Abstract
AIM: To determine free and conjugated serum bile acid (BA) levels in inflammatory bowel disease (IBD) subgroups with defined clinical manifestations.
METHODS: Comprehensive serum BA profiling was performed in 358 IBD patients and 310 healthy controls by liquid chromatography coupled to electrospray ionization tandem mass spectrometry.
RESULTS: Serum levels of hyodeoxycholic acid, the CYP3A4-mediated detoxification product of the secondary BA lithocholic acid (LCA), was increased significantly in Crohn’s disease (CD) and ulcerative colitis (UC), while most other serum BA species were decreased significantly. Total BA, total BA conjugate, and total BA glycoconjugate levels were decreased only in CD, whereas total unconjugated BA levels were decreased only in UC. In UC patients with hepatobiliary manifestations, the conjugated primary BAs glycocholic acid, taurocholic acid, and glycochenodeoxycholic acid were as significantly increased as the secondary BAs LCA, ursodeoxycholic acid, and tauroursodeoxycholic acid compared to UC patients without hepatobiliary manifestations. Finally, we found that in ileocecal resected CD patients, the unconjugated primary BAs, cholic acid and chenodeoxycholic acid, were increased significantly compared to controls and patients without surgical interventions.
CONCLUSION: Serum BA profiling in IBD patients that indicates impaired intestinal barrier function and increased detoxification is suitable for advanced diagnostic characterization and differentiation of IBD subgroups with defined clinical manifestations.
Keywords: Bile acids, Liquid chromatography, Tandem mass spectrometry, Inflammatory bowel disease, Crohn’s disease, Ulcerative colitis
INTRODUCTION
The pathophysiology of inflammatory bowel disease (IBD), Crohn’s disease (CD) and ulcerative colitis (UC) is not yet completely understood. In recent years, it has become obvious that genetic, immunological, environmental and microbial factors contribute to the etiology of IBD[1–5]. The concept of multilevel dysfunction of the intestinal detoxification system is accepted as an important aspect of the pathophysiology of IBD[6]. Intestinal epithelial cells are of major importance as a physiological barrier against components of the intestinal lumen such as bacteria, nutrients and toxins. Protective mechanisms that maintain intestinal barrier function include detoxification and biotransformation of luminal substances, as well as the control of junctional proteins in cell-cell contacts. These processes are influenced by lipids and the availability of adequate cellular lipid processing[7].
Bile acids (BAs) are involved in these processes. First of all, intestinal reabsorption is a critical step in enterohepatic circulation (EHC) of BAs[8]. Once synthesized in the liver and secreted via the bile duct into the duodenum, BAs are effectively absorbed in the distal ileum and transported back to the liver via the portal vein, which contributes to the pool of BAs in the blood[9,10]. Absorption in the distal ileum may be hampered in CD by terminal ileitis or by ileocecal resection, which tends towards decreased fasting and slightly increased postprandial blood BA levels[11–15]. There have also been indications of abnormal blood BA levels in UC[16,17]. Furthermore, EHC and enterohepatic detoxification of BAs depend on a carefully adjusted regulatory network of BA-binding transcription factors, including farnesoid X receptor (FXR) and pregnane X receptor (PXR)[9,18]. For instance, like ursodeoxycholic acid (UDCA), the endogenous toxic lithocholic acid (LCA) belongs to a group of potent PXR agonists[19–21] that comprises steroid hormones, vitamins and β-carotene[22]. Several detoxification genes and ATP-binding cassette transporters are down-regulated in intestinal cells of IBD patients[23], and PXR as a major transcriptional regulator of these detoxification genes is decreased in UC patients[23]. Finally, despite their potential toxic activities, BAs can also confer gut mucosal protection against bacteria and other cell damaging constituents of the gut lumen by two mechanisms[10]. In the proximal small intestine, BAs inhibit bacterial growth directly via their pharmacological properties, whereas in the distal small intestine, BAs mediate their antimicrobial effect indirectly via activation of FXR[24,25]. For instance, binding of chenodeoxycholic acid (CDCA) to FXR and subsequent activation of the receptor is followed by up-regulation of genes that are involved in the prevention of bacterial overgrowth and promotion of epithelial integrity[26].
In the present multicenter study, serum BA profiling was performed retrospectively in 358 IBD patients and in 310 age-matched healthy controls to assess the influence of different IBD phenotypes with various clinical manifestations on BA composition. The results further elucidate the intestinal contribution to BA homeostasis and detoxification, which is much less understood compared to corresponding processes in the liver[24,27].
MATERIALS AND METHODS
Patients and specimens
Blood samples of IBD patients and healthy volunteers were from the University Hospitals of Regensburg and Heidelberg (Germany), the Konventhospital Barmherzige Brueder Linz (Austria), and the Innsbruck Medical University (Austria). Informed consent was obtained from all patients, and the study was approved by the respective ethics committees. Samples of the Regensburg patients were from the serum bank of the German IBD network of excellence (“Kompetenznetz CED”). Blood samples were collected irrespective of the individual diet. For BA analysis, sera and clinical data were obtained from 197 CD patients (62% females; aged 16-84 years, mean age, 40 years; 46 with active disease, 43 with chronic active disease, 108 in remission) and 161 UC patients (63% females; aged 17-90 years, mean age, 40 years; 42 with active disease, 40 with chronic active disease, 79 in remission). The diagnosis was based on clinical, radiological and pathological criteria according to the guidelines of the German Gastroenterological Association (DGVS). CD patients were subgrouped according to the Vienna Classification with respect to disease behavior and localization. A CD activity index (CDAI) > 150 was regarded as “active CD”, duration of CDAI > 150 for > 3 mo as “chronic active CD”, and CDAI < 150 as “CD in remission”. UC patients were classified according to the Truelove-Witts index: A Truelove-Witts index > 6 was regarded as “active UC”, a Truelove-Witts index > 6 for > 3 mo as “chronic active UC”, and a Truelove-Witts index < 6 as “UC in remission”. Sera from 310 healthy blood donors (60% females; aged 19-66 years, mean age, 40 years) served as controls. All samples were stored frozen at -20°C until analysis.
Materials for BA analysis
Cholic acid (CA), CDCA, deoxycholic acid (DCA), LCA, UDCA, hyodeoxycholic acid (HDCA), glycocholic acid (GCA), glycochenodeoxycholic acid (GCDCA), glycodeoxycholic acid (GDCA), glycolithocholic acid (GLCA), glycoursodeoxycholic acid (GUDCA), glycohyodeoxycholic acid (GHDCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), taurolithocholic acid (TLCA), tauroursodeoxycholic acid (TUDCA), taurohyodeoxycholic acid (THDCA) standard substances, as well as deuterated BA internal standard (IS) substances (D4-CA, -CDCA, -DCA, -LCA, -UDCA, -GCA, -GCDCA) were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany), Steraloids Inc. (Newport, RI, USA), Campro Scientific GmbH (Berlin, Germany), Larodan Fine Chemicals AB (Malmø, Sweden), and were at least of 95% purity. Ammonium acetate (98%), hydrochloric acid (p.a.), as well as HPLC grade methanol and acetonitrile were purchased from VWR Int. GmbH (Darmstadt, Germany). Ultra pure water (18.2 MΩcm) was obtained from a Milli-Q Plus system (Millipore GmbH, Schwalbach, Germany).
BA extraction
For serum BA extraction, the method of Tagliacozzi et al[28] was applied with some modifications. Twenty-five microliters of a mixed IS BA solution (6-140 μmol/L in methanol) was pipetted into a 1.5-mL reaction tube and vacuum-evaporated. Two hundred and fifty microliters serum and 30 μL 1 mol/L hydrochloric acid were added (pH < 1), and the mixture was shaken for 1 min. After addition of 1 mL acetonitrile, the mixture was shaken for 2 min and centrifuged at 14 000 g for 15 min. The acetonitrile supernatant was transferred to a new reaction tube and vacuum-evaporated. The residue was dissolved in 250 μL methanol/water 1:1 (v/v) that contained 10 mmol/L ammonium acetate by shaking and sonication, and centrifuged at 14 000 g for 5 min. Ten microliters of the clear methanolic supernatant was used for analysis. Calibration samples were prepared by spiking pooled control serum with 0, 25, 50, 75 and 100 μL of a combined BA standard solution that contained appropriate amounts of each BA (0.5-70.5 μmol/L).
Liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) determination of BAs
BAs were analyzed by LC-ESI-MS/MS using the following instrumentation. An HTS PAL autosampler (CTC Analytics AG, Zwingen, Switzerland), an Agilent 1100 series binary HPLC pump (Agilent Technologies Sales & Services GmbH & Co KG, Waldbronn, Germany) combined with a Micromass Quattro Ultima tandem MS (Waters GmbH, Eschborn, Germany) operated in negative-ion mode. BA separation was performed on a Symmetry C18 reversed-phase HPLC column (50 mm × 2.1 mm, 3.5 μm particle size; Waters GmbH) by gradient elution at a flow rate of 0.3 mL/min. The 25-min elution cycle consisted of a stepwise linear change from 90% solvent A (methanol/water 1:1, v/v, 10 mmol/L ammonium acetate) + 10% solvent B (methanol, 10 mmol/L ammonium acetate) to 100% B; in detail: 0-6 min A/B = 90/10; 6-12 min A/B = 72/28; 12-16 min A/B = 60/40; 16-22 min A/B = 0/100; 22-25 min A/B = 90/10. The mass spectrometer was operated in multiple-reaction monitoring (MRM) with a cone voltage of 80 V and a collision gas pressure of 250 kPa argon. Unconjugated BAs were detected unfragmented using a collision energy of 10 eV. Glycine- and taurine-conjugated BAs were analyzed by their specific product ions at m/z 74 and 80 using collision energies of 35 and 85 eV, respectively. In detail, the retention times and MRM transitions were as follows: TCA (6.0 min, 514→80), TUDCA (4.0 min, 498→80), THDCA (4.8 min, 498→80), TCDCA (9.3 min, 498→80), TDCA (10.2 min, 498→80), TLCA (13.7 min, 482→80), GCA (6.2 min, 464→74), GUDCA (4.1 min, 448→74), GHDCA (4.9 min, 448→74), GCDCA (9.5 min, 448→74), GDCA (10.4 min, 448→74), GLCA (13.9 min, 432→74), CA (7.1 min, 407→407), UDCA (5.1 min, 391→391), HDCA (6.0 min, 391→391), CDCA (11.0 min, 391→391), DCA (11.8 min, 391→391), LCA (14.8 min, 375→375). Quantification was performed by peak ratios of BA peak areas and corresponding IS peak areas. BAs without identical deuterated ISs were related to the IS with the nearest retention time, as well as the similar MRM transition.
Statistical analysis
The significance of differences in BA concentrations was determined between cohorts with n ≥ 10 by Mann-Whitney U test for non-normally distributed data using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL, USA). A two-sided P value < 0.05 was considered statistically significant.
RESULTS
Differentiation of CD, UC and controls by characteristic BA profiles
Based on previous findings on BA metabolism in IBD and our earlier results on dysregulation of xenobiotic nuclear receptors including PXR in IBD[23], serum BA levels and composition were determined in the two major IBD phenotypes, CD and UC, and in healthy controls. The most significant differences in serum BA concentrations were found in comparison to controls. BA concentrations were decreased predominantly in both IBD subgroups, CD and UC (Tables 1, 2 and 3). Considering individual BA species, as in CD, most BAs were decreased significantly in UC patients compared with controls, but several BA conjugates, for instance TCDCA, GCDCA and GDCA, were decreased more significantly in CD than in UC patients (Table 1).
Table 1.
Serum bile acids and conjugates in CD, UC and control cohorts (nmol/L)
| BA class | BA/BA-conjugate | Control | CD | UC |
| Primary | CA | 62.5 | 72.0 | 58.0 |
| TCA | 23.6 | 0.0a | 0.0ab | |
| GCA | 383.5 | 234.0a | 377.0b | |
| CDCA | 196.5 | 190.0 | 145.0ab | |
| TCDCA | 230.5 | 46.0a | 93.0ab | |
| GCDCA | 1446.0 | 848.0a | 1243.0ab | |
| Secondary | DCA | 239.8 | 53.0a | 64.0a |
| TDCA | 48.2 | 0.0a | 0.0a | |
| GDCA | 238.6 | 26.0a | 80.0ab | |
| LCA | 15.0 | 6.2a | 8.0a | |
| TLCA | 0.0 | 0.0a | 0.0a | |
| GLCA1 | 17.4 | 0.0a | 0.4a | |
| UDCA | 28.5 | 22.0a | 17.0a | |
| TUDCA | 0.0 | 0.0a | 0.0b | |
| GUDCA1 | 137.9 | 75.6a | 60.6a | |
| Tertiary | HDCA | 0.0 | 16.0a | 10.0a |
| THDCA | 0.0 | 0.0a | 0.0a | |
| GHDCA1 | 0.0 | 0.0a | 0.0a |
Control: n = 310; CD: n = 197, n = 731; UC: n = 161, n = 441. Data expressed as medians.
P < 0.05 vs control,
P < 0.05 vs CD (Mann-Whitney U test).
Table 2.
Effect of extraintestinal manifestations on serum bile acids and conjugates in UC cohorts (nmol/L)
| BA class | BA/BA-conjugate | Control | No EM | Arthralgia/arthritis | Hepatobiliary diseases |
| Primary | CA | 62.5 | 67.5 | 88.5 | 59.0 |
| TCA | 23.6 | 0.0 | 0.0 | 67.9bc | |
| GCA | 383.5 | 360.0 | 408.5 | 722.5ab | |
| CDCA | 196.5 | 37.5 | 175.5 | 252.0 | |
| TCDCA | 230.5 | 140.0a | 92.5a | 163.0c | |
| GCDCA | 1446.0 | 1327.0 | 1281.5 | 2403.0bc | |
| Secondary | DCA | 239.8 | 78.0a | 28.0a | 62.5a |
| TDCA | 48.2 | 0.0a | 0.0a | 0.0a | |
| GDCA | 38.6 | 82.2a | 14.0a | 131.7c | |
| LCA | 15.0 | 8.0a | 6.1a | 18.0bc | |
| TLCA | 0.0 | 0.0a | 0.0a | 0.0 | |
| GLCA1 | 17.4 | 2.0a | 0.0 | 6.5 | |
| UDCA | 28.5 | 18.0a | 7.0ab | 131.5bc | |
| TUDCA | 0.0 | 0.0 | 0.0ab | 30.9abc | |
| GUDCA1 | 137.9 | 96.5 | 54.1 | 75.1 | |
| Tertiary | HDCA | 0.0 | 9.5a | 8.0a | 28.0a |
| THDCA | 0.0 | 0.0a | 0.0 | 0.0 | |
| GHDCA1 | 0.0 | 0.0 | 0.0 | 0.0 |
Control: n = 310; No EM: n = 50, n = 211; Arthralgia/arthritis: n = 30, n = 71; Hepatobiliary diseases: n = 16, n = 61. Data expressed as medians.
P < 0.05 vs control,
P < 0.05 vs no EM,
P < 0.05 vs arthralgia/arthritis (Mann-Whitney U test).
Table 3.
Effect of surgical interventions on serum bile acids and conjugates in CD cohorts (nmol/L)
| BA class | BA/BA-conjugate | Control | No surgery | Ileocecal resection | Colectomy | Other surgery | Ileocecal resection + other surgery |
| Primary | CA | 62.5 | 52.0 | 160.0ab | 71.0 | 57.5c | 89.0b |
| TCA | 23.6 | 0.0a | 0.0ab | 9.5c | 8.0c | 0.0 | |
| GCA | 383.5 | 240.5a | 156.0ab | 375.0c | 212.0 | 221.5a | |
| CDCA | 196.5 | 144.0a | 505.0ab | 174.5 | 153.0 | 121.0 | |
| TCDCA | 230.5 | 81.0a | 19.0ab | 37.5ab | 55.5a | 17.0ab | |
| GCDCA | 1446.0 | 848.5a | 767.0a | 1059.0 | 663.5a | 772.0 | |
| Secondary | DCA | 239.8 | 90.5a | 11.0a | 6.5ab | 17.5a | 53.0d |
| TDCA | 48.2 | 5.9a | 0.0ab | 0.0a | 0.0ab | 0.0a | |
| GDCA | 238.6 | 64.0a | 0.0ab | 0.0ab | 0.0ab | 43.5a | |
| LCA | 15.0 | 7.0a | 5.9a | 3.0a | 4.7a | 2.9a | |
| TLCA | 0.0 | 0.0 | 0.0ab | 0.0ab | 0.0 | 0.0 | |
| GLCA1 | 17.4 | 3.6a | 0.0a | 0.0 | 2.3 | 0.0 | |
| UDCA | 28.5 | 14.5a | 26.0b | 12.7 | 53.5 | 22.5 | |
| TUDCA | 0.0 | 0.0 | 0.0a | 0.0a | 0.0a | 0.0a | |
| GUDCA1 | 137.9 | 95.2a | 59.7 | 92.1 | 323.5 | 75.6 | |
| Tertiary | HDCA | 0.0 | 27.0a | 10.0a | 9.5a | 9.0a | 2.0a |
| THDCA | 0.0 | 0.0a | 0.0 | 0.0 | 0.0 | 0.0 | |
| GHDCA1 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 |
Control: n = 310; No surgery: n = 64, n = 311; Ileocecal resection: n = 41, n = 161; Colectomy: n = 22, n = 81; Other surgery: n = 12, n = 41; Ileocecal resection + other surgery: n = 12, n = 91. Data expressed as medians.
P < 0.05 vs control,
P < 0.05 vs no surgery,
P < 0.05 vs ileocecal resection,
P < 0.05 vs colectomy (Mann-Whitney U test).
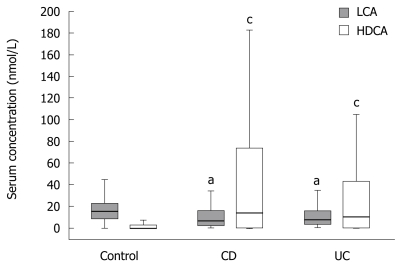
While serum levels of LCA, known to be the strongest PXR agonist, were significantly lower, serum levels of HDCA, the CYP3A4-mediated detoxification product of LCA, were always significantly higher in CD and UC patients compared to controls (Figure 1).
Figure 1.
Decrease of serum LCA but increase of serum HDCA in CD and UC vs healthy control cohorts. LCA and HDCA were analyzed using LC-ESI-MS/MS. Box plots represent interquartile ranges containing medians (boxes) and minimum/maximum bars. aP < 0.05 vs LCA in controls; cP < 0.05 vs HDCA in controls (Mann-Whitney U test).
If total serum BA levels were considered (Table 4), we found that total unconjugated and total BA tauroconjugate levels, respectively, were decreased significantly in UC patients, but other than in CD patients, there was no decrease in total BA, total BA conjugate, and total BA glycoconjugate levels compared to controls. Moreover, total BA conjugate levels, as well as total BA glycoconjugate and tauroconjugate levels alone, were increased significantly in UC vs CD patients. However, if levels relative to total BA conjugate levels were considered, total BA tauroconjugate levels were increased significantly but total BA glycoconjugate levels were decreased significantly in UC vs CD patients. In addition, UC patients were characterized by a significantly decreased ratio of total deoxy-BA, LCA, and LCA conjugate levels to total BA levels compared to CD patients and controls (Table 4).
Table 4.
Total serum bile acids and conjugates in CD, UC and control cohorts
| Control | CD | UC | |
| Total BAs (nmol/L) | 3752.0 | 2563.3a | 3010.2 |
| Total unconjugated BAs1 (nmol/L) | 644.1 | 631.1 | 471.0ab |
| Total conjugated BAs (nmol/L) | 2763.6 | 1526.9a | 2529.6b |
| Total BA glycoconjugates (nmol/L) | 2423.6 | 1407.6a | 2298.9b |
| Total BA glycoconjugates/total conjugated BAs (%) | 87.4 | 95.7a | 92.7ab |
| Total BA tauroconjugates1 (nmol/L) | 344.2 | 68.0a | 145.0ab |
| Total BA tauroconjugates/ total conjugated BAs (%) | 12.6 | 4.3a | 7.3ab |
| (Deoxy-BAs + TLCA + GLCA + LCA)/total BAs (%) | 83.7 | 84.1 | 77.4ab |
Control: n = 310; CD: n = 73, n = 1971; UC: n = 44, n = 1611. Data expressed as medians.
P < 0.05 vs control,
P < 0.05 vs CD (Mann-Whitney U test).
Hepatobiliary manifestations influence BA composition in UC
IBD is often accompanied by extraintestinal manifestations (EMs), such as hepatobiliary diseases, and eye, joint and skin affections[29,30]. We therefore investigated whether IBD patients with EMs showed different serum BA profiles. While we found no influence of EMs on serum BA levels in CD patients (data not shown), UC patients with hepatobiliary diseases, e.g. primary sclerosing cholangitis (PSC), hepatitis, or cholelithiasis, had significantly increased BA concentrations compared to UC patients without EMs, especially levels of the primary BAs TCA, GCA and GCDCA, as well as the secondary BAs LCA, UDCA and TUDCA (Table 2).
Previous bowel resection influences BA composition in CD
Intestinal reabsorption of BAs is a physiological function of the terminal ileum, therefore, surgical interventions in this region may influence serum BA levels caused by impaired EHC. While there were no UC patients with surgical interventions included in the present study, CD patients showed significant variations in serum BA concentrations correlated to previous bowel resection (Table 3). Overall, compared to controls and CD patients without surgical interventions, ileocecal resection alone was associated with the most intensive decrease of primary and secondary BA conjugates, such as TCDCA, TDCA, GCA and GDCA, as well as a marked increase in the unconjugated primary BAs, CA and CDCA. In addition, CD patients with ileocecal resection and other surgical interventions, e.g. ileostomy, sigmoidostomy, transversostomy, fistula excision, and hemicolectomy, had significantly decreased TCDCA compared to controls and CD patients without surgical interventions. Furthermore, in CD patients with colectomy, TCDCA, as well as the secondary BAs GDCA and DCA, were decreased significantly compared to those in controls and patients without surgical interventions.
No effects of disease activity and medical treatment on BA composition in CD and UC
We also investigated whether serum BA composition was influenced by disease activity and different therapeutic medications in IBD patients, since mucosal inflammation, as well as pharmacologically induced changes in the inflammatory process in IBD may influence BA reabsorption, which results in changed serum BA profiles. Overall, serum BA composition in CD and UC were independent of disease activity and medical treatment (data not shown).
DISCUSSION
During the past three decades, BA analysis in IBD has been achieved in serum, bile or feces from small patient cohorts by radioimmunoassay or gas-liquid chromatography detecting total BAs and selected individual BAs, respectively[17,31–34]. In the present study, we applied a sensitive high-throughput LC-ESI-MS/MS method with minimal sample preparation steps for simultaneous determination of 18 different BA species as serum BA profiles in a large cohort of IBD patients and controls. We analyzed the main unconjugated human primary, secondary and tertiary BAs, i.e. CA, CDCA, DCA, LCA, UDCA, HDCA, and their respective glycine and taurine conjugates. Comparing a whole profile of BA subspecies in various IBD phenotypes may reflect intestinal malfunction and disease states more sensitively than just considering total or selected individual BA levels.
Thus, we showed that decreased serum BA levels were not restricted to CD alone, as previously reported[13–15,35], but were also found in UC if a defined set of specific BAs were considered. This is contradictory to the few reports on abnormal blood BA levels in UC patients. Ejderhamm and Strandvik[17] have reported increased primary serum BAs, CA and CDCA, in juvenile active UC patients compared to healthy controls, while there were no significant differences for CA, but decreased CDCA levels in our UC cohort compared to controls. Kostic et al[16] have reported decreased total plasma BA levels in CD and UC patients compared to controls, but we did not find significant differences in total BA, total BA conjugate, and total BA glycoconjugate levels between UC patients and the control group. Only total BA tauroconjugate and unconjugated BA levels were reduced significantly in UC patients vs controls. However, because of their relatively low contribution to total BA and BA conjugate levels compared to the most abundant BA glycoconjugates, this effect was not dominant and may explain the missing reports on decreased total BA levels in UC patients. In summary, our data confirm studies that have shown a decrease in serum BA levels in CD patients, which reflects the strongest impact on intestinal BA reabsorption during EHC. This can be explained by the fact that BA reabsorption takes place predominantly in the distal small intestine[10], which is usually more affected in CD in contrast to colon-restricted UC. Therefore, most serum BA levels in UC patients are not decreased as much as in CD patients.
Furthermore, it is noteworthy that the levels of the unconjugated primary BAs, CA and CDCA, in CD and UC patients were not significantly different from the controls, except for decreased CDCA in UC patients. The explanation that there is an increased compensatory synthesis of primary BAs in IBD, as suggested by Rutgeerts et al[13] in Crohn’s ileitis, assumes an accelerated bacterial deconjugation of the respective glyco- and tauroconjugates in the intestine. Indeed, the reduced serum levels of TCA, TCDCA, GCA, and GCDCA shown in Table 1 support this assumption, which is in agreement with previous findings of unusually high intestinal BA deconjugation in CD and UC[36,37]. Apart from deconjugation, the quantitatively most important bacterial biotransformation of BAs is 7α-deoxidation of CA and CDCA by Eubacteria in the colon, which yields DCA and LCA, respectively[38,39]. In UC patients, we found significantly reduced ratios of total deoxy-BA (including DCA, LCA, and its conjugates) to total BA levels compared to those in CD patients and controls, which may reflect an abnormal colonic bacterial flora with reduced deoxidation capacity. In addition, bacterial overgrowth in the small intestine and colon of IBD patients may enhance the described BA biotransformation processes and contribute to the imbalance of BA species distribution in the EHC. Decreased intestinal BA levels, especially of conjugated BAs, may promote bacterial overgrowth because of a loss of their antimicrobial properties[10,26]. Since IBD patients included in this study were not stratified according to the use of antibiotics, this effect has not been evaluated and needs further systematic investigation in well-defined patient cohorts.
The invariably increased HDCA and decreased LCA in IBD compared to control sera, irrespective of the clinical findings (EMs, surgical interventions, disease activity or medication), indicates accelerated enterohepatic LCA detoxification via CYP3A4[18]. Whether serum HDCA elevation is additionally caused by increased intestinal reabsorption or impaired hepatic excretion cannot be resolved by the present data and has to be further investigated.
Moreover, the influence of hepatobiliary EMs on serum BA levels in IBD is demonstrated clearly in UC patients, who showed a significant increase in primary and secondary BAs compared to EM-free patients. This confirms previous observations when elevated serum levels of total primary BA conjugates have been seen in IBD patients with liver diseases[31,40]. Although serum LCA levels in UC patients with hepatobiliary EMs were found to be normal compared to controls, in accordance with the findings of Dew et al[40], they were significantly higher compared to those in UC patients without EMs and with arthralgia/arthritis. Elevated BA levels are particularly found in patients with PSC, which is often associated with IBD[29,41]. However, it cannot be ruled out that the significantly increased serum levels of TUDCA, UDCA and LCA found in our UC cohort with hepatobiliary EMs were caused by therapeutic administration of UDCA, which is being used increasingly for the treatment of cholestatic liver diseases[42–44] and PSC-associated UC[45,46]. UDCA medication not only causes increased primary BA biosynthesis, but UDCA is also metabolized to additional TUDCA and LCA[47], which yields increased serum levels in these patients. Nonetheless, we assume that disturbed EHC of BAs in IBD is highly susceptible to additional hepatobiliary EMs.
With surgical interventions predominantly appearing in CD patients, we found that ileocecal resection exerts the strongest impact on serum BA levels in CD patients, since BA reabsorption is located predominantly in the terminal ileum[10]. Compared to patients without surgical interventions, the finding that patients with ileocecal resection showed significantly decreased conjugated BAs but increased unconjugated primary BAs, i.e. CA and CDCA, may be explained by an increased compensatory synthesis of primary BAs in IBD, as previously suggested[13], associated with an enhanced bacterial deconjugation of the respective glyco- and tauroconjugates in the remaining intestinal sections[36,37]. In addition, concerning the elevated CA levels in patients with ileocecal resection, we confirm the previous findings of Tougaard et al[48]. As expected, unlike ileocecal resection, the influence of colectomy on serum BA levels in CD was less significant, since only small amounts of BAs are reabsorbed in this intestinal region.
To summarize, using mass spectrometric BA species profiling instead of total BA determination, we showed the characteristic impact of different IBD phenotypes with intestinal and hepatobiliary manifestations on BA homeostasis and detoxification. Further prospective studies on prominent BAs in well-defined IBD cohorts are necessary to confirm their diagnostic and prognostic value.
COMMENTS
Background
Inflammatory bowel disease (IBD) is a multifactorial disorder with as yet incompletely elucidated causes. Since bile acids (BAs) derived from the liver are directly involved in intestinal processes primarily by facilitating lipid digestion, IBD has an impact on BA metabolism. This correlation may be reflected in unusual BA blood levels that differentiate between the two clinical IBD phenotypes, Crohn’s disease (CD) and ulcerative colitis (UC), as well as between CD and UC subgroups with diverse clinical manifestations.
Research frontiers
Besides their digestive functions, BAs have recently been found to play an important regulatory role in numerous metabolic processes, e.g. energy and lipid balance and elimination of harmful substances. They are mediated by binding appropriate nuclear receptors in the cell that depend on the molecular type of BA, which can be differentiated by means of high performance mass spectrometry. Thus, quantifying diverse BAs simultaneously, a characteristic profile of main and rare BAs is available that reflects medical conditions far better than measuring total BA levels or individual abundant BAs.
Innovations and breakthroughs
Applying BA profiling in IBD patients, the authors showed that most but not all BA species are decreased in CD and UC patient sera, but with different intensity. BA decrease is highly pronounced in CD patients with surgical interventions in the gut except for unconjugated primary BAs. On the other hand, UC patients with additional liver and gallbladder diseases show clearly increased levels of primary and secondary BAs. Finally, the authors found a marked decrease in the toxic BA lithocholic acid, together with a marked increase in its physiological detoxification product, hyodeoxycholic acid, irrespective of the IBD phenotype or clinical manifestation, which shows accelerated detoxification activity in IBD patients.
Applications
Serum BA profiling may serve as an additional diagnostic tool for IBD characterization and differentiation. In combination with expression profiles of pregnane X receptor (PXR) -regulated genes, it may allow us to estimate the BA detoxification potential of IBD patients.
Terminology
Primary BAs are directly synthesized in the liver and secondary BAs are derived from primary BAs by biochemical modification by intestinal bacteria. BAs can be conjugated, mainly with the amino acids glycine and taurine, or unconjugated. The enterohepatic circulation leads to a maximum physiological recycling of BAs and comprises liver synthesis, intestinal excretion via the bile duct, intestinal reabsorption, and return transport to the liver via the portal vein. Liquid chromatography tandem mass spectrometry is a sensitive analytical method for simultaneous determination of structural related biomolecules like BAs. The nuclear BA receptors farnesoid X receptor and PXR mediate most of the physiological effects of BAs, e.g. expression of detoxification genes by PXR.
Peer review
This work expand the knowledge about the role of BA metabolism in IBD. It is a well-conducted study.
Acknowledgments
The authors thank Eva Balogh, Helga Staudner, Andrea Dirmeier, Barbara Effenberger, Tanja Spöttl, Sabine Tuma, and Michael Scharl for their excellent technical assistance.
Supported by A grant from the Deutsche Forschungsgemeinschaft (SFB585-A1/A4), the Stiftung für Pathobiochemie und Molekulare Diagnostik (TL), the Dietmar Hopp Foundation, the EU FP 6 funded SSA “ELIfe” project (The European Lipidomics Initiative; Shaping the life sciences; proposal number 013032), and the EU FP 7 funded project “LipidomicNet” (lipid droplets as dynamic organelles of fat deposition and release: translational research towards human disease; proposal number 202272)
Peer reviewer: Eldon Shaffer, Professor of Medicine, Division of Gastroenterology, Department of Medicine, Health Science Centre, University of Calgary, 3330 Hospital Dr N.W., Calgary, AB, T2N4N1, Canada
S- Editor Li LF L- Editor Kerr C E- Editor Ma WH
References
- 1.Scholmerich J. New developments in aetiological mechanisms of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2003;15:585–586. doi: 10.1097/00042737-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Rogler G. Update in inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2004;20:311–317. doi: 10.1097/00001574-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt C, Stallmach A. Etiology and pathogenesis of inflammatory bowel disease. Minerva Gastroenterol Dietol. 2005;51:127–145. [PubMed] [Google Scholar]
- 4.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed FE. Role of genes, the environment and their interactions in the etiology of inflammatory bowel diseases. Expert Rev Mol Diagn. 2006;6:345–363. doi: 10.1586/14737159.6.3.345. [DOI] [PubMed] [Google Scholar]
- 6.Langmann T, Schmitz G. Loss of detoxification in inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:358–359. doi: 10.1038/ncpgasthep0545. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz G, Langmann T, Heimerl S. Role of ABCG1 and other ABCG family members in lipid metabolism. J Lipid Res. 2001;42:1513–1520. [PubMed] [Google Scholar]
- 8.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Houten SM, Auwerx J. The enterohepatic nuclear receptors are major regulators of the enterohepatic circulation of bile salts. Ann Med. 2004;36:482–491. doi: 10.1080/07853890410018790. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci USA. 2006;103:4333–4334. doi: 10.1073/pnas.0600780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuelson K, Johansson C, Norman A. Serum bile acids after a test meal in Crohn’s disease. Scand J Clin Lab Invest. 1979;39:511–518. doi: 10.1080/00365517909108828. [DOI] [PubMed] [Google Scholar]
- 12.Heuman R, Sjodahl R, Tobiasson P, Tagesson C. Postprandial serum bile acids in resected and non-resected patients with Crohn's disease. Scand J Gastroenterol. 1982;17:137–140. doi: 10.3109/00365528209181058. [DOI] [PubMed] [Google Scholar]
- 13.Rutgeerts P, Ghoos Y, Vantrappen G. Kinetics of primary bile acids in patients with non-operated Crohn’s disease. Eur J Clin Invest. 1982;12:135–143. doi: 10.1111/j.1365-2362.1982.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 14.Heuman R, Sjodahl R, Tobiasson P, Tagesson C. Decreased absorption of ingested unconjugated chenodeoxycholic acid in patients with Crohn's disease. Scand J Gastroenterol. 1983;18:23–26. doi: 10.3109/00365528309181553. [DOI] [PubMed] [Google Scholar]
- 15.Linnet K, Mertz Nielsen A. Fasting and postprandial serum concentrations of glycine- and taurine-conjugated bile acids in Crohn’s disease. Scand J Gastroenterol. 1983;18:433–438. doi: 10.3109/00365528309181619. [DOI] [PubMed] [Google Scholar]
- 16.Kostic N, Bozanic M, Cvetkovic R, Adamov A. [Lipids and total bile acids in the blood of patients with inflammatory bowel diseases] Srp Arh Celok Lek. 1990;118:43–46. [PubMed] [Google Scholar]
- 17.Ejderhamn J, Strandvik B. Serum bile acids in relation to disease activity and intake of dietary fibers in juvenile ulcerative colitis. Digestion. 1991;50:162–169. doi: 10.1159/000200757. [DOI] [PubMed] [Google Scholar]
- 18.Makishima M. Nuclear receptors as targets for drug development: regulation of cholesterol and bile acid metabolism by nuclear receptors. J Pharmacol Sci. 2005;97:177–183. doi: 10.1254/jphs.fmj04008x4. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 20.Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 2005;129:735–740. doi: 10.1016/j.gastro.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 21.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40:539–551. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Ruhl R. Induction of PXR-mediated metabolism by beta-carotene. Biochim Biophys Acta. 2005;1740:162–169. doi: 10.1016/j.bbadis.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:289–303. doi: 10.2174/1568008054863781. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang JY. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G349–G356. doi: 10.1152/ajpgi.00417.2002. [DOI] [PubMed] [Google Scholar]
- 28.Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, Urbani A, Federici G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 29.Raj V, Lichtenstein DR. Hepatobiliary manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:491–513. doi: 10.1016/s0889-8553(05)70067-4. [DOI] [PubMed] [Google Scholar]
- 30.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, Gasbarrini G, Gasbarrini A. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–7236. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihas AA, Gibson RG, Levy N, Hirschowitz BI. Serum-bile-acids in inflammatory bowel disease. Lancet. 1977;2:405–406. doi: 10.1016/s0140-6736(77)90337-3. [DOI] [PubMed] [Google Scholar]
- 32.Samuelson K, Aly A, Johansson C, Norman A. Evaluation of fasting serum bile acid concentration in patients with liver and gastrointestinal disorders. Scand J Gastroenterol. 1981;16:225–234. doi: 10.3109/00365528109181960. [DOI] [PubMed] [Google Scholar]
- 33.Kruis W, Kalek HD, Stellaard F, Paumgartner G. Altered fecal bile acid pattern in patients with inflammatory bowel disease. Digestion. 1986;35:189–198. doi: 10.1159/000199367. [DOI] [PubMed] [Google Scholar]
- 34.Lapidus A, Akerlund JE, Einarsson C. Gallbladder bile composition in patients with Crohn’s disease. World J Gastroenterol. 2006;12:70–74. doi: 10.3748/wjg.v12.i1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutgeerts P, Ghoos Y, Vantrappen G. Bile acid studies in patients with Crohn’s colitis. Gut. 1979;20:1072–1077. doi: 10.1136/gut.20.12.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton KW. Disturbances of bile acid metabolism in intestinal disease. Clin Gastroenterol. 1977;6:69–89. [PubMed] [Google Scholar]
- 37.Lenz K. Bile acid metabolism and vitamin B12 absorption in ulcerative colitis. Scand J Gastroenterol. 1976;11:769–775. [PubMed] [Google Scholar]
- 38.Hylemon PB, Harder J. Biotransformation of monoterpenes, bile acids, and other isoprenoids in anaerobic ecosystems. FEMS Microbiol Rev. 1998;22:475–488. doi: 10.1111/j.1574-6976.1998.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 39.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Dew MJ, van Berge Henegouwen GP, Huybregts AW, Allan RN. Hepatotoxic effect of bile acids in inflammatory bowel disease. Gastroenterology. 1980;78:1393–1401. [PubMed] [Google Scholar]
- 41.Jorge A, Findor J, Esley C, Bruch E. Primary sclerosing cholangitis. Z Gastroenterol. 1988;26:322–330. [PubMed] [Google Scholar]
- 42.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 43.Paumgartner G, Beuers U. Mechanisms of action and therapeutic efficacy of ursodeoxycholic acid in cholestatic liver disease. Clin Liver Dis. 2004;8:67–81, vi. doi: 10.1016/S1089-3261(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 44.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 45.Pardi DS, Loftus EV Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 46.Wolf JM, Rybicki LA, Lashner BA. The impact of ursodeoxycholic acid on cancer, dysplasia and mortality in ulcerative colitis patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. 2005;22:783–788. doi: 10.1111/j.1365-2036.2005.02650.x. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1–15. doi: 10.3109/00365529409103618. [DOI] [PubMed] [Google Scholar]
- 48.Tougaard L, Giese B, Pedersen BH, Binder V. Bile acid metabolism in patients with Crohn’s disease in terminal ileum. Scand J Gastroenterol. 1986;21:627–633. doi: 10.3109/00365528609003110. [DOI] [PubMed] [Google Scholar]