each for 500 ms. Two examples of arm- and leg-action–related sentences are given. SOA between 2 consecutive critical words (indicated in bold) was 6.6 s. A fixed delay of 2.1 s, where a fixation cross remained on the screen, was inserted between 2 consecutive trials, so that the ISI varied between 2.6 and 5.1 s. The oblique axis on the right illustrates the temporal sequence of the trials and gives the onset of the corresponding stimulus (in milliseconds). TR of the EPI sequence is also represented (TR = 2 s).
Subsequent to the main experiment, participants were asked to perform a motor localizer task. The localizer scans always followed the sentence experiment to avoid any attentional bias toward action-related aspects of the stimuli. Instructions on which extremity to move (right or left index finger, right or left foot) were presented visually on the computer screen. Instructions remained on the screen for 20 s each and were repeated 4 times in pseudorandomized order (see Hauk et al. 2004).
Imaging Methods
Subjects were scanned in a 3-T Siemens Tim Trio magnetic resonance system using a head coil. Echo-planar imaging (EPI) sequence parameters were time repetition (TR) = 2 s, time echo = 30 ms and flip angle = 78°. The functional images consisted of 32 slices covering the whole brain (slice thickness 3 mm, interslice distance 0.75 mm, in-plane resolution 3 × 3 mm). Imaging data were processed using SPM5 software (Wellcome Department of Cognitive Neurology, London, UK).
Images were corrected for slice timing and then realigned to the first image using sinc interpolation. Any nonbrain parts were removed from the T1-weighted structural images by using a surface-model approach (“skull-stripping”; Smith 2002). The EPI images were coregistered to these skull-stripped structural T1 images by using a mutual information coregistration procedure (Maes et al. 1997). The structural MRI was normalized to the 152-subject T1 template of the Montreal Neurological Institute (MNI). The resulting transformation parameters were applied to the coregistered EPI images. During the spatial normalization process, images were resampled with a spatial resolution of 2 × 2 × 2 mm3. Finally, all normalized images were spatially smoothed with a 10-mm full-width half-maximum Gaussian kernel, and single-subject statistical contrasts were computed by using the general linear model, including 3 orthogonal basis functions (canonical Haemodynamic Response Function [HRF], its time derivative and dispersion as implemented in SPM5; Friston et al. 1998). Only the estimate for the canonical HRF was used for the second level statistics, which is a measure for the amplitude of the brain response. Low-frequency noise was removed with a high-pass filter (time constant 128 s). We modeled the 4 experimental conditions (arm idiomatic, leg idiomatic, arm literal, and leg literal sentences) with the onset of the HRF response time-locked to the onset of the critical words of the sentences (i.e., early analysis window) and to a point delayed by 3 s from their offset (i.e., late analysis window). The probe sentences were modeled as separate events, though the corresponding data were not analyzed. Results are presented for both analysis windows. Group data were analyzed with a random-effects analysis. For visual display, Figures report results at P = 0.001, uncorrected. Tables report activations that passed the threshold of P = 0.001, uncorrected; activations that survived false discovery rate (FDR) correction (Genovese et al. 2002) at P < 0.05 are also indicated. Stereotaxic coordinates for voxels with maximal Z values within activation clusters are reported in the MNI standard space (which resembles very closely the standardized space of Talairach and Tournoux 1988; see Brett, Anton, et al. 2002).
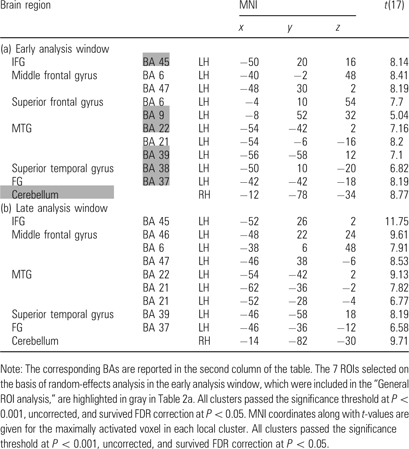
Several statistical analyses aimed at testing different hypotheses were performed. First, to assess whether reading of idioms and literal sentences activated a common cortical network and/or possibly additional selective brain areas, we carried out an analysis with 7 regions of interest (ROIs; “General ROI analysis”). On the basis of random-effects analysis in the early analysis window, we defined 7 ROIs activated by all sentences compared with the baseline (see Table 2; left perisylvian language areas—i.e., inferior frontal gyrus [IFG] Brodmann area [BA] 45, middle temporal gyrus [MTG] BA 22, angular gyrus [AG] BA 39, fusiform gyrus [FG] BA 37, and temporal pole [TP] BA 38—dorso-lateral prefrontal cortex BA 9 [known as being involved in idiom processing, see Lauro et al. 2007] and right cerebellum). This definition was done by using MARSBAR software utility (Brett, Johnsrude, et al. 2002). For each subject and each of these 7 ROIs, average parameter estimates over voxels were calculated for spheres of radius 10 mm. This was done in both early and late analysis windows. Cortical activity in the 7 ROIs was compared between the early and late windows using a 4-way ANOVA with the design Time-Window (early vs. late) × ROI × Idiomaticity (idiomatic vs. literal) × Body Part (arm vs. leg). To further analyze potential interactions, time-windows were then examined separately with a 3-way ANOVA (ROI × Idiomaticity × Body Part). Significant effects are reported in the text only if they survived Greenhouse–Geisser correction.
Table 2.
Coordinates and statistics for activation peaks produced by all sentences (idiomatic and literal) versus the baseline (hash-marks strings) in the (a) early and (b) late analysis windows
 |
Second, we directly tested the hypothesis that both idioms and literal sentences that include action words activate the motor cortex somatotopically. To this aim, we performed an analysis including the 2 ROIs selected from the motor localizer task (“motor localizer ROI analysis”). Because idiomatic and literal sentences elicited mainly left-lateralized activity, we selected left-hemispheric ROIs from the right finger and right foot conditions only. Right finger movements yielded activity in the left postcentral gyrus (BA 2: −50 −22 44, t(17) = 9.54), whereas activity in a left dorsal area on the midline was observed during execution of right foot movements (BA 6: −4 −26 72, t(17) = 16.34). Note that these postcentral activations (for which maximal t-values were obtained) may be related to somatosensory self-stimulation during motor performance (see also Hauk et al. 2004). Activity in these regions was compared between the early and late analysis windows with a 4-way ANOVA (Time-Window × ROI × Idiomaticity × Body Part). Values were then subjected to a 3-way ANOVA (ROI × Idiomaticity × Body Part) where the 2 time-windows were analyzed separately.
Because ROIs from the motor localizer ended up in postcentral cortex, we subsequently performed an additional statistical analysis with a priori selected ROIs along the central sulcus and precentral gyrus (“motor strip ROI analysis”; as in Pulvermüller et al. 2006). A chain of 9 spheres with a radius of 10 mm was aligned along the central sulcus of the standard MNI brain between vertical z-coordinates 25 and 76 mm. An additional line of 9 regions just 1 cm anterior to the central ROIs was defined in the same way in the precentral gyrus. These central and precentral regions were selected a priori as belonging to the motor strip (Penfield and Rasmussen 1952; see also Pulvermüller et al. 2006 for similar methods). The subdivision of precentral and central cortex resulted in an array of 2 × 9 = 18 regions, for each of which activation values were obtained for each condition (idiomatic vs. literal, and arm- vs. leg-action relatedness) and subject. A 4-way ANOVA with the design Time-Window (early vs. late) × Dorsality (9 regions, inferior to superior) × Idiomaticity × Body Part was used to compare cortical activity along the motor strip between both analysis windows. Activity was then analyzed in each of these windows with an additional 4-way ANOVA (Frontality [precentral vs. central] × Dorsality × Idiomaticity × Body Part).
Results
Behavioral Results
To ensure that the 18 participants were attentive to the silent reading task, they were asked to answer yes/no questions about probe sentences by pressing one of 2 buttons with left index or middle finger. Mean error rate was small (8.3%, SE = 2.45), indicating that they paid attention to the sentences.
fMRI General Activation
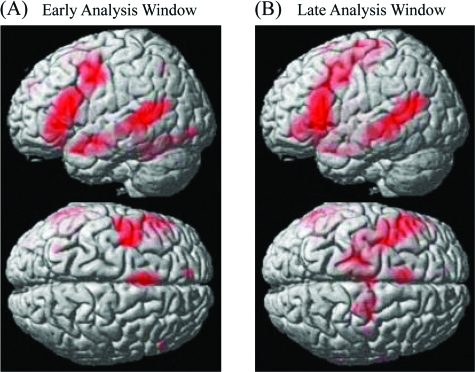
In both early and late analysis windows, comparison of all sentences (literal and idiomatic) to the baseline (hash-mark strings) revealed left-lateralized activation in core language areas, that is, the IFG and the MTG (Table 2 and Fig. 2). Activity was also observed in left FG, left AG, left TP, left dorsolateral prefrontal cortex (DLPC), and right cerebellum. Regions in the primary motor and premotor cortex were further activated during reading of action-related sentences. As can be seen from Figure 2, this activity along the motor strip tended to be more distributed and to extend further in dorsal motor areas (z-coordinates > 50 mm) in the late analysis window.
Figure 2.
Cortical activation during silent reading of all sentences (idiomatic and literal) relative to the baseline (hash-mark strings) in the (a) early and (b) late analysis windows (P < 0.001, uncorrected). Results are rendered on a standard brain surface. Top panel: lateral view of the brain, Bottom panel: top view. Note the greater activation of precentral areas in the late window.
Idiomatic versus Literal Sentence Processing
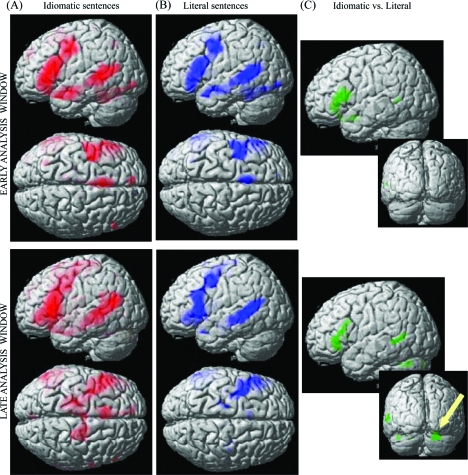
The results broken down for the idiomatic and literal sentences, compared with the baseline, are presented in Figure 3a,b, respectively. A common network of cortical activity was observed for both conditions in both analysis windows, with the idioms eliciting overall more distributed activation. This network included core perisylvian language areas as well as the right cerebellum (Table 3). Importantly, the precentral and middle frontal gyri including the premotor and motor cortex were activated when both literal and idiomatic sentences were being processed.
Figure 3.
Cortical activation during silent reading of (a) idioms and (b) literal sentences (P < 0.001, uncorrected), compared with the baseline (hash-marks strings), in the early (top panel) and late analysis windows (bottom panel). Results are rendered on a standard brain surface. Specific activations for the direct contrast between idioms and literal sentences are reported for both windows in (c). The inset in (c) highlights the specific activation observed in the right cerebellum for idioms, compared with literal sentences, in the late window (bottom panel).
Table 3.
Coordinates and statistics for activation peaks produced by literal and idiomatic sentences in the (a) early and (b) late analysis windows, compared with the baseline (hash-marks strings)
| Brain region | MNI |
t(17) | ||||
| x | y | z | ||||
| (a) Early analysis window | ||||||
| Literal sentences | ||||||
| IFG | BA 45 | LH | −50 | 20 | 16 | 7.49 |
| BA 47 | LH | −48 | 30 | 2 | 6.37 | |
| Middle frontal gyrus | BA 6 | LH | −40 | −2 | 48 | 8.71 |
| BA 9 | LH | −38 | 10 | 24 | 4.81 | |
| Superior frontal gyrus | BA 6 | LH | −4 | 10 | 54 | 7.27 |
| BA 6 | LH | −6 | 12 | 70 | 4.29 | |
| MTG | BA 21 | LH | −54 | −6 | −14 | 7.08 |
| BA 39 | LH | −56 | −58 | 12 | 6.82 | |
| Superior temporal gyrus | BA 22 | LH | −64 | −46 | 8 | 7.2 |
| BA 38 | LH | −52 | 12 | −18 | 5.61 | |
| FG | BA 37 | LH | −42 | 40 | −16 | 7.96 |
| Cerebellum | RH | 12 | −78 | −34 | 8.46 | |
| Idiomatic sentences | ||||||
| IFG | BA 45 | LH | −48 | 30 | 4 | 9.52 |
| BA 47 | LH | −36 | 32 | −16 | 6.68 | |
| BA 46 | LH | −52 | 26 | 12 | 8.74 | |
| BA 9 | LH | −36 | 10 | 24 | 5.97 | |
| Medial frontal gyrus | BA 6 | LH | −6 | −14 | 70 | 3.89 |
| Superior frontal gyrus | BA 6 | LH | −4 | 12 | 58 | 7.38 |
| BA 9 | LH | −8 | 50 | 30 | 5.9 | |
| MTG | BA 21 | LH | −54 | −6 | −16 | 8.37 |
| BA 39 | LH | −56 | −60 | 12 | 7.02 | |
| Superior temporal gyrus | BA 22 | LH | −64 | −46 | 8 | 7.78 |
| BA 38 | LH | −50 | 12 | −22 | 7.5 | |
| Precental gyrus | BA 6 | LH | −42 | −4 | 48 | 7.59 |
| BA 6 | LH | −38 | 0 | 32 | 7.42 | |
| Cerebellum | RH | 12 | −78 | −34 | 7.72 | |
| Idiomatic > literal | ||||||
| IFG | BA 45 | LH | −48 | 28 | 6* | 5.61 |
| BA 44 | LH | −58 | 16 | 10* | 4.78 | |
| (b) Late analysis window | ||||||
| Literal sentences | ||||||
| IFG | BA 45 | LH | −48 | 18 | 12 | 5.74 |
| BA 47 | LH | −50 | 20 | 0 | 6.29 | |
| Middle frontal gyrus | BA 47 | LH | −48 | 36 | −6 | 4.98 |
| BA 6 | LH | −40 | 4 | 48 | 7.63 | |
| BA 46 | LH | −50 | 24 | 26 | 5.85 | |
| MTG | BA 22 | LH | −52 | −40 | 2 | 6.91 |
| BA 21 | LH | −60 | −48 | 6 | 5.98 | |
| Superior temporal gyrus | BA 22 | LH | −58 | −54 | 12 | 5.48 |
| BA 21 | LH | −50 | −22 | −6 | 5.12 | |
| Cerebellum | RH | −16 | −80 | −34 | 6.44 | |
| Idiomatic sentences | ||||||
| IFG | BA 45 | LH | −48 | 22 | 12 | 10.49 |
| Middle frontal gyrus | BA 47 | LH | −44 | 36 | −4 | 10.68 |
| BA 46 | LH | −52 | 24 | 4 | 11.65 | |
| BA 6 | LH | −34 | −4 | 54 | 7.34 | |
| Medial frontal gyrus | BA 8 | LH | −6 | 42 | 40 | 5.18 |
| Superior frontal gyrus | BA 6 | LH | −12 | 18 | 60 | 6.93 |
| BA 9 | LH | −12 | 52 | 28 | 4.93 | |
| MTG | BA 21 | LH | −52 | −28 | −4 | 6.78 |
| BA 22 | LH | −56 | −40 | 2 | 8.87 | |
| BA 39 | LH | −58 | −58 | 20 | 11.01 | |
| FG | BA 37 | LH | −46 | −36 | −10 | 6.79 |
| Precental gyrus | BA 6 | RH | 16 | −18 | 68 | 8.17 |
| Cerebellum | RH | 16 | −82 | −30 | 10.19 | |
| Idiomatic > literal | ||||||
| IFG | BA 45 | LH | −44 | 30 | 2* | 4.84 |
| Middle frontal gyrus | BA 9 | LH | −56 | 20 | 26* | 4.37 |
| MTG | BA21 | LH | −62 | −56 | 6* | 4.69 |
| Cerebellum | LH | 20 | −82 | −32* | 5.13 | |
Note: Activation peaks for the contrast idiomatic vs. literal stimuli are also reported. The corresponding BAs are indicated in the second column of the table. All clusters passed the significance threshold at P < 0.001, uncorrected. Activations that did not survive FDR correction at P < 0.05 are indexed by asterisks. MNI coordinates along with t-values are given for the maximally activated voxel in each local cluster. All clusters passed the significance threshold at P < 0.001, uncorrected. Activations that did not survive FDR correction at P < 0.05 are indexed by asterisks.
Direct comparisons between the 2 activation conditions showed that literal sentences failed to elicit stronger activation than idioms in any brain area. In contrast, stronger activation to idioms than to literal sentences was seen in IFG (pars triangularis of Broca's area) in both early and late analysis windows, in the pars opercularis of Broca's area in the early window only, and in MTG, right cerebellum and DLPC in the late window only (Fig. 3c and Table 3).
General ROI Analysis
To test activity dynamics related to Time-Window, Idiomaticity, and Body Part reference of action verbs, along with their possible interactive effects, activity in 7 ROIs was compared between arm/leg-related idiomatic/literal sentences in the early and late analysis windows (“General ROI analysis,” see Imaging Methods). A 4-way ANOVA (Time-Window × ROI × Idiomaticity × Body Part) revealed a significant main effect of Time-Window (F1,17 = 18.09, P = 0.001) and a significant Time-Window × ROI interaction (F1,17 = 12.31, P < 0.001), indicating that cortical activity was weaker in the late than in the early analysis window in a range of areas (IFG, MTG, FG, TP, and AG, P values < 0.003), whereas in other regions (cerebellum and DLPC), no significant change was observed (i.e., prolonged activation).
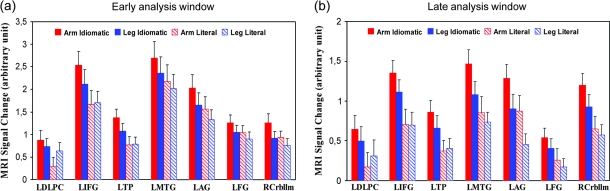
To further analyze these complex interactions, the analysis windows were examined with separate 3-way ANOVAs (see Imaging Methods). Results, which are presented in Figure 4, first revealed a significant main effect of ROI (early window: F1,17 = 17.24, P = 0.001; late window: F1,17 = 11.65, P = 0.001), indicating that cortical activity was particularly strong in left perisylvian areas (IFG, MTG, and AG). A significant main effect of Idiomaticity (early: F1,17 = 11.92; P = 0.003; late: F1,17 = 16.42, P = 0.001) and a significant ROI × Idiomaticity interaction (in the early window only: F1,17 = 4.46, P = 0.002) also emerged, suggesting stronger activity for idioms than for literal sentences, especially in IFG, MTG, TP, and AG in the early analysis window (P values < 0.025). In the late analysis window, the right cerebellum was strongly activated also showing enhancement of activity to idioms (P = 0.001). There was a significant ROI × Body Part interaction (early: F1,17 = 3.17, P = 0.02; late: F1,17 = 3.56, P = 0.01), documenting that activity in the 7 defined ROIs depended on the body part reference of the action verbs. In the early analysis window, arm-related sentences generally activated more strongly the defined ROIs than leg-related sentences (P values < 0.035; with exceptions however in the IFG and DLPC). In the late window, activity was stronger for arm sentences than for leg sentences in the AG (P = 0.017).
Figure 4.
Mean parameter estimates (in arbitrary units) for the 7 ROIs in the 4 experimental conditions (arm idiomatic, leg idiomatic, arm literal, and leg literal sentences) in the (a) early and (b) late analysis windows. Error bars are reported. LDLPC, left DLPC; LIFG, left IFG; LTP, left TP; LMTG, left MTG; LAG, left AG; LFG, left FG; RCrbllm, right cerebellum.
Semantic Somatotopy
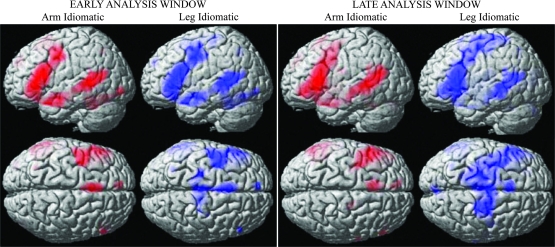
Inspection of Figure 5 shows that in the critical comparison between idioms including arm- and leg-related action words, different activation patterns were obtained. Already in the early analysis window, only idioms that include leg words led to spreading of activity to leg areas in dorsocentral cortex compared with the baseline (right hemisphere [RH]: 10 −24 60, t(17) = 7.1; left hemisphere [LH]: −22 −26 60, t(17) = 5.3). This effect seemed even more pronounced in the late window (LH: −18 −28 72, t(17) = 7.19; RH: 22 −26 70, t(17) = 9; LH: −12 −20 72, t(17) = 7.71; RH: 20 −12 68, t(17) = 8.59). A 4-way ANOVA (Time-Window × ROI × Idiomaticity × Body Part) including the 2 ROIs selected from the motor localizer experiment (“motor localizer ROI analysis,” see Imaging Methods) revealed a significant interaction between Time-Window, ROI, and Body Part (F1,17 = 7.6, P = 0.013). This establishes that cortical activity in motor areas was modulated by the body part relationship of words included in the sentences differently for the 2 time-windows.
Figure 5.
Somatotopic activation for idioms including arm- (in red) and leg-related action words (in blue), compared with the baseline (hash-mark strings), in the early (left panel) and late analysis windows (right panel; P < 0.001, uncorrected). Results are rendered on a standard brain surface.
To analyze this triple interaction, activity in finger and foot areas was examined separately for early and late analysis windows (see Imaging Methods). Whereas the early analysis window failed to show any ROI × Body Part interaction, the late window revealed a significant interaction of these 2 factors (F1,17 = 7.42, P = 0.014). This result shows that sentences including leg-related action words elicited stronger activity in the left foot dorsal area, whereas arm-related sentences recruited more strongly the left finger lateral area. Note that there was overlap, but not absolute congruency, between movement and action-sentence-related focal activations in the motor system, an observation which is in very good agreement with previous work on single action words (Hauk et al. 2004; Kemmerer et al. 2007) where also overlap, but not exact congruency, between the brain loci processing movements and action-related language was found (Hauk et al. 2004 reported the highest t-value to arm words at z = 48 mm but that for finger movements at z = 60 mm, 12 mm dorsal to it). A significant ROI × Idiomaticity interaction (F1,17 = 7.99, P = 0.012) finally documented that idioms produced greater activation than literal sentences particularly in the left foot dorsal area. Note again that the ROIs from the motor localizer were however located in the postcentral cortex, possibly due to somatosensory self-stimulation during movement execution (for discussion, see also Hauk et al. 2004).
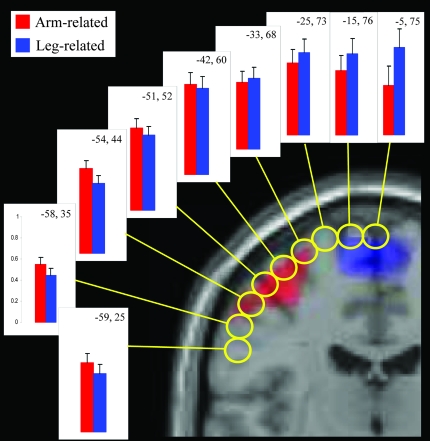
To further examine semantic somatotopy to arm- and leg-related sentences, we carried out an additional analysis for both analysis windows in regions along the motor strip (“motor strip ROI analysis,” see Imaging Methods). A 4-way ANOVA (Time-Window × Dorsality × Idiomaticity × Body Part) showed a significant interaction between Time-Window, Dorsality, and Body Part (F8,136 = 4.09, P < 0.001), documenting that the body part reference of action words modulated cortical activity in motor areas differently for the 2 time-windows. Given that no significant indication of somatotopy was found in the early analysis window with the “motor localizer ROI analysis” (see above), the “motor strip ROI analysis” including the chain of 2 × 9 regions along the motor strip was performed for the late window only. A 4-way ANOVA (Frontality × Dorsality × Idiomaticity × Body Part) revealed a significant interaction between the Dorsality and Body Part factors (F8,136 = 4.40, P < 0.001). As can be seen in Figure 6, in dorsal ROIs (z-coordinate ∼75 mm), leg sentences elicited stronger activation than arm sentences, and the opposite pattern, relatively stronger arm-sentence activation, was seen in lateral ROIs (25 mm ≤ z ≤ 50 mm). Semantic somatotopy (Dorsality × Body Part interaction) was also found in separate analyses for central (P = 0.002) and precentral regions (P = 0.05), suggesting that both motor and premotor cortex contributed to this effect. A 2-way ANOVA (ROI × Body Part), including a dorsal motor ROI (computed over the 3 regions with 73 ≤ z ≤ 76 mm) and a lateral motor ROI (computed over the 3 regions with 35 ≤ z ≤ 52 mm), confirmed this result by showing a significant ROI × Body Part interaction (F1,17 = 6.55, P = 0.02). Finally, additional analyses on local differences revealed stronger leg- than arm-sentence responses in the most dorsal ROI (z = 75 mm; P = 0.033), whereas stronger activity for arm sentences was found in a more lateral ROI (z = 44 mm; P = 0.037). These regions are in good agreement with those reported by Hauk et al. (2004) who found maximal activation probabilities (t-values) in precentral gyrus for arm words at z = 48 mm and for leg words at z = 64 mm.
Figure 6.
Semantic somatotopy for literal and idiomatic sentences along the motor strip in the late analysis window. Bar graphs show mean parameter estimates (in arbitrary units) for the 9 ROIs aligned along the central sulcus and the precentral gyrus that are reported for sentences including arm- (in red) and leg-related action words (in blue). For each graph, the /x/ and /z/ coordinates are indicated at the top right (x, z). The locations of the ROIs are reported (yellow circles) on a coronal slice of the brain. Somatotopic activations elicited during finger (in red) and foot movements (in blue) during the localizer experiment are also shown.
A significant Frontality × Idiomaticity × Body Part interaction also emerged (F8,136 = 23.61, P < 0.001), suggesting that activity along the central sulcus and in the precentral gyrus was differentially modulated by the idiomatic nature of the sentences as well as by the body part reference of the component action words. This interaction, which was not influenced by body part representations (superior vs. lateral ROIs), was due to generally enhanced blood oxygenation level–dependent (BOLD) signals to idioms in precentral cortex and an additional tendency for leg-idiomatic sentences to more strongly activate central areas.
Discussion
Silent reading of sentences including action words activated a range of left perisylvian fronto-temporal areas with a well-known role in language processing, along with the FG and the right cerebellum. Idioms activated most of these areas more strongly than literal sentences, both in early and late analysis windows. Activity patterns critically depended on the body part reference of action-related words embedded into both idioms and literal sentences. Semantic somatotopy with stronger dorsal motor cortex activation for “leg-action” idioms (“He kicked the habit”) and relatively stronger lateral motor cortex activation for “arm-action” idioms (“He grasped the idea”) was evident, especially when the modeled metabolic response was adjusted to a time period after critical word ending, to capture the metabolic indexes of sentence-level meaning processing (Figs 5 and 6). These results establish for the first time the differential involvement of motor and premotor cortex in idiom processing and support theories that view abstract semantics as grounded in action-perception systems (Pulvermüller 2005; Barsalou 2007; Glenberg 2007; Martin 2007).
Materials putting a particularly heavy burden on the language system are known to activate the left fronto-temporal language network more strongly and in a more widespread fashion than relatively simple language stimuli. For instance, it is well known that the N400 brain response is enlarged to sentences including semantically unexpected constituent words (Kutas and Hillyard 1984; Van Berkum et al. 1999), the main generators of this effect being localized in the posterior perisylvian cortex (Van Petten and Luka 2006). Rodd et al. (2005) presented sentences with unexpected ambiguities and found enhanced and more distributed fronto-temporal fMRI activation relative to unambiguous control sentences. Lauro et al. (2007) probed literal and idiomatic sentences and found left fronto-temporal activation also extending into anterior inferior frontal cortex, anterior temporal cortex and AG (see also Rapp et al. 2004; Lee and Dapretto 2006; Zempleni et al. 2007 for fMRI studies on idiom and metaphor processing). Our present activity enhancements to idioms at left inferior frontal and middle temporal sites are consistent with this pattern of results. They may, however, be best explained as an index of increased workload on the language system rather than as specific brain signature of idiom processing.
In the late analysis window examined here, we found additional idiom-related activation enhancement in the cerebellum and in the middle frontal gyrus extending into frontocentral motor and premotor cortex. The stronger cerebellar activity to idioms extends previous findings on the role of this structure in perceptual and language processing (Ivry and Keele 1989; Braitenberg et al. 1997; De Smet et al. 2007; Ackermann 2008). The co-occurrence of cerebellar and motor cortex increased activation to idiomatic sentences further suggests a consorted role of these structures in motor cognition (Jeannerod 2006) brought about by abstract action-related language.
For both idiomatic and literal sentences, we observed differences that reflected the meaning of their constituent arm- and leg-related action words. This influence became evident in a range of analyses, most notably with the significant ROI × Body Part interaction in the analysis of motor and premotor cortex activation (“motor strip ROI analysis”; Fig. 6). This interaction did not involve the “Idiomaticity” factor, thus documenting that the well-known semantic somatotopy found for concrete action words and sentences (Pulvermüller et al. 2001; Hauk et al. 2004; Tettamanti et al. 2005; Aziz-Zadeh et al. 2006) can be replicated for abstract sentences including action words. Activation of frontocentral motor and premotor areas was relatively weak at the onset of critical words but was strong after their offset (Fig. 5). If the fMRI brain response reflecting sentence meaning is delayed relative to that of single words (Humphries et al. 2007), this late activation of motor areas can be linked to the sentence processing stage. Any contribution of individual action words would have been expected to arise at action word onset (on average 1.2 s before critical word onset and thus 4.7 s before the late analysis window) and to decrease with time. Our results therefore suggest that the orchestration of abstract meaning in the human brain is not solely explained by the activation of unspecific semantic centers in fronto-temporal cortex, but that it involves late complementary activations in the sensory–motor system. These referentially grounded activations may play a specific functional role in the composition of sentence meaning. However, further work using techniques such as TMS and neuropsychological studies in brain-damaged patients are necessary to draw firm conclusions on functional contributions of the motor system to idiom comprehension.
The present results support a compositional perspective on semantic processing postulating that idiom meaning is computed from the semantics of constituent words and from combinatorial information. Semantic somatotopy to idioms indeed suggests that meaning aspects of words included in these sentences are being re-accessed and combined in the relatively late construction of sentence meaning. Access to concrete referential aspects of constituent words, as it regularly occurs in language comprehension, appears not as an irrelevant by-product but rather as an important step in the comprehension process, which may play a role in the comprehension of figurative language too (Gibbs et al. 1989; Gibbs and O'Brien 1990; Titone and Connine 1999). In the context of the present study, one may argue that the activation of dorsal and lateral motor and premotor cortex was related to the processing of leg- and arm-related words per se and not to the comprehension of sentence meaning. We should remind the reader, however, that somatotopic semantic grounding of constituent arm/leg words in lateral/dorsal frontocentral cortex, respectively, was relatively weak at action word onset and also at presentation of critical words (Fig. 5). It became pronounced about 3 s after sentence ending, suggesting its specificity to a late stage of sentence processing. At such a late stage, it would be extremely unlikely that one particular word from the several ones included in the sentence is still processed in depth in isolation and dominates the brain response. Rather, it appears plausible that semantic integration at the sentence level underlies metabolic changes, which might occur especially late for highly abstract sentences. This pattern of results is therefore consistent with a gradual emergence of semantic somatotopy in the processing of idiom meaning and is not explained by word-related activation.
Previous electrophysiological studies have shown instant spreading of activity to motor regions during action word recognition (<200 ms; Pulvermüller et al. 1999; Hauk and Pulvermüller 2004; Pulvermüller, Shtyrov, et al. 2005; Kiefer et al. 2007; Boulenger, Silber, et al. 2008; for a review, see Hauk et al. 2008). Despite the apparent discrepancy between these results and those of the present study, the reader should however be reminded that 1) previous neurophysiological work focused on single words while we here used complex sentences, where action words had to be integrated into their context; 2) as opposed to fMRI, electrophysiological techniques offer high temporal resolution that allows precise tracking of the time-course of brain activation during cognitive processes. Future research, using electroencephalography and/or magnetoencephalography for instance, is therefore necessary to address the question of when grasping ideas activates the motor system. Comparing the present results with previous fMRI studies of brain activation to action-related sentences (Tettamanti et al. 2005; Aziz-Zadeh et al. 2006), it is still noteworthy that BOLD signal changes occurred relatively late in the present study. This indicates that, if idioms appear in the context of literal sentences, as they do in normal language use, semantic and any postunderstanding processes may be delayed relative to an experimental context where only literal sentences are presented. We note however that early brain reflections of semantic processing of word pairs (Shtyrov and Pulvermüller 2007; Hoenig et al. 2008) have recently been reported early-on (100–150 ms) and even the first effects of sentence-level semantics have been found between 100–200 ms (Sereno et al. 2003; Penolazzi et al. 2007). It may therefore be suggested that the idiomaticity of sentence stimuli in the present experiment critically contributed to the lateness of the hemodynamic brain response. To further explore the temporal structure of idiom comprehension, we would like to re-emphasize the need for future neurophysiological work.
The late effects we observed here also raise the issue that motor activity could be epiphenomenal with respect to sentence comprehension or could reflect motor imagery after semantic access. It is indeed possible that postunderstanding processes (Glenberg and Kaschak 2002), following semantic sentence-level analysis, are reflected (e.g., imagining a picture or a scene matching the sentence content). If this is true, semantic somatotopy of this secondary process triggered by sentence meaning would still argue against an abstract symbolic perspective. Indeed, in such an abstract symbolic framework, the meaning of the phrase “grasp an idea” is semantically unrelated to grasping. The only way to account for such secondary somatotopic activation would be through semantic somatotopy in sentence meaning analysis and consequent somatotopy of the secondary (imagery or the like) process.
Models assuming storage of idiom meanings as whole units unrelated to the meaning of their constituent words do not provide an explanation of the observed differences between idioms that include arm and leg action words. To provide such an explanation, 2 assumptions are necessary: 1) Idiom meaning must be computed on-line from the meaning of constituent words, and 2) Semantic aspects grounded in action–perception knowledge must play a critical role in the composition process yielding idiom meaning (Gibbs and O'Brien 1990). In this sense, the present results support both semantic compositionality and the grounding of figurative/abstract language in concrete sensory–motor information and in their corresponding specific brain circuits. Motor systems of the brain, including motor and premotor cortex, and the motor cognitions they process (Jeannerod 2006) appear to be central for understanding idioms. When “grasping ideas,” the motor system is engaged in a specific manner.
Funding
Medical Research Council (UK; U1055.04.003.00001.01, U1055.04.003.00003.01) and by the European Community under the “New and Emerging Science and Technologies” Programme (NEST-2005-PATH-HUM contract 043374, NESTCOM) to F.P. V.B. was supported by a postdoctoral fellowship from the Fyssen Foundation. Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council (UK).
Acknowledgments
We thank Elisabeth Fonteneau and Kambiz Tavabi for helpful comments on a previous version of this paper, as well as Matthew H. Davis for his help with the SPM analysis. We also thank 4 anonymous Referees for their efforts and help in improving this work. Conflict of Interest: None declared.
References
- Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31(6):265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Wilson SM, Rizzolatti G, Iacoboni M. Congruent embodied representations for visually presented actions and linguistic phrases describing actions. Curr Biol. 2006;16:1818–1823. doi: 10.1016/j.cub.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Bak TH, Yancopoulou D, Nestor PJ, Xuereb JH, Spillantini MG, Pulvermüller F, Hodges JR. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129(2):321–332. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Barber HA, Kutas M. Interplay between computational models and cognitive electrophysiology in visual word recognition. Brain Res Rev. 2006;53(1):98–123. doi: 10.1016/j.brainresrev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22(4):577–609. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annu Rev Psychol. 2007;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43(3):461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Bobrow S, Bell S. On catching on to idiomatic expressions. Mem Cognit. 1973;1:343–346. doi: 10.3758/BF03198118. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson's disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Déprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 ms of processing. J Cogn Neurosci. 2006;18(10):1607–1615. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Silber BY, Roy AC, Paulignan Y, Jeannerod M, Nazir TA. Subliminal display of action words interferes with motor planning: a combined EEG and kinematic study. J Physiol Paris. 2008;102(1–3):130–136. doi: 10.1016/j.jphysparis.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Heck D, Sultan F. The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav Brain Sci. 1997;20:229–245. [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. In: Paper presented at: 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. Neuroimage. 2002;16(2):372–373. [Google Scholar]
- Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Res Cogn Brain Res. 2005;24(3):355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Cacciari C, Tabossi P. The comprehension of idioms. J Mem Lang. 1988;27:668–683. [Google Scholar]
- Davidson D. Truth and meaning. Synthese. 1967;17:304–323. [Google Scholar]
- De Smet HJ, Baillieux H, De Deyn PP, Marlen P, Paquier P. The cerebellum and language: the story so far. Folia Phoniatr Logop. 2007;59(4):165–170. doi: 10.1159/000102927. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gibbs RW. Spilling the beans on understanding and memory for idioms in conversation. Mem Cognit. 1980;8:449–456. doi: 10.3758/bf03213418. [DOI] [PubMed] [Google Scholar]
- Gibbs RW, Nayak NP, Bolton JL, Keppel ME. Speakers' assumptions about the lexical flexibility of idioms. Mem Cognit. 1989;17(1):58–68. doi: 10.3758/bf03199557. [DOI] [PubMed] [Google Scholar]
- Gibbs RW, O'Brien JE. Idioms and mental imagery: the metaphorical motivation for idiomatic meaning. Cognition. 1990;36(1):35–68. doi: 10.1016/0010-0277(90)90053-m. [DOI] [PubMed] [Google Scholar]
- Glenberg AM. What memory is for. Behav Brain Sci. 1997;20(1):1–19. doi: 10.1017/s0140525x97000010. [DOI] [PubMed] [Google Scholar]
- Glenberg AM. Language and action: creating sensible combination of ideas. In: Gaskell G, editor. Handbook of psycholinguistics. Oxford: Oxford University Press. p. 361–371; 2007. [Google Scholar]
- Glenberg AM, Kaschak MP. Grounding language in action. Psychon Bull Rev. 2002;9(3):558–565. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp. 2004;21(3):191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Shtyrov Y, Pulvermüller F. The time course of action and action-word comprehension in the human brain as revealed by neurophysiology. J Physiol Paris. 2008;102(1–3):50–58. doi: 10.1016/j.jphysparis.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Sim EJ, Bochev V, Hernberger B, Kiefer M. Conceptual flexibility in the human brain: dynamic recruitment of semantic maps from visual, motor, and motion-related areas. J Cogn Neurosci. 2008;20(10):1799–1814. doi: 10.1162/jocn.2008.20123. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time-course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36:924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008;107(1):16–43. doi: 10.1016/j.bandl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Sin EJ, Liebich S, Hauk O, Tanaka J. Experience-dependent plasticity of conceptual representations in human sensory-motor areas. J Cogn Neurosci. 2007;19(3):525–542. doi: 10.1162/jocn.2007.19.3.525. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Motor cognition: what action tells the self. New York: Oxford University Press; 2006. [Google Scholar]
- Lakoff G, Johnson M. Philosophy in the flesh: the Embodied mind and its challenge to western thought. New York: Basic Books; 1999. [Google Scholar]
- Lauro LJ, Tettamanti M, Cappa SF, Papagno C. Idiom comprehension: a prefrontal task? Cereb Cortex. 2007;18(1):162–170. doi: 10.1093/cercor/bhm042. [DOI] [PubMed] [Google Scholar]
- Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. Neuroimage. 2006;29(2):536–544. doi: 10.1016/j.neuroimage.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Moscoso del Prado Martin F, Hauk O, Pulvermüller F. Category specificity in the processing of color-related and form-related words: an ERP study. Neuroimage. 2006;29(1):29–37. doi: 10.1016/j.neuroimage.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Nazir TA, Boulenger V, Roy AC, Silber BY, Jeannerod M, Paulignan Y. Language-induced motor perturbations during the execution of a reaching movement. Q J Exp Psychol. 2008;61(6):933–943. doi: 10.1080/17470210701625667. [DOI] [PubMed] [Google Scholar]
- Neininger B, Pulvermüller F. Word-category specific deficits after lesions in the right hemisphere. Neuropsychologia. 2003;41(1):53–70. doi: 10.1016/s0028-3932(02)00126-4. [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The cerebral cortex in man. New York: Macmillan; 1952. [Google Scholar]
- Penolazzi B, Hauk O, Pulvermüller F. Early semantic context integration and lexical access as revealed by event-related brain potentials. Biol Psychol. 2007;74:374–388. doi: 10.1016/j.biopsycho.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain reflections of words and their meaning. Trends Cogn Sci. 2001;5:517–525. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nat Rev Neurosci. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain processes of word recognition as revealed by neurophysiological imaging. In: Gaskell G, editor. Handbook of psycholinguistics. Oxford: Oxford University Press p. 119–141; 2007. [Google Scholar]
- Pulvermüller F, Harle M, Hummel F. Walking or talking? Behavioral and neurophysiological correlates of action verb processing. Brain Lang. 2001;78(2):143–168. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O. Category-specific conceptual processing of color and form in left fronto-temporal cortex. Cereb Cortex. 2006;16(8):1193–1201. doi: 10.1093/cercor/bhj060. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21(3):793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Nat Acad Sci USA. 2006;103:7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Luzenberger W, Preissl H. Nouns and verbs in the intact brain: evidence from event-related potentials and high-frequency cortical responses. Cereb Cortex. 1999;9(5):497–506. doi: 10.1093/cercor/9.5.497. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Ilmoniemi RJ. Brain signatures of meaning access in action word recognition. J Cogn Neurosci. 2005;17(6):884–892. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- Rapp AM, Leube DT, Erb M, Grodd W, Kircher TT. Neural correlates of metaphor processing. Brain Res Cogn Brain Res. 2004;20(3):395–402. doi: 10.1016/j.cogbrainres.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude JS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005;15(8):1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Sereno SC, Brewer CC, O'Donnell PJ. Context effects in word recognition: evidence for early interactive processing. Psychol Sci. 2003;14(4):328–333. doi: 10.1111/1467-9280.14471. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Pulvermüller F. Early activation dynamics in the left temporal and inferior-frontal cortex reflect semantic context integration. J Cogn Neurosci. 2007;19(10):1633–1642. doi: 10.1162/jocn.2007.19.10.1633. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Hamann SB, Harenski CL, Hu XP, Barsalou LW. fMRI evidence for word association and situated simulation in conceptual processing. J Physiol Paris. 2008;102(1–3):106–119. doi: 10.1016/j.jphysparis.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D. Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci. 2005;17:273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Titone DA, Connine CM. On the compositional and noncompositional nature of idiomatic expressions. J Pragm. 1999;31:1655–1674. [Google Scholar]
- Van Berkum JJ, Hagoort P, Brown CM. Semantic integration in sentences and discourse: evidence from the N400. J Cogn Neurosci. 1999;11(6):657–671. doi: 10.1162/089892999563724. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang. 2006;97(3):279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Zempleni MZ, Haverkort M, Renken R, Stowe L. Evidence for bilateral involvement in idiom comprehension: an fMRI study. Neuroimage. 2007;34(3):1280–1291. doi: 10.1016/j.neuroimage.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Zwaan RA, Taylor LJ. Seeing, acting, understanding: motor resonance in language comprehension. J Exp Psychol Gen. 2006;135(1):1–11. doi: 10.1037/0096-3445.135.1.1. [DOI] [PubMed] [Google Scholar]