Abstract
Hepatitis B virus (HBV) is a common viral pathogen that causes a substantial health burden worldwide. Remarkable progress has been made in our understanding of the natural stages of chronic HBV infection. A dynamic balance between viral replication and host immune response is pivotal to the pathogenesis of liver disease. Knowledge of the HBV genome organization and replication cycle can unravel HBV genotypes and molecular variants, which contribute to the heterogeneity in outcome of chronic HBV infection. Most HBV infections are spontaneously resolved in immunocompetent adults, whereas they become chronic in most neonates and infants at a great risk of developing complications such as cirrhosis and hepatocellular carcinoma (HCC). Those with chronic HBV infection may present in one of the four phases of infection: immune tolerance, immune clearance [hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB)], inactive carrier state, and reactivation (HBeAg-negative CHB). Understanding the dynamic nature of chronic HBV infection is crucial in the management of HBV carriers. Long-term monitoring and optimal timing of antiviral therapy for chronic HBV infection help to prevent progression of HBV-related liver disease to its later stage, particularly in patients with higher risk markers of HCC, such as serum DNA concentration, HBeAg status, serum aminotransferase, HBV genotypes, and pre-core or core mutants.
Keywords: Hepatitis B virus, Pathology, Immune tolerance, Immune clearance, Inactive hepatitis B surface antigen carriers, Reactivation, T-cell response, Cytokines
INTRODUCTION
Hepatitis B virus (HBV) infection is a major health problem worldwide. Some individuals can develop acute HBV infection and achieve complete immune clearance of virus, yielding a life-long immunity, while others can develop chronic HBV infection depending on the host immune response. Chronic HBV infection is associated with a wide range of clinical manifestations, from an asymptomatic carrier state with a normal liver histology to severe and chronic liver diseases, including cirrhosis and hepatocellular carcinoma (HCC)[1,2].
There is a particular concern in the Asia-Pacific region, where chronic HBV infection is prevalent, with a carrier rate of approximately 10% of chronic HBV carriers. About 25%-40% of them will eventually die of liver disease (cirrhosis with and without HCC) with a death rate of 50% for male carriers and 15% for female carriers, respectively. Chronic HBV infection is a dynamic process with a replicative or a non-replicative (or low replicative) phase based on virus-host interaction which is pivotal to the pathogenesis of liver disease. Understanding the dynamic nature of chronic HBV infection is crucial in the management of HBV carriers. Long-term monitoring and optimal timing of antiviral therapy for chronic HBV infected patients can help to prevent progression of HBV-related liver disease to its later stage[3,4].
PATHOLOGY OF HBV INFLAMMATORY REACTION
Viral hepatitis, characterized by diffused inflammatory reaction, is associated with cell damage and death. It has been recently reported that HBV replication is associated with cell death, which is different from the widely accepted non-cytopathic characteristics of HBV[5]. The mechanism of cell damage is generally defined as the result of cytotoxic T-lymphocyte (CTL)-mediated immune responses to viral infection[6]. Another typical process causing cell death is apoptosis. It has been shown that HBV proteins, such as HBx and HBsP, can induce apoptosis[7]. A careful light microscopic examination of HBV genotypes A-C transfected cells can reveal rounded up and death cells which are apoptotic signs. To identify the observed cell death, FACs is used because apoptotic cells can show phoplaridylserine on cell membrane. HePG2 cells can be transfected with HBV genotypes A-C. Cells observed under a phase contrast microscope, can be stained with apoptosis markers and analyzed by flow cytometry. HBsP expression can be detected by Western blotting assay. BH3 sequences can be aligned and analyzed with the vector NT1. HBV genotypes A-C transfected cells display cell death which has been further proved as apoptosis. HBsP, a pro-apoptotic protein, is detectable during transfection of virus genomes. Different apoptotic effects are correlated with the expression of different genomes. Alignment and analysis of the HB3 domains of three virus genomes can reveal a slight variance. It has been reported that variant HBsP expression and BH3 sequence of HBV genotype may be involved in differential apoptotic effects on transfected cells[8]. However, HBV can also directly cause death of hepatocytes[6].
HBV TRANSMISSION AND INFECTION
In high endemic regions, such as Asia, Africa, Pacific Islands and the Arctic, early perinatal and horizontal infection in childhood is the main route of HBV transmission with a hepatitis B surface antigen (HBsAg) positive rate of 8%-15%, while in low endemic areas, such as Western countries, HBV is a predominant disease in adolescents and adults due to high risk sexual behaviors or drug injections, with a HBsAg positive rate of less than 2%[9].
The vast majority of early perinatal or horizontal infections in childhood are the main route of HBV transmission in untreated infants whose mothers are hepatitis B e antigen (HBeAg) positive, and over 90% of them will become chronic HBV carriers. In contrast, about 90% of HBV infections may occur as acute infection and only 5%-10% may occur as chronic infection in adults. This dramatic difference in chronic rates is believed to reflect the host immunologic status and the time of infection.
Although infants whose mothers are HBeAg-positive HBV carriers are at a high risk of developing infection and subsequently become viral persistence, the age of infants at the time of HBV infection is inversely correlated with the chronic rate[10,11]. A HBc/HBe-specific Th-cell tolerance model can show the reversibility of T-cell tolerance. It has been shown that a single prenatal dose of HBeAg can result in apparent T-cell tolerance in mice at the age of 8-12 wk, but the tolerance may disappear at the age of 16 wk[12], suggesting that T-cell tolerance can be maintained and HBcAg/HBeAg will continuously present. HBc/HBe-specific thymocytes are absent in thymus and this “repertoire renewal process” requires about 16 wk. Similarly, human fetus may be exposed to tolerogenic HBeAg in uterus not infected at birth. The longer the elapsed time before HBV infection is, the greater the probability of renewing HBc/HBe-specific T-cell repertoire is, because the neonate would no longer be exposed to HBeAg[13].
There is an obvious difference between patients infected with HBV in adolescence or adulthood immediately entering immune clearance phase, and short duration and tendency quiescent after seroconversion from HBeAg to antibody against HBeAg (anti-HBe). Such patients are termed “healthy” carriers. In contrast, patients with early HBV infection have a prolonged immune tolerance phase and a prolonged immune clearance phase, indicating that their diseases tend to progress after HBeAg seroconversion.
STAGES OF HBV INFECTION
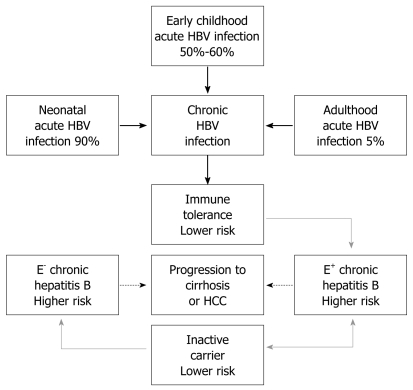
Remarkable progress has been made in our understanding of the four natural stages of chronic hepatitis B (CHB): immune tolerance stage, immune clearance stage, inactive HBsAg carrier stage, and reactivation stage. However, not all CHB infection patients go through all the four stages (Figure 1).
Figure 1.
Phases of chronic hepatitis B virus (HBV) infection. Thick arrows indicate HBV infection rates in different age groups, dotted arrows indicate changes in histology, grey arrows indicate changes at risk of progressing to cirrhosis or HCC (E+: HBeAg positive; E-: HBeAg negative).
Immune tolerance stage
Patients with perinatal or early childhood-acquired HBV infection have an initial tolerance stage characterized by the presence of HBeAg, high serum DNA level, normal serum aminotransferase level, and minimal or no inflammation on liver biopsy[14]. Such manifestations can rarely be seen in those who are infected with HBV in later childhood or adulthood and whose infection subsequently develop into chronic HBV infection[14].
Although a high serum DNA level in liver disease patients with minimal or no inflammation is considered as a sequela of immune tolerance to HBeAg, it has been shown that HBeAg may promote HBV chronicity by functioning as an immunoregulatory protein[15]. For example, in transgenic mice, transplacental transfer of maternal HBeAg may preferentially behave as a tolerogen and inactive HBe/HBc-specific Th cells through at least central deletion of high-affinity HBe/HBcAg-specific CD4 T cells or clonal ignorance and anergy in periphery blood, resulting in ineffective cytotoxic T cell lysis of infected hepatocytes. Such a mechanism may be responsible for the high chronic HBV infection rate (-90%) observed in babies infected by their HBeAg positive mothers accounting for the inability of infants to clear perinatal HBV infection. After neonatal or prenatal HBV infection (absent in uterus tolerance), secretion of monomeric HBeAg in the relatively Th2-bised neonatal immune system may also have an anti-inflammatory influence on nucleoprotein-specific immune response by eliciting Th2-like cytokines. Secreted HBeAg can also enter thymus. It has been subsequently reported that HBeAg specific Th2-like cells can preferentially survive tolerance production to a greater extent than HBeAg-specific Th1-like cells[16]. Therefore, chronicity resulting from vertical transmission of HBV characterized by the predominance of HBeAg-specific Th2-like cells and secretion of anti-inflammatory cytokines, such as IL-4, IL-5, and IL-10, can enhance antibody production, and viral persistence would characterize the HBeAg-specific T-cell response. If the status quo of various clonal tolerance phenotypes could be maintained as long as the HBeAg concentration and/or the non-inflammatory hepatic environment remain unchanged, the immune tolerance would last 1-4 decades. During this phase, the rate of spontaneous or treatment-induced HBeAg seroconversion is less than 5%[17,18]. Patients in the immune tolerance phase are considered at a low risk of progressing to cirrhosis or HCC based on serial monitoring of virological, clinical and ultrasonographic assessments.
Although antiviral therapy is not recommended for immune tolerance patients, they should be closely monitored for progression to the immune clearance phase. Once this occurs, antiviral therapy should be considered more diligently after 6-12 mo if HBeAg seroconversion does not occur because disease progression can occur in the immune clearance phase.
Immune clearance stage
As the host immune system matures, a nonspecific increase in hepatic inflammation or decrease in HBeAg serum concentration, perhaps due to the emergence of core promoter region or pre-core region mutants, may allow activation of intermediate-or low-avidity HBeAg-specific T cell clones that are not physically deleted and/or reverse the anergic state of others[17]. Therefore, treatment modalities for chronic HBV infection should be directed at activating the relatively low-avidity HBeAg-specific T cells. Such a shift from HBeAg-specific Th2 cell tolerance to Th1 cell activation may recognize HBV-related epitopes on hepatocytes, and immune-mediated hepatocellular injury ensues, the so called clearance phase of CHB infection. Then, IL-2, INF and tumor necrosis factor are secreted following inflammation. The HBcAg-specific T-cell response is characterized by CTL induction, liver injury and inhibition of viral replication[6]. In patients with prenatally acquired HBV infection, transition from immune tolerance phase to immune clearance phase occurs during the second or third decade of life. Although HBV replication and viremia continue in the liver, the serum virus level becomes lower in immune clearance phase than in immune tolerance phase when viral replication is completely unopposed. The active phase of CHB is often marked by increased levels of alanine aminotransferase (ALT), necrotic inflammatory activity, and cycling HBV-DNA and HBeAg due to liver injury. Pre-core minus mutants and mutations within the core gene begin to accumulate at the time of ALT flare up because they have a better ability to evade immune clearance[19], suggesting that nucleoprotein antigens are the major immune attacking foci during chronic HBV infection, perhaps because the nucleoprotein-specific T-cell repertoire has been eroded to a lesser extent than the envelope-specific repertoire simply due to the lower concentration of HBe/HBcAgs. CD4+ HBeAg-specific T cells, identified in HBeAg-single-Tg and TCR-HBeAg-Tg mice, are not deleted or anergized and remain quiescent in the presence of serum HBeAg, but can mediate seroconversion and liver injury once they are activated. These HBeAg-specific T cells escape tolerance induction due to their low avidity and/or low TCR density[18].
This active phase is characterized by the presence of HBeAg, high serum HBV DNA and aminotransferase levels, as well as active inflammations and fibrosis in the liver. A key event in the natural history of HBeAg positive CHB patients is HBeAg seroconversion[20]. Several studies have shown that seroconversion with a marked reduction in HBV replication is associated with biochemical and histological remission of inflammatory activity in the majority of patients[2,10,20]. Most studies showed that the mean annual rate of spontaneous HBeAg seroconversion ranges 8%-15% in children or adults with an elevated ALT level[20]. Although the ALT level is normal in most Asian children, their spontaneous HBeAg seroconversion rate is less than 2% during the first 3 years of age and then increases to 4%-5%. In some cases, spontaneous flare up of hepatitis is not frequently recognized because it is usually asymptomatic. Since subsequent HBeAg seroconversion would not occur in such flare up of hepatitis, it can thus be viewed as an abortive attempt at seroconversion. However, some patients present with a symptomatic flare up of hepatitis that mimics acute hepatitis and even present with fulminant hepatic failure. Regression of fibrosis occurs several months or years after HBeAg seroconversion. These flare ups of hepatitis may precede the disappearance of HBeAg and development of HBeAg antibody, culminating in the remission of hepatitis activity. It has been recognized that the duration of immune clearance phase and the frequency and severity of flare ups are correlated with the risk of progressing to cirrhosis and HCC[21–23].
Inactive HBsAg carrier stage
This inflammatory phase of HBV infection also leads to HBeAg seroconversion and enters into inactive HBsAg carrier status. Inactive carriers form the largest group of chronic HBV infection patients. After seroconversion, most patients remain negative for HBeAg and positive for anti-HBe antibody with an undetectable or a low HBV DNA level, while the minority have undetectable viral loads. Biopsy findings can range from mild inflammation and minimal fibrosis to inactive cirrhosis if the disease is severe during immune clearance[20].
The progress of inactive HBsAg carrier state is usually benign. A long-term follow-up (up to 18 years) of these carriers can indicate a sustained biochemical remission and a very low risk of developing cirrhosis or HCC in them[19]. Patients even with no cirrhosis may develop live cancer in their inactive HBsAg carrier state. In addition, approximately 20%-30% of inactive HBsAg carriers may undergo spontaneous reactivation of hepatitis B during the follow-up. Multiple episodes of reactivation or sustained reactivation can cause progressive liver damage and even decompensation. HBV reactivation is usually asymptomatic but can occasionally mimic acute viral hepatitis. Some carriers eventually become HBsAg negative and develop anti-HBs. The estimated incidence of delayed HBsAg clearance is 1%-2% per year in Western countries where HBV infection is acquired in adulthood, and 0.05%-0.8% per year in endemic areas where HBV infection is mostly acquired perinatally or in early childhood. Prognosis can be improved by loss of HBsAg as liver disease is inactive or non-progressive, but HBsAg clearance does not completely prevent occurrence of liver decompensation or HCC in patients with cirrhosis[23–25].
Reactivation stage
Chronic HBeAg-negative patients can be divided into chronic inactive HBsAg carriers and CHB patients with biochemical and intermittent virological activity[5]. HBeAg-negative chronic hepatitis may recur in one third of inactive HBV carriers without serum reversion of HBeAg[22,23].
It is believed that seroconversion of HBeAg to HBeAb is accompanied with cessation of HBV replication and remission of liver disease. However, HBsAg-negative CHB has been recognized as an important form of chronic hepatitis, and e-antigen negativity is due to mutations in pre-core and core promoter regions. The most frequent pre-core mutation is a G-A change in nucleotide 1896 (G1896A) which creates a stop codon[23] and the most common core promoter mutation involves a substitution of nucleotides 1762 and 1764, which can result in loss of HBeAg synthesis. Loss of circulating HBeAg can decrease the induction of HBeAg-specific Th2 cell activity and result in a predominance of inflammatory Th1-like cells[16]. HBeAg-negative CHB (pre-core mutant) occurs as the predominant species during typical HBV infection with wild-type virus which is selected during the immune clearance phase (HBeAg seroconversion). Several studies have shown that HBeAg may be a target antigen on HBV-infected hepatocytes[5,15,18]. Failure to produce a target antigen may be a means of evading immune clearance. The clonal heterogeneity of HBeAg-specific T-cell tolerance may explain how a primarily tolerogenic protein can exert its pressure on the immune response to the selection of HBeAg negative mutant. For example, high-avidity HBeAg-specific T-cell clones may be tolerized and low-activity T-cell clones may be activated and involved in selecting HBeAg-negative mutant in the same patient[18,24–26]. The occurrence of HBeAg-negative mutants during chronic active HBV infection, especially in the presence of a high viral load, is correlated with an exacerbation of liver injury and a worse prognosis. Serum HBeAg can act as an efficient T-cell tolerogen which reduces the frequency of liver injury and down-regulates anti-HBc production. Anergy of HBc/HBeAg and HBeAg specific T-cells depends on HBeAg concentration and is reversible in the absence of HBeAg, which may explain the correlation between pre-core and core promoter mutations and severe liver injury[19,22,27,28].
Progress to this phase occurs spontaneously or to inactive carriers during immune suppression. Some patients can progress directly from HBeAg positive to HBeAg negative CHB. Identification of pre-core/core promoter mutations and recognition of HBeAg negative CHB indicate that the disease occurs after HBeAg seroconversion[19]. Age is significantly higher in HBeAg-negative patients than in HBeAg-positive patients. ALT and HBV DNA levels are significantly lower in e-antigen negative patients than in e-antigen positive patients. However, spontaneous recovery is rarer, long-term prognosis is poorer, and histological lesions are more severe in HBeAg-negative patients than in HBeAg-positive patients. Necrotic inflammatory activity is almost identical in both HBeAg-negative and positive patients. However, fibrotic activity is higher in e-antigen negative patients than in e-antigen positive patients. The estimated annual incidence of cirrhosis is 2%-6% in HBeAg positive CHB patients and 8%-10% in HBeAg negative CHB patients. The higher incidence of cirrhosis in HBeAg-negative patients is related to age and fibrosis stage, suggesting that HBeAg-negative chronic hepatitis can progress to cirrhosis and HCC in the natural history of HBV infection rather than de nove infection with HBV variants that do not produce HBeAg[29–31]. HBeAg-specific T cell tolerance is reversible in the absence of tolerogen. Since antiviral treatment can reduce HBeAg and viral load possibly in combination with HBc/HBeAg-specific immunization, it can alleviate chronic HBV infection by shifting the cytokine profile from Th2 to Th0/1[26,32].
Occult HBV infection
Occult HBV infection is defined as the existence of HBV DNA in serum, although it is not considered as a phase of CHB[33,34].
In addition to a symptomatic and serologically evident infection, occult persistent HBV carriage has been identified since nucleic acid amplification assay enhances its sensitivity to hepadnaviral genomes and their replicative intermediates. There is evidence that occult HBV infection is a common and long-term consequence of acute hepatitis B resolution. This form of residual infection is termed as secondary occult infection (SOI). The data from the woodchuck model of HBV infection indicate that exposure to a small amount of hepadnavirus can also cause primary occult infection where virus genome but not serological makers of virus exposure are detectable without liver involvement. However, both forms of virus replicate at a low level in the lymphatic system. Serological testing for SOI can reveal the presence of antibodies to HBV core antigen (anti-HBc), which has been recognized not only as a valuable marker of prior HBV exposure but also as an indicator of progressing occult HBV infection[35]. It has been recently reported that up to 20% of individuals with occult HBV carriage are not reactive to anti-HBx or any other serological indicators of HBV exposure, and detection of naturally acquired antibodies to HBsAg (anti-HBc) does not exclude the existence of occult HBV infection[33,36].
The severe consequences of occult HBV infection have not been fully recognized. There is evidence that occult HBV can be a source of virus contamination in blood and organ donations, as well as a reservoir from which full blown hepatitis can arise[37]. Case reports also indicate that immunosuppression caused by chemotherapy or immunomodulatory agents or immunodeficiency due to HIV infection or hematological malignancies can induce reactive occult infection[38,39]. Mild necrotic inflammation has been documented in liver samples obtained from acute hepatitis B patients many years after recovery[40]. Liver fibrosis and cirrhosis of unknown origin have been explained by occult HBV infection in many retrospective studies[35,41,42]. The oncogenic potency of occult HBV persistence becomes progressively evident and is further elevated in alcoholics and patients with other liver ailments like hepatitis C[41,42]. No reports are available on the treatment of occult HBV infection (Table 1).
Table 1.
Characteristics of chronic hepatitis B at different stages
| Phase | ALT | HBsAg | HBeAg | HBeAb | HBV DNA (IU/mL) | Th cell biased | Liver histology |
| Immune tolerance | Usually normal | Present | Present | Absent | ≥ 20 000 | Th2 cell | Normal or mild inflammation |
| Immune clearance | Elevated | Present | Present | Absent | ≥ 20 000 | Th2/Th1 cell | Active inflammation |
| Inactive HBsAg carrier | Usually normal; can have flares | Present | Absent | Present | > 20 000 | Th1 cell | Mild inflammation or inactive cirrhosis |
| HBeAg- CHB | Perioid flares | Present | Absent | Present | > 20 000 | Th1 cell | Active inflammation |
| < 20 000 | |||||||
| Occult hepatitis B | Rarely elevated | Absent | Absent | Present when HBV recovered | < 20 000 | Th1 cell | From normal to cirrhosis HCC |
CONSEQUENCES OF CHRONIC HBV INFECTION
Individuals with chronic HBV infection are at an increased risk of developing end-stage liver diseases including cirrhosis, hepatic failure, and HCC. It has recently been estimated that about 53% of HCC cases in the world are related to HBV infection. The lifetime risk of developing HCC is increased even in patients with cleared HBsAg or occult HBV infection. Further risk factors include chronic HCV infection, exposure to aflatoxin B1, alcohol abuse, obesity and diabetes[4,43]. Thus, it is important to identify HBV-infected patients at a higher risk of progressing to HCC.
The reason why some CHB patients progress to HCC remains unknown. Host factors, such as immune response to HBV, genetic predisposition to HCC, high HBV replication rate, mutations within the HBV genome, are related with HCC. Many observations revealed that the major factor for the development of HBV-associated HCC is the immune system[41,43,45]. Development of hepatitis, chronic hepatitis, and HCC could be exclusively observed in mice reconstituted with bone marrow and in non-transgenic animals, but not in controls, suggesting that ineffective immune response is the principle oncogenic factor during chronic HBV infection of human beings. In other words, the same T-cell response has different effects. If T cell response is strong enough, HBV can be eliminated from the liver. If not, a pro-carcinogenic effect can be induced by triggering necrotic inflammatory disease without final eradication of HBV from the liver. It can, thus, be concluded that the immune system-mediated chronic inflammation of the liver, continuous cell death and subsequent cell proliferation may increase the frequency of genetic alteration and the risk of developing cancer. However, the molecular basis of inflammatory liver carcinogenesis caused by HBV remains largely unsolved. Cytokines modulate inflammation and the presence of inflammatory cells with the production of inflammatory cytokines activates cellular oxidant-generating pathways. Reactive oxygen species that are generated in inflammatory conditions induce oxidative DNA damage and increased oxidative stress caused by chronic inflammation can produce genetic mutations and gross chromosomal alterations[4,44,45]. Extensively oxidative DNA damage has been detected in hepatocytes of HBV-transgenic mice and humans with chronic hepatitis[46].
HBV genotype C infection is associated with a higher risk of developing HCC than HBV genotype B infection[29]. The BCP A1762T/G1764A mutant is associated with an increased risk of developing HCC compared with the double wild type variant, whereas the pre-core G1896A mutation is associated with a decreased risk of developing HCC compared with the wild-type variant. Several mechanisms of liver carcinogen are related to the BCP A1762T/G1764A mutation which may enhance HBV virulence by increasing host immune response and viral replication, or by altering the coding region of the X antigen. Mutant BCP may augment the host immune response to HBV-infected hepatocytes by diminishing circulating HBeAg and increasing hepatocyte apoptosis and regeneration, thus leading to liver injury[47,48]. The BCP mutation appears to enhance the efficacy of viral replication either by modulating the relative levels of pre-core and core RNAs or by creating a transcription factor binding site for hepatocyte nuclear factor 1. Mutations in the BCP region over lapping the coding sequence of the X antigen of HBV may result in changes of amino acids, K130M and V131I, in the X gene. These amino acid changes may interfere with cell growth control and DNA repair, thus leading to HCC[49,50]. There is experimental evidence that HBx, a multifunctional protein with oncogenic potentials, can interact with a large number of cellular factors and modulate their normal function, thus leading to deregulation of normal cell activities and HCC[46,51]. Despite its importance in HCC development, the clinical significance of genetic variability in the x genetic region still remains poorly understood[52].
Several factors, including age, male gender, repeated episodes of severe acute exacerbation, and HBV reactivation after HBeAg seroconversion, are related with the risk of developing advanced liver disease in patients with CHB. Previous studies showed that HBV genotype C infection is associated with later HBeAg seroconversion and multiple episodes of acute exacerbation without HBeAg seroconversion than genotype B HBV infection[9,21,25,30,49]. The delayed HBeAg seroconversion may prolong the inflammation process and subsequently result in more severe liver damage[30]. Several nucleotide mutations in the pre-core and core promoter regions may reduce HBeAg production and are associated with advanced liver disease[47]. In Asia, genotype C and T1762 and A1764 mutants may play a role in HBV-related liver cirrhosis, and can be used in predicting the clinical outcome of patients with chronic HBV infection.
Supported by Science and Technology Department of Qingdao Government 07-2-1-15-nsh
Peer reviewer: Thomas Bock, PhD, Professor, Department of Molecular Pathology, Institute of Pathology, University Hospital of Tuebingen, D-72076 Tuebingen, Germany
S- Editor Li LF L- Editor Wang XL E- Editor Ma WH
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 3.He YL, Zhao YR, Zhang SL, Lin SM. Host susceptibility to persistent hepatitis B virus infection. World J Gastroenterol. 2006;12:4788–4793. doi: 10.3748/wjg.v12.i30.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis. World J Gastroenterol. 2007;13:74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai SL, Chen PJ, Lai MY, Yang PM, Sung JL, Huang JH, Hwang LH, Chang TH, Chen DS. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest. 1992;89:87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu YW, Tan TL, Zhang J, Chen WN. Cellular apoptosis induced by replication of hepatitis B virus: possible link between viral genotype and clinical outcome. Virol J. 2007;4:117. doi: 10.1186/1743-422X-4-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milich DR, Schodel F, Hughes JL, Jones JE, Peterson DL. The hepatitis B virus core and e antigens elicit different Th cell subsets: antigen structure can affect Th cell phenotype. J Virol. 1997;71:2192–2201. doi: 10.1128/jvi.71.3.2192-2201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu YW, Tan TL, Chan V, Chen WN. The HBSP gene is expressed during HBV replication, and its coded BH3-containing spliced viral protein induces apoptosis in HepG2 cells. Biochem Biophys Res Commun. 2006;351:64–70. doi: 10.1016/j.bbrc.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Mahtab MA, Rahman S, Khan M, Karim F. Hepatitis B virus genotypes: an overview. Hepatobiliary Pancreat Dis Int. 2008;7:457–464. [PubMed] [Google Scholar]
- 10.Elgouhari HM, Abu-Rajab Tamimi TI, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med. 2008;75:881–889. doi: 10.3949/ccjm.75a.07019. [DOI] [PubMed] [Google Scholar]
- 11.Xu YY, Yu JY, Zhong YW, Song HB, Liu HH, Jia LL, Li SL, Xu JQ, Li Q. Association between the frequency of class II HLA antigens and the susceptibility to intrauterine infection of hepatitis B virus. Int J Biol Sci. 2008;4:111–115. doi: 10.7150/ijbs.4.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roh S, Kim K. Overcoming tolerance in hepatitis B virus transgenic mice: a possible involvement of regulatory T cells. Microbiol Immunol. 2003;47:453–460. doi: 10.1111/j.1348-0421.2003.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 13.Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci USA. 1990;87:6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashima H, Araki K, Miyazaki J, Yamamura K, Kimoto M. Characterization of T-cell tolerance to hepatitis B virus (HBV) antigen in transgenic mice. Immunology. 1992;75:398–405. [PMC free article] [PubMed] [Google Scholar]
- 15.Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075–1086. doi: 10.1053/jhep.2003.50453. [DOI] [PubMed] [Google Scholar]
- 16.Huang CF, Lin SS, Ho YC, Chen FL, Yang CC. The immune response induced by hepatitis B virus principal antigens. Cell Mol Immunol. 2006;3:97–106. [PubMed] [Google Scholar]
- 17.Milich DR, Peterson DL, Schodel F, Jones JE, Hughes JL. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 1995;69:2776–2785. doi: 10.1128/jvi.69.5.2776-2785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Sallberg M, Hughes J, Jones J, Guidotti LG, Chisari FV, Billaud JN, Milich DR. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunetto MR, Giarin MM, Oliveri F, Chiaberge E, Baldi M, Alfarano A, Serra A, Saracco G, Verme G, Will H. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci USA. 1991;88:4186–4190. doi: 10.1073/pnas.88.10.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J. 2005;2:82. doi: 10.1186/1743-422X-2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furusyo N, Nakashima H, Kashiwagi K, Kubo N, Hayashida K, Usuda S, Mishiro S, Kashiwagi S, Hayashi J. Clinical outcomes of hepatitis B virus (HBV) genotypes B and C in Japanese patients with chronic HBV infection. Am J Trop Med Hyg. 2002;67:151–157. doi: 10.4269/ajtmh.2002.67.151. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama T, Kuwata S, Koike K, Iino S, Yasuda K, Yotsuyanagi H, Moriya K, Maekawa H, Yamada H, Shibata Y, et al. Precore wild-type DNA and immune complexes persist in chronic hepatitis B after seroconversion: no association between genome conversion and seroconversion. Hepatology. 1998;27:245–253. doi: 10.1002/hep.510270137. [DOI] [PubMed] [Google Scholar]
- 23.Chan HL, Hussain M, Lok AS. Different hepatitis B virus genotypes are associated with different mutations in the core promoter and precore regions during hepatitis B e antigen seroconversion. Hepatology. 1999;29:976–984. doi: 10.1002/hep.510290352. [DOI] [PubMed] [Google Scholar]
- 24.Chen MT, Billaud JN, Sallberg M, Guidotti LG, Chisari FV, Jones J, Hughes J, Milich DR. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci USA. 2004;101:14913–14918. doi: 10.1073/pnas.0406282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CH, Lee CM, Lu SN, Changchien CS, Eng HL, Huang CM, Wang JH, Hung CH, Hu TH. Clinical significance of hepatitis B virus (HBV) genotypes and precore and core promoter mutations affecting HBV e antigen expression in Taiwan. J Clin Microbiol. 2005;43:6000–6006. doi: 10.1128/JCM.43.12.6000-6006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milich DR. Influence of T-helper cell subsets and crossregulation in hepatitis B virus infection. J Viral Hepat. 1997;4 Suppl 2:48–59. doi: 10.1111/j.1365-2893.1997.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 27.Lok AS, Akarca US, Greene S. Predictive value of precore hepatitis B virus mutations in spontaneous and interferon-induced hepatitis B e antigen clearance. Hepatology. 1995;21:19–24. [PubMed] [Google Scholar]
- 28.Milich DR, Jones J, Hughes J, Maruyama T. Role of T-cell tolerance in the persistence of hepatitis B virus infection. J Immunother Emphasis Tumor Immunol. 1993;14:226–233. doi: 10.1097/00002371-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609–8620. doi: 10.1128/JVI.76.17.8609-8620.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You J, Sriplung H, Chongsuvivatwong V, Geater A, Zhuang L, Huang JH, Chen HY, Yu L, Tang BZ. Profile, spectrum and significance of hepatitis B virus genotypes in chronic HBV-infected patients in Yunnan, China. Hepatobiliary Pancreat Dis Int. 2008;7:271–279. [PubMed] [Google Scholar]
- 31.Victoria Fda S, Oliveira CM, Victoria MB, Victoria CB, Ferreira LC. Characterization of HBeAg-negative chronic hepatitis B in western Brazilian Amazonia. Braz J Infect Dis. 2008;12:27–37. doi: 10.1590/s1413-86702008000100008. [DOI] [PubMed] [Google Scholar]
- 32.Menne S, Cote PJ. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J Gastroenterol. 2007;13:104–124. doi: 10.3748/wjg.v13.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–486. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 34.Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001;34:204–206. doi: 10.1053/jhep.2001.25225. [DOI] [PubMed] [Google Scholar]
- 35.Mulrooney-Cousins PM, Michalak TI. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J Gastroenterol. 2007;13:5682–5686. doi: 10.3748/wjg.v13.i43.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang Y, Teng X, Xu WZ, Li D, Zhao HW, Fu LJ, Zhang FM, Gu HX. Molecular characterization and functional analysis of occult hepatitis B virus infection in Chinese patients infected with genotype C. J Med Virol. 2009;81:826–835. doi: 10.1002/jmv.21463. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 38.Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, Luk JM, Lie AK, Kwong YL, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59–68. doi: 10.1053/j.gastro.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Chamorro AJ, Casado JL, Bellido D, Moreno S. Reactivation of hepatitis B in an HIV-infected patient with antibodies against hepatitis B core antigen as the only serological marker. Eur J Clin Microbiol Infect Dis. 2005;24:492–494. doi: 10.1007/s10096-005-1355-1. [DOI] [PubMed] [Google Scholar]
- 40.Menne S, Cote PJ, Butler SD, Toshkov IA, Gerin JL, Tennant BC. Immunosuppression reactivates viral replication long after resolution of woodchuck hepatitis virus infection. Hepatology. 2007;45:614–622. doi: 10.1002/hep.21558. [DOI] [PubMed] [Google Scholar]
- 41.Korba BE, Wells FV, Baldwin B, Cote PJ, Tennant BC, Popper H, Gerin JL. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology. 1989;9:461–470. doi: 10.1002/hep.1840090321. [DOI] [PubMed] [Google Scholar]
- 42.Pollicino T, Raffa G, Costantino L, Lisa A, Campello C, Squadrito G, Levrero M, Raimondo G. Molecular and functional analysis of occult hepatitis B virus isolates from patients with hepatocellular carcinoma. Hepatology. 2007;45:277–285. doi: 10.1002/hep.21529. [DOI] [PubMed] [Google Scholar]
- 43.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 44.Takagi A, Deguchi R, Kobayashi K, Miwa T. Cytokine expressions and H. pylori-associated gastric mucosal lesion. Keio J Med. 2002;51 Suppl 2:51–52. doi: 10.2302/kjm.51.supplement2_51. [DOI] [PubMed] [Google Scholar]
- 45.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagen TM, Huang S, Curnutte J, Fowler P, Martinez V, Wehr CM, Ames BN, Chisari FV. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci USA. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan JM, Ambinder A, Fan Y, Gao YT, Yu MC, Groopman JD. Prospective evaluation of hepatitis B 1762(T)/1764(A) mutations on hepatocellular carcinoma development in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2009;18:590–594. doi: 10.1158/1055-9965.EPI-08-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong MJ, Blatt LM, Kao JH, Cheng JT, Corey WG. Basal core promoter T1762/A1764 and precore A1896 gene mutations in hepatitis B surface antigen-positive hepatocellular carcinoma: a comparison with chronic carriers. Liver Int. 2007;27:1356–1363. doi: 10.1111/j.1478-3231.2007.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shinkai N, Tanaka Y, Ito K, Mukaide M, Hasegawa I, Asahina Y, Izumi N, Yatsuhashi H, Orito E, Joh T, et al. Influence of hepatitis B virus X and core promoter mutations on hepatocellular carcinoma among patients infected with subgenotype C2. J Clin Microbiol. 2007;45:3191–3197. doi: 10.1128/JCM.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725–12734. doi: 10.1128/JVI.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kidd-Ljunggren K, Oberg M, Kidd AH. The hepatitis B virus X gene: analysis of functional domain variation and gene phylogeny using multiple sequences. J Gen Virol. 1995;76(Pt 9):2119–2130. doi: 10.1099/0022-1317-76-9-2119. [DOI] [PubMed] [Google Scholar]