Abstract
Purpose
To test for associations between urine markers, bladder biopsy features and bladder ulcers in interstitial cystitis/painful bladder syndrome (IC/PBS).
Materials and Methods
Subjects were 72 patients with IC/PBS undergoing bladder distention and biopsy. Urine was collected before the procedure. Urine marker levels were correlated with biopsy and cystoscopic findings. Patients with no previous IC/PBS treatments (n=47) were analyzed separately from previously treated patients (n=25).
Results
For untreated patients, urine IL-6 and cGMP were associated with urothelial EGF receptor staining (for IL-6 r=0.29, 95% CI (0.07, 0.51), p=0.01; for cGMP r=0.34, 95% CI (0.13, 0.55), p=0.002). Urine IL-8 was negatively associated with urothelial HB-EGF staining (r=-0.34, 95% CI (-0.55, -0.12), p=0.002) and positively associated with lamina propria mast cell count (r=0.29, 95% CI (0.06, 0.52), p=0.01). The latter association also was seen in treated patients (r=0.46, 95% CI (0.20, 0.73), p<0.001). None of the urine markers was significantly different for ulcer vs. nonulcer patients. All of the ulcer patients had extensive inflammation on bladder biopsy: severe mononuclear cell infiltration, moderate or strong IL-6 staining in the urothelium and lamina propria, and LCA staining in >10% of the lamina propria. However, these features also were seen in 24-76% of the nonulcer patients.
Conclusions
Overall, urine markers did not associate robustly with biopsy findings. The strongest association was a positive association between urine IL-8 levels and bladder mast cell count. Ulcer patients consistently had bladder inflammation, but the cystoscopic finding of ulcers was not a sensitive indicator of inflammation on bladder biopsy.
Keywords: interstitial cystitis, urine; interstitial cystitis, pathology; interstitial cystitis, physiopathology
Introduction
Interstitial cystitis/painful bladder syndrome (IC/PBS) includes pelvic/perineal pain, urgency and frequent voiding, and is often difficult to treat. Clinicians are hampered by the lack of biomarkers to use in selecting treatments, evaluating treatment effects, and deciding when and how to modify treatments. Most treatments are hypothesized to affect one or more specific abnormalities in IC/PBS, e.g. bladder epithelial deficiency (as a primary or secondary problem), bladder inflammation, bladder mast cell activation or altered innervation. Unfortunately, it is difficult to determine whether any of these processes is occurring in an individual patient. The only well-established information comes from cystoscopy and bladder biopsies, but this is invasive, expensive, and not feasible to repeat over time. In contrast, urine collection is painless and repeatable. Therefore, we evaluated a panel of urine markers to determine whether they associated with specific bladder biopsy findings or the presence of cystoscopically visible ulcers. We also tested whether ulcers were a sensitive indicator of bladder inflammation on biopsy.
Materials and Methods
Subjects
Subject criteria and procedures were previously described in detail.1 Briefly, patients with IC/PBS underwent cystoscopy with bladder distention by one of three investigators (DRE, KMP, ESR). Ulcer patients were defined as those with visible ulcers on cystoscopy, i.e. Hunner's ulcer. Nonulcer patients had no visible ulcers. Among the nonulcer patients, some had sufficient glomerulations to met the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK)2 cystoscopic criteria, while others did not. We did not require patients to meet the cystoscopic criteria because we previously found that meeting the criteria had no effect on urine marker levels or bladder biopsy findings.1
Urine measurements
Before cystoscopy the subjects provided voided urine specimens, which were processed and analyzed as previously described.3 The urine markers included anti-proliferative factor (APF), epidermal growth factor (EGF), heparin-binding EGF-like growth factor (HB-EGF), cyclic guanosine monophosphate (cGMP), interleukins 6 and 8 (IL-6, IL-8). All markers except APF were normalized to urine creatinine concentration. For APF, urine was diluted to a standard pH and osmolarity, then subjected to thymidine uptake assay. APF activity was read as either positive (> 2 standard deviations of the inhibition seen on the control cells on the same plate) or negative.
Bladder biopsies
After distention, the urologists' usual practice was to take cold-cup bladder biopsies from one to three sites (excluding the trigone). After preparing slides for clinical purposes, extra slides were made for research purposes and analyzed by one of us (JET) as previously described.1 If more than one biopsy was taken and the findings differed, the score for the more intense staining or the most severe pathology was used. Staining for EGF, HB-EGF, EGF receptor and IL-6 was semi-quantitatively graded from 0 (none) to 3 (intense). Mast cells were counted after tryptase stain as previously described.1 Overall bladder inflammation was classified as either mild (<100 mononuclear cells/HPF and no lymphoid aggregates) or severe (≥100 mononuclear cells/HPF or lymphoid aggregates) as previously described.1 The IC/PBS Database Study definitions were used for grading other biopsy features (trichrome staining for detrusor fibrosis, F8 stain for vessels in lamina propria, LCA (leukocyte common antigen) staining for leukocytes in the lamina propria, submucosal hemorrhage, submucosal granulation tissue and percent of epithelium denuded).4
Statistical analysis
Since treatments might affect urine marker levels or biopsy findings, patients with no previous IC/PBS treatments were analyzed separately from treated patients. Continuous data are reported as the median (25th percentile, 75th percentile). Confidence interval is abbreviated as CI. Kendall's tau-b correlation coefficient was used to assess association between urine markers and ordinal or cardinal biopsy variables. The Wilcoxon-Mann-Whitney test was used to compare urine marker levels between two independent groups. Fisher's exact test was used to compare presence/absence of ulcers with binary variables. The exact version of the Mantel-Haenszel chi-square test was used for comparisons between ulcer status and ordinal biopsy features. All data were analyzed using SAS software version 9.1 (SAS Institute Inc., Cary, NC). Significance was established at the α=0.01 level to account for possible artifacts due to multiple hypothesis tests.
Results
Demographics
The untreated patients included two Caucasian men and 45 women (38 Caucasian, one Asian, two African-American, one bi-racial, three Hispanic). Their median age was 36 years (range 22-72) and their median symptom duration was 6 years (range, 0.3-40). The treated patients were three men (two Caucasian, one Asian) and 22 women (18 Caucasian, three African-American, one with race not stated). Their median age was 52 years (range 20-76), significantly older than the untreated patients (p=0.004). Their median symptom duration was 7 years (range, 0.2-47), not significantly different from untreated patients (p=0.35).
The treated patients included 17 nonulcer patients and eight ulcer patients. The ulcer patients were significantly older than the nonulcer patients (median age 68, range, 35-76 vs median age 39, range 20-73, p=0.03). However, the symptom durations were not significantly different (ulcer patients median 7 years, range 0.2-47 vs. nonulcer patients median 11 years, range 0.9-45, p=0.93). Among the untreated patients, only one had ulcers (her age was 46, symptom duration 6 years).
Urine markers vs. individual biopsy findings in untreated patients
For urine APF activity, all patients were positive except one. Frequency distributions for most relevant biopsy features are as follows. Percent of epithelium denuded: no denudation (1 patient), 1-25% (19 patients), 26-50% (10 patients), 51-99% (15 patients, including the APF-negative patient) and 100% (two patients). HB-EGF staining in urothelium: zero (4 patients), 1 (11 patients), 2 (9 patients) and 3 (intense): (23 patients, including the APF-negative patient).
For the other urine markers, four statistically significant associations were found (Table 1). However, the marker values had too much scatter to be reliable surrogates for the bladder biopsy findings (Figures 1-4). Since the role of the NIDDK criteria is still controversial, we repeated the analysis using only patients who met the cystoscopic criteria. Overall the results were similar, except the association between mast cell count and urine IL-8 was no longer statistically significant.
Table 1. Significant Associations Between Urine Markers and Biopsy Findings in Untreated IC Patients.
| Cystoscopic criteria required | Criteria not required | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biopsy | Urine | n* | r** | 95% CI | P value | n* | r** | 95% CI | P value |
| HB-EGF in urothelium | IL-8 | 22 | -0.30 | -0.51, -0.10 | 0.005 | 34 | -0.34 | -0.55, -0.12 | 0.002 |
| EGF receptor in urothelium | cGMP | 22 | 0.35 | 0.13, 0.57 | 0.002 | 35 | 0.34 | 0.13, 0.55 | 0.002 |
| EGF receptor in urothelium | IL-6 | 30 | 0.29 | 0.02, 0.57 | 0.04 | 45 | 0.29 | 0.07, 0.51 | 0.01 |
| Mast cells in lamina propria | IL-8 | 22 | 0.13 | -0.18, 0.45 | 0.40 | 34 | 0.29 | 0.06, 0.52 | 0.01 |
some values are missing, due to loss or insufficiency of the relevant urine aliquot or slide.
Kendall's tau-b correlation coefficient
Figure 1.
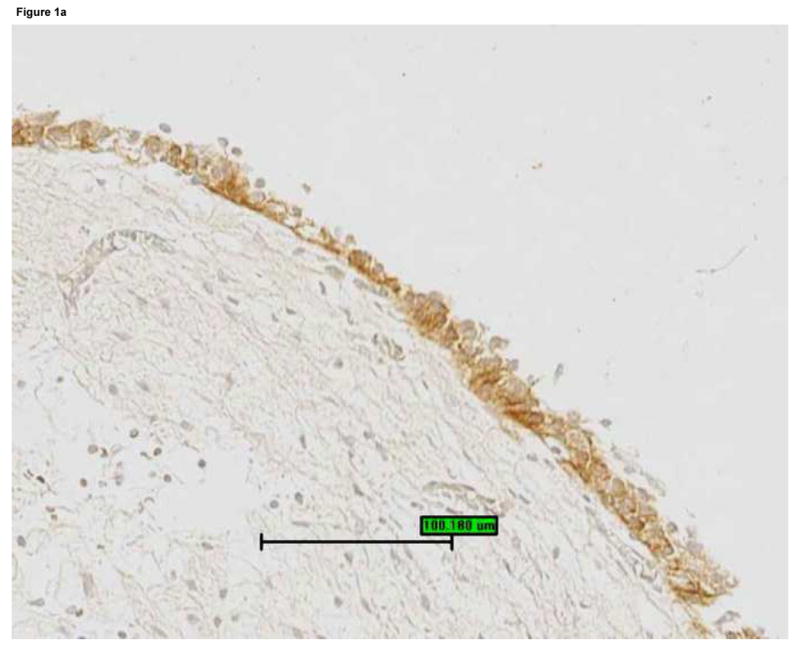
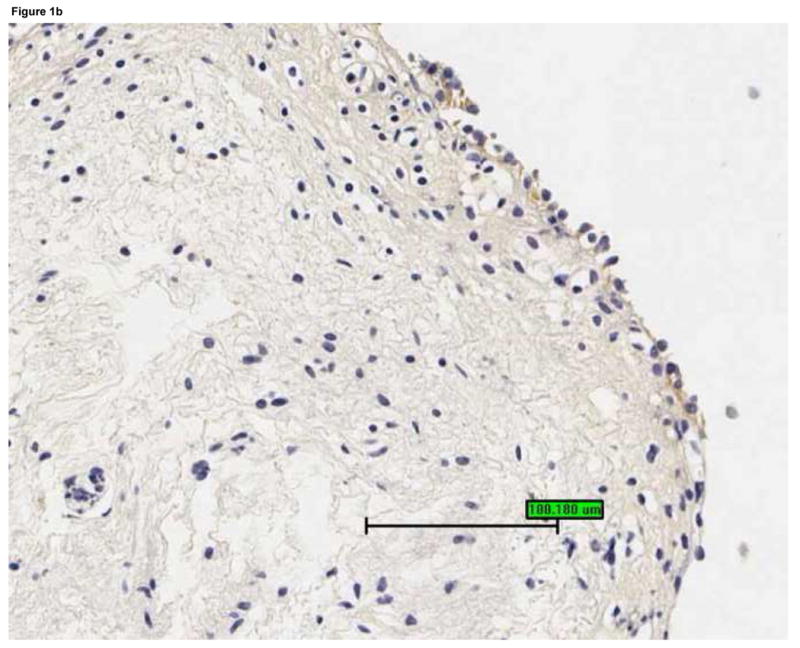
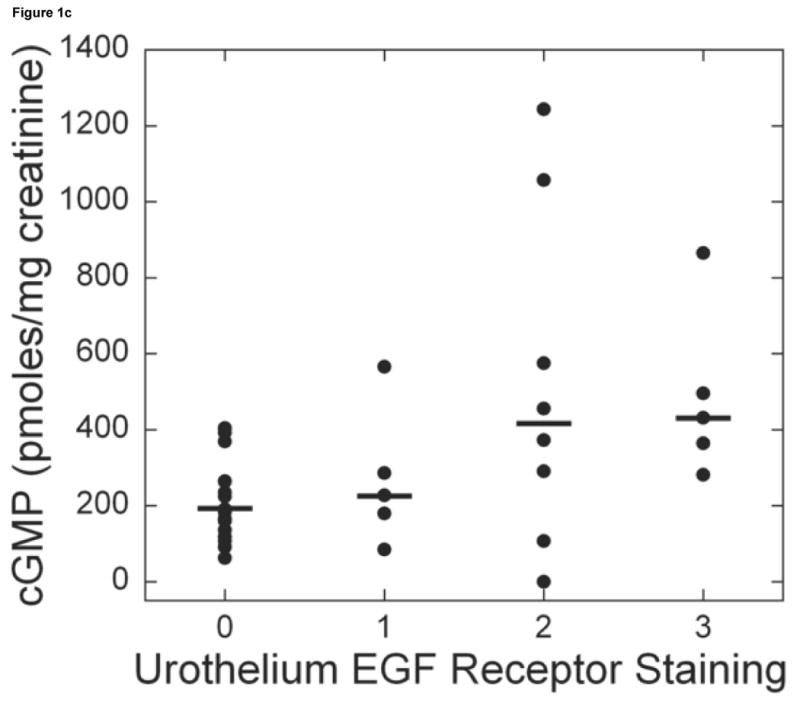
Bladder biopsies were stained with anti-EGFR primary antibody, followed by a standard streptavidin method. EGFR staining was graded semiquantitatively from 0 (none) to 3 (intense). On each biopsy the urothelium and lamina propria were graded separately. a: Grade 3 staining for EGFR in the urothelium (magnification 200×). b: Grade 1 staining for EGFR in the urothelium (magnification 200×). c: Urothelial EGFR staining intensity was significantly associated with urine cGMP levels in untreated IC/PBS patients. Urine cGMP was analyzed by radioimmunoassay and normalized to urine creatinine. Bars represent median values for cGMP in each staining category.
Figure 4.
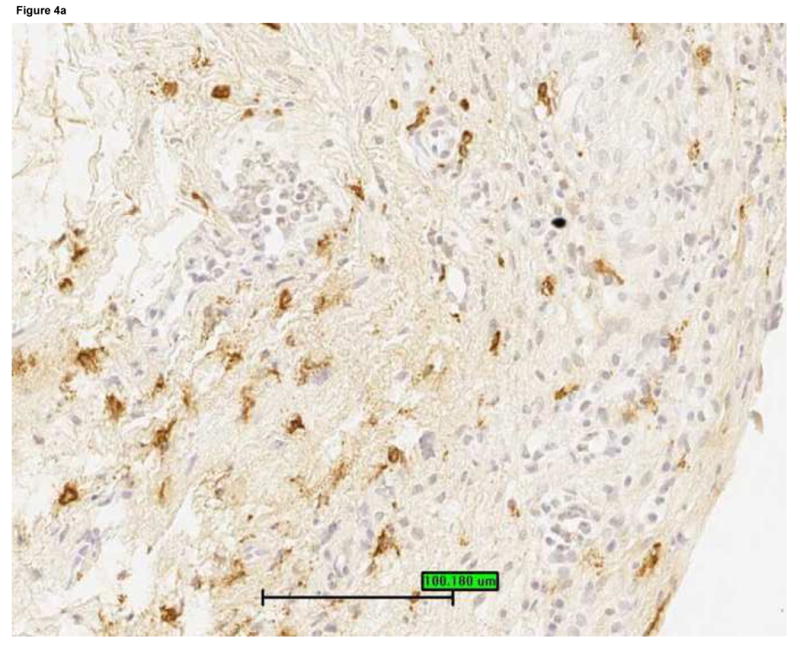
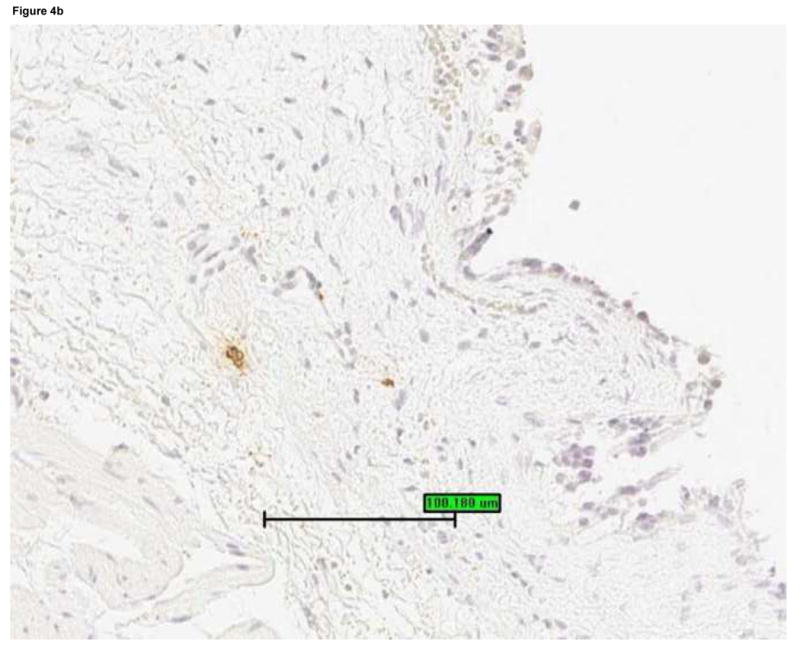
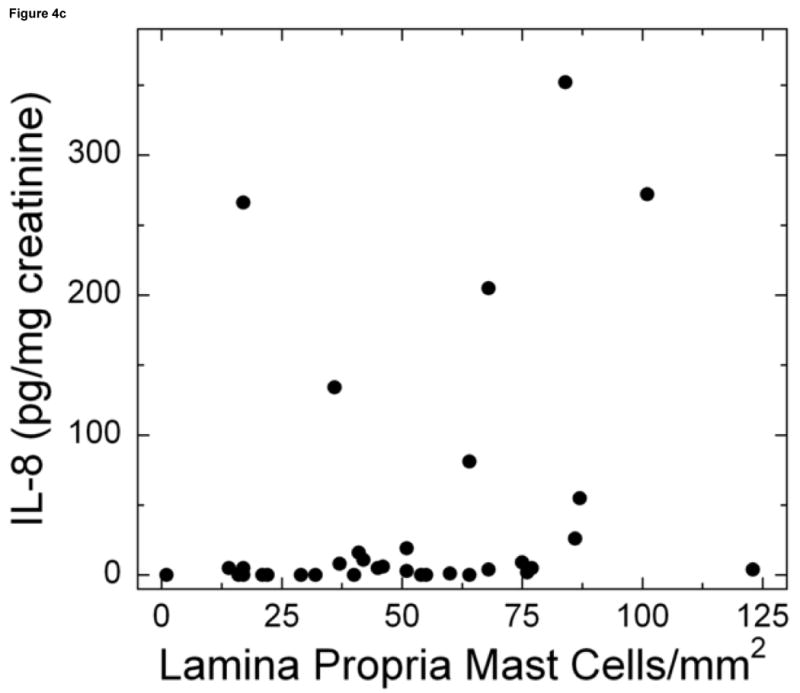
Mast cells were detected by staining bladder biopsies with anti-tryptase primary antibody followed by a standard streptavidin method. An optical reticle was used to count mast cells in the urothelium, lamina propria and detrusor. a: Brisk mastocytosis in the lamina propria (magnification 200×). b: Minimal mastocytosis in the lamina propria (magnification 200×). c: Scatter plot of urine IL-8 levels vs. mast cell count in the lamina propria for untreated IC/PBS patients. Lamina propria mast cell count was significantly associated with urine IL-8, which was analyzed by ELISA and normalized to urine creatinine.
Urine markers vs. individual biopsy findings in treated patients
For urine APF activity, one patient was borderline and the rest were positive. Percent epithelium denuded was distributed as follows: none (1 patient), 1-25% (9 patients), 26-50% (5 patients), 51-99% (5 patients) and 100% (5 patients). HB-EGF staining in urothelium was distributed as: zero (4 patients), 1 (7 patients), 2 (7 patients) and 3 (intense) (7 patients).
For the other urine markers, the only clear association was between urine IL-8 and bladder lamina propria mast cell count (r=0.46, 95% CI (0.20, 0.73), p<0.001) (Figure 5).
Figure 5.
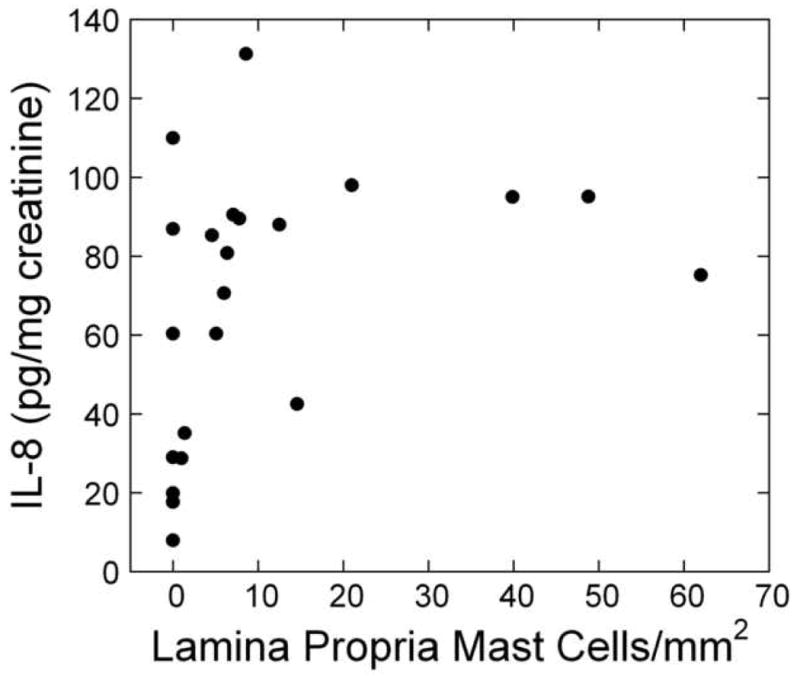
Scatter plot of urine IL-8 levels vs. mast cell count in the lamina propria for chronically treated IC/PBS patients. Mast cells were detected by staining bladder biopsies with anti-tryptase primary antibody followed by a standard streptavidin method. An optical reticle was used to count mast cells in the urothelium, lamina propria and detrusor. (For examples of high and low mast cell counts, see Figure 4.) Lamina propria mast cell count was significantly associated with urine IL-8, which was analyzed by ELISA and normalized to urine creatinine.
Urine markers in ulcer vs. nonulcer patients
The treated patients included eight ulcer patients and 17 nonulcer patients. Urine APF activity was positive in all ulcer patients and 16 nonulcer patient (one patient was borderline). For the other urine markers, ulcer patients had a trend to higher urine HB-EGF, but there were no significant differences between ulcer and nonulcer patients (Table 2).
Table 2. Urine Marker Levels for Ulcer vs Nonulcer Patients.
| Marker** | Nonulcer, untreated | Nonulcer, treated | Ulcer* | ||
|---|---|---|---|---|---|
| Median++ | P value+ | Median++ | P value+ | Median++ | |
| HB-EGF (ng) | 0.37 (0.07, 0.85) | 0.05 | 0.28 (0.16, 0.49) | 0.04 | 1.62 (0.37, 4.0) |
| EGF (ng) | 27 (17, 57) | 0.82 | 27 (23, 39) | 0.25 | 20 (14, 139) |
| IL-6 (pg) | 3.5 (1.9, 6.5) | 0.15 | 3.6 (3.1, 7.1) | 0.34 | 5.5 (3.6, 19.4) |
| IL-8 (pg) | 4.5 (0, 19.4) | 0.40 | 3.0 (0, 7.1) | 0.14 | 9.25 (3.1, 30.4) |
| cGMP (pmole) | 264 (160, 404) | 0.55 | 239 (219, 359) | 0.39 | 363 (172, 710) |
All ulcer patients in this table were previously treated.
Markers are expressed as the unit in parentheses per mg urine creatinine.
Compared to ulcer patients, Wilcoxon-Mann-Whitney test.
Data are given as median (25th percentile, 75th percentile).
Of the untreated patients, only one had ulcer IC/PBS. Therefore, the 46 untreated nonulcer patients were compared with the 8 treated ulcer patients. This comparison also showed a trend for higher urine HB-EGF in the ulcer patients, but no significant differences (Table 2).
Bladder inflammation in nonulcer patients
Although ulcer patients generally have more intense bladder inflammation than nonulcer patients,5 we suspected that a cystoscopically visible ulcer might not be a sensitive indicator of bladder inflammation. Therefore, we selected the inflammatory features that were present in all of the ulcer patients, and tested their frequencies in the nonulcer patients. As shown in Table 3, these inflammatory features were seen in 24-76% of the nonulcer patients.
TABLE 3. Frequencies of Inflammatory Features in Nonulcer IC Patients*.
| Bladder Biopsy Feature | Untreated | Treated |
|---|---|---|
| Severe inflammation (100 mononuclear cells/HPF or lymphoid aggregates) | 24% | 35% |
| Urothelium IL-6 staining grade 2 or 3** | 70% | 76% |
| Lamina propria IL-6 staining grade 2 or 3** | 45% | 41% |
| LCA stain positive in >10% of the lamina propria | 36% | 53% |
All of the ulcer patients had these bladder biopsy features.
Staining intensity was graded from zero (none) to 3 (intense).
Discussion
While the pathophysiologies of IC/PBS are still not completely defined, some objective information can be obtained from cystoscopy and bladder histopathology. However, since an invasive procedure is needed to gain this information, it cannot be easily used for clinical decision-making, especially if repeated evaluation over time is needed. Therefore, we tested whether certain noninvasive urine markers were associated with visible ulcers or with specific biopsy findings (including lamina propria and urothelium).
For both treated and untreated patients, lamina propria mast cell count was significantly associated with urine IL-8. This may have physiologic relevance because mast cells secrete IL-8 when stimulated6 and they also induce urothelial cells to secrete IL-8.7 The association was not robust enough for urine IL-8 to be a surrogate for bladder mast cell count, possibly because mast cell density may vary in different bladder areas, or urine IL-8 might be a marker of bladder mast cell activation rather than mast cell number. Mast cell activation is difficult to quantify on bladder biopsy because degranulated mast cells may be missed on conventional histology (they can be seen by electron microscopy), and also because mast cells can secrete IL-8 and other mediators without degranulating.7 To clarify the role of mast cell activation in IC/PBS, a combination of conventional histology, electron microscopy and urine assay for multiple mast cell mediators will probably be required.
Overall, we found few and weak associations between urine markers and bladder biopsy findings. Several possible explanations can be considered. First, IC/PBS bladders may be heterogeneous, so biopsy findings may differ for different bladder areas in the same patient. Second, some urine markers (e.g. EGF, cGMP) are also secreted and/or excreted by the kidneys, so their urine levels are a combined reflection of bladder and kidney physiology and/or pathophysiology.8,9 Third, the urine markers we selected may not have accurately reflected the bladder biopsy features that we focused on. For example, urine contains numerous cytokines and inflammatory mediators, any one of which might reflect underlying bladder inflammation. IL-6, IL-8 and cGMP were well documented to be altered in IC/PBS at the time the study began, but it is possible that other biomarkers might reflect bladder inflammation more robustly. A recent publication showed that bladder nitric oxide accurately predicted the presence of ulcers (a sign of inflammation) and decreased with clinical response to corticosteroid treatment.10 Unfortunately, nitric oxide measurement requires specialized equipment and is not practical for routine clinical use.
APF has several adverse effects on urothelial cells in vitro, some of which are ameliorated by adding HB-EGF.11-13 Therefore, positive urine APF activity and/or low urine HB-EGF should associate with urothelial abnormalities. It turned out that 70 patients had positive APF activity and 68 patients had extremely low HB-EGF (0-3 ng/mg creatinine), so we expected almost all patients to have urothelial abnormalities. We found some degree of epithelial denudation in all but two patients, and 38% of our patients had over half of the epithelium denuded. However, epithelial denudation may be mechanical from the biopsy or embedding process, and since normal bladders are rarely biopsied, there are no well-established cutpoints for how much epithelial denudation is “abnormal.” Based on prior experience as a genitourinary pathology specialist (JET), complete denudation of epithelium is very unusual in “normal” bladder mucosa from IC/PBS patients. Interestingly, in spite of the low urine HB-EGF levels in our patients, 42% had intense urothelial staining for HB-EGF. Thus, the urothelium in IC/PBS may still synthesize HB-EGF, but not release it into the urine. This finding is compatible with the previous finding that explanted IC/PBS cells do not have less proHB-EGF mRNA than normal control cells even though they produce less HB-EGF in vitro.11
IC/PBS may involve other urothelial abnormalities that we did not include in this study. For example, rather than complete urothelial denudation, specific urothelial layers may be decreased or lost. Also, a recent study described increased E-cadherin staining and decreased ZO-1 staining in IC/PBS bladder biopsies,14 which is of great interest because these same changes occur in vitro if cultured urothelial cells are treated with APF.11,12 Other bladder epithelial abnormalities reported in IC/PBS include abnormal cellular architecture on electron microscopy 15 and abnormal uroplakin expression assessed by reverse-transcriptase polymerase chain reaction.16
To our knowledge, no previous studies have compared urine markers with bladder biopsy findings. Three studies compared urine markers with presence/absence of ulcers seen on cystoscopy. First, histamine levels and mast cell counts in bladder washings were higher for ulcer patients than nonulcer patients.17 Second, bladder nitric oxide was high in ulcer patients and negligible in nonulcer patients.10 Third, urine APF and HB-EGF were similar for ulcer vs. nonulcer patients, but ulcer patients had higher EGF levels (mean 21.90 ± 1.19 ng/ml vs. mean 16.32 ± 1.44 ng/ml, p <0.004).18 Since that study reported EGF concentrations rather than normalizing to creatinine, and reported mean +/- SEM rather than median and percentiles, we reanalyzed our EGF results accordingly. Our EGF concentrations were not increased in ulcer patients (mean 29.2 ± 7.7 ng/ml for ulcer patients, mean 29.4 ± 1.9 ng/ml for nonulcer patients). Our EGF levels were slightly higher than those in the previous study, which may be related to assay differences, or to the fact that all their patients were Chinese, while ours were American and mostly Caucasian, and the effect of race on these urine growth factor levels is not known.
It remains an important goal to properly select IC/PBS patients for treatments based on individual patient features. Currently, the most common stratification is based on presence/absence of visible ulcers on cystoscopy. This is a useful distinction because visible ulcers can be fulgurated.19 Also, two groups of investigators have used corticosteroids to treat ulcer IC/PBS,10,20 with the rationale that ulcers signify underlying bladder inflammation.5 Our study also showed that ulcers signified underlying inflammation. 100% of our ulcer patients had bladder inflammation as evidenced by mononuclear infiltrate, IL-6 staining and LCA staining (caveat: normal bladders are rarely biopsied, so there are no well-established cutpoints at which bladder inflammation is defined as “abnormal.”) Importantly, some of our nonulcer patients also had these biopsy findings. Whether inflammation contributes to denudation, or whether denudation contributes to the development of inflammation, is unknown. However, our results suggest that some nonulcer patients also might respond to anti-inflammatory or immunosuppressive treatment. There are many indicators of bladder inflammation in IC/PBS (ulcers, nitric oxide, urine markers, bladder biopsy findings) and it is unknown which of these are most predictive of treatment response.
This study was limited by the finite number of biopsy features chosen, the finite number of urine markers chosen, and the variability of biopsies at different sites in the bladder. In future research, the first two problems could be improved by including different urine markers and biopsy features, but the variability issue might require extensive bladder mapping to overcome. On the other hand, even if no urine markers are ever found to accurately reflect bladder biopsy findings, they may still predict response to individual treatments, especially for treatments that are directed towards a specific urine component. For example, urine APF may predict response to an APF inhibitor, or urine substance P may predict response to a neurokinin receptor blocker.
Conclusions
Urine IL-8 levels were positively associated with bladder mast cell count, and may indicate bladder mast cell activation in IC/PBS. No other urine markers in this study were strongly associated with bladder biopsy findings or presence of ulcers. Presence of ulcers was not a sensitive indicator of inflammation on bladder biopsy.
Figure 2.
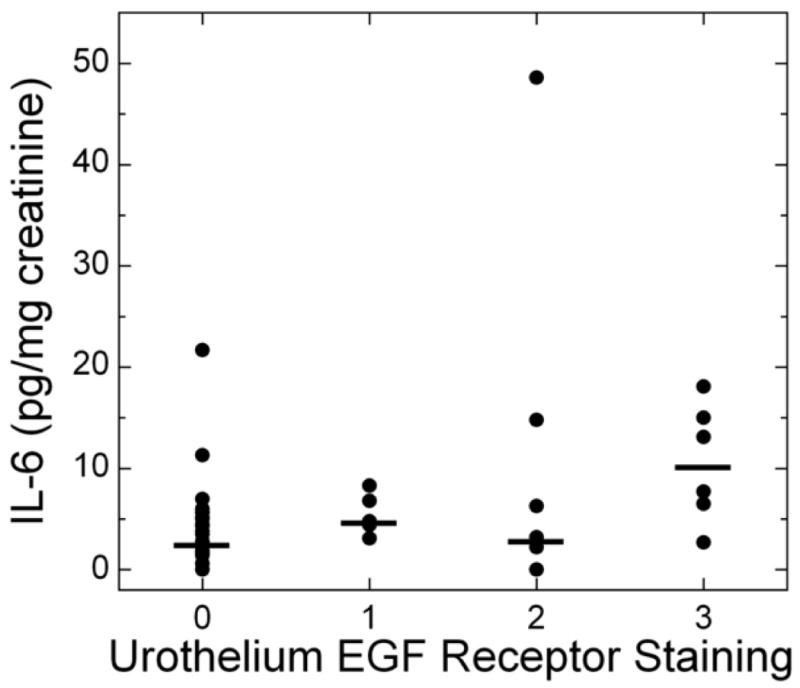
Urine IL-6 levels vs. EGF receptor staining in the urothelium for untreated patients. Bladder biopsies were stained with anti-EGFR primary antibody followed by a standard streptavidin method. EGFR staining was graded semiquantitatively from 0 (none) to 3 (intense). (For examples of strong and weak EGFR staining, see Figure 1). On each biopsy the urothelium and lamina propria were graded separately. Urothelial EGFR staining intensity was significantly associated with urine IL-6 levels in untreated IC/PBS patients. Urine IL-6 was analyzed by ELISA and normalized to urine creatinine. Bars represent median values for IL-6 in each staining category.
Figure 3.
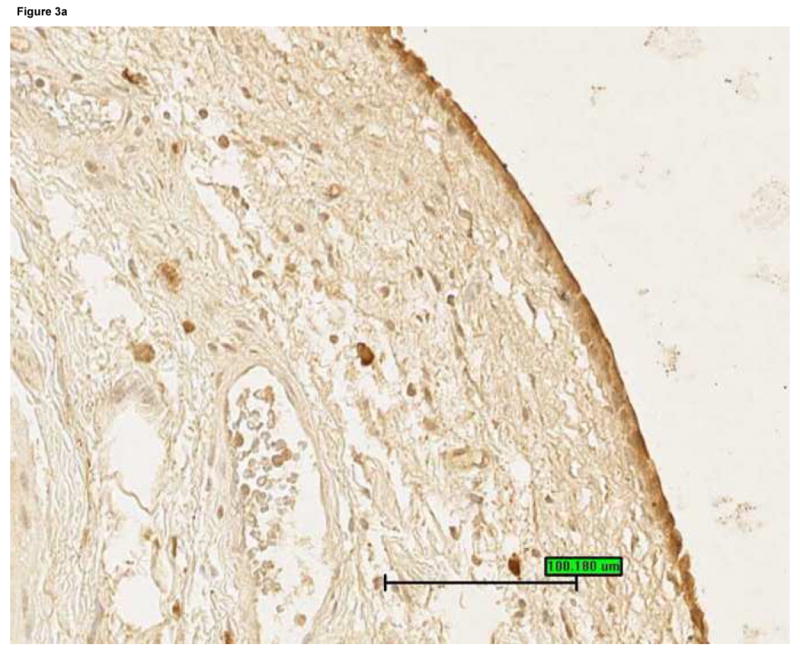
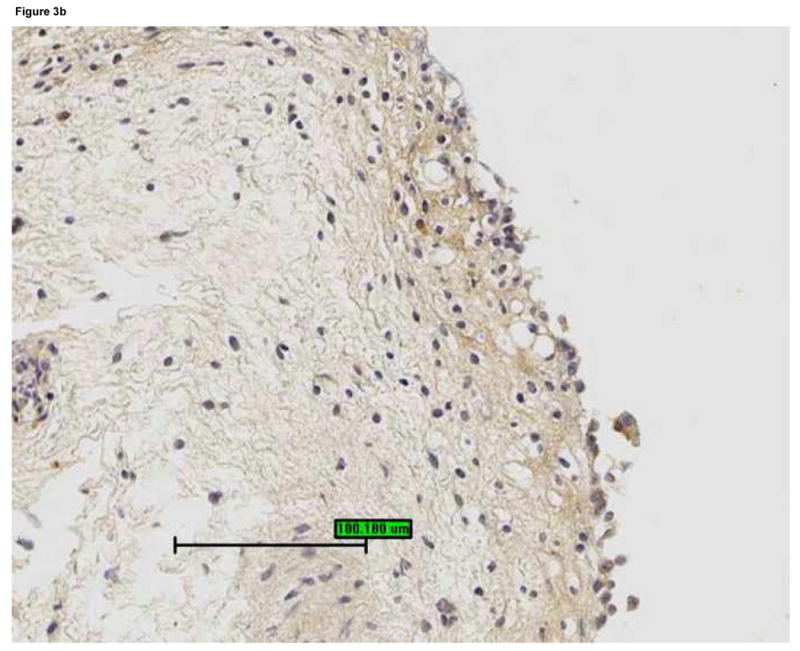
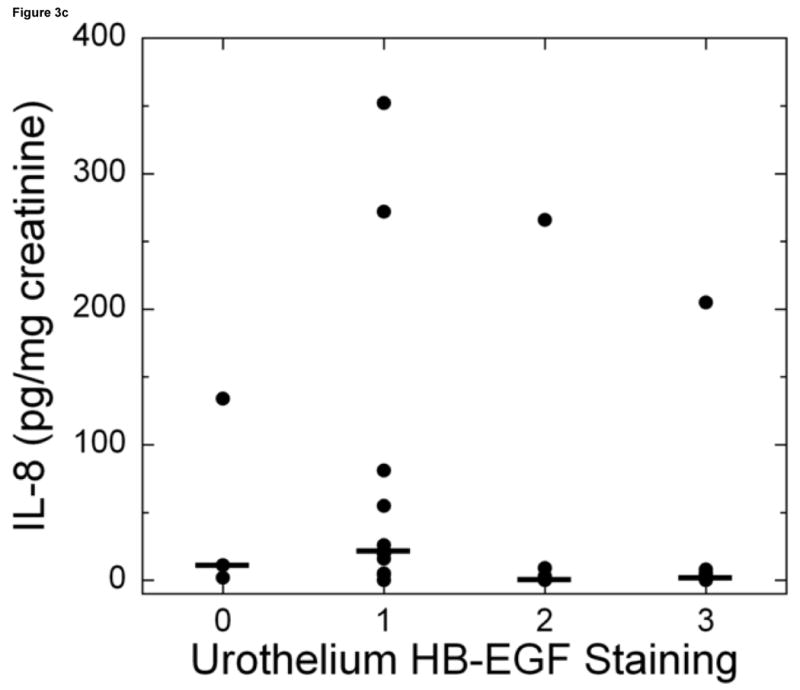
Bladder biopsies were stained with anti-HB-EGF primary antibody followed by a standard streptavidin method. HB-EGF staining was graded semiquantitatively from 0 (none) to 3 (intense). On each biopsy the urothelium and lamina propria were graded separately. a: Grade 3 staining for HB-EGF in the urothelium and lamina propria (magnification 200×). b: Grade 1 staining for HB-EGF in the urothelium and lamina propria (magnification 200×). c: Urothelial HB-EGF staining intensity was significantly associated with urine IL-8 levels in untreated IC/PBS patients. Urine IL-8 was analyzed by ELISA and normalized to urine creatinine. Bars represent median values for IL-8 in each staining category.
Acknowledgments
This study was funded by the National Institute of Diabetes, Digestive and Kidney Diseases, RO1DK57281.
List of Abbreviations
- IC/PBS
Interstitial cystitis
- NIDDK
National Institute of Diabetes, Digestive and Kidney Diseases
- APF
Anti-proliferative factor
- EGF
Epidermal growth factor
- HB-EGF
Heparin-binding EGF-like growth factor
- cGMP
Cyclic guanosine monophosphate (cGMP)
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- LCA
Leukocyte common antigen
- HPF
High power field
- CI
Confidence interval
- SEM
Standard error of the mean
- FDA
United States Food and Drug Administration
- ELISA
Enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erickson DR, Tomaszewski JE, Kunselman AR, Bentley CM, Peters KM, Rovner ES, Demers LM, Wheeler MA, Keay SK. Do the National Institute of Diabetes and Digestive and Kidney Diseases cystoscopic criteria associate with other clinical and objective features of interstitial cystitis? J Urol. 2005;173:93. doi: 10.1097/01.ju.0000146466.71311.ab. [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L the Interstitial Cystitis Database Study Group. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database Study. J Urol. 1999;161:553. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 3.Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, Demers LM, Kushner L, Keay SK. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461. [PubMed] [Google Scholar]
- 4.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM The Interstitial Cystitis Database Study Group. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57(6 Suppl 1):67. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 5.Peeker R, Fall M. Toward a precise definition of interstitial cystitis: further evidence of differences in classic and nonulcer disease. J Urol. 2002;167:2470. [PubMed] [Google Scholar]
- 6.Fischer M, Harvima IT, Carvalho RF, Moller C, Naukkarinen A, Enblad G, et al. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest. 2006;116:2748. doi: 10.1172/JCI24274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batler RA, Sengupta S, Forrestal SG, Schaeffer AJ, Klumpp DJ. Mast cell activation triggers a urothelial inflammatory response mediated by tumor necrosis factor-alpha. J Urol. 2002;168:819. [PubMed] [Google Scholar]
- 8.Lev-Ran A, Hwang DL, Ben-Ezra J, Williams LE. Origin of urinary epidermal growth factor in humans: excretion of endogenous EGF and infused [131I]-human EGF and kidney histochemistry. Clin Exp Pharmacol Physiol. 1992;19:667. doi: 10.1111/j.1440-1681.1992.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 9.Gennari C, Toccafondi R, Rotella CM, Francini G, Brandi ML, Maioli E. Salmon calcitonin and cGMP production by human kidney: studies in vivo and in vitro. Calcif Tissue Int. 1983;35:273. doi: 10.1007/BF02405045. [DOI] [PubMed] [Google Scholar]
- 10.Hosseini A, Ehren I, Wiklund NP. Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol. 2004;172:2261. doi: 10.1097/01.ju.0000144761.69398.be. [DOI] [PubMed] [Google Scholar]
- 11.Keay S, Seillier-Moiseiwitsch F, Zhang CO, Chai TC, Zhang J. Changes in human bladder epithelial cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiol Genomics. 2003;14:107. doi: 10.1152/physiolgenomics.00055.2003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CO, Wang JY, Koch KR, Keay S. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from patients with interstitial cystitis. J Urol. 2005;174:2382. doi: 10.1097/01.ju.0000180417.11976.99. [DOI] [PubMed] [Google Scholar]
- 13.Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol. 2000;164:2112. [PubMed] [Google Scholar]
- 14.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 15.Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology. 1997;49(Suppl 5A):14. doi: 10.1016/s0090-4295(99)80329-x. [DOI] [PubMed] [Google Scholar]
- 16.Zeng Y, Wu XX, Homma Y, Yoshimura N, Iwaki H, Kageyama S, et al. Uroplakin III-delta4 messenger RNA as a promising marker to identify nonulcerative type interstitial cystitis. J Urol. 2007 doi: 10.1016/j.juro.2007.05.125. in press. [DOI] [PubMed] [Google Scholar]
- 17.Enerback L, Fall M, Aldenborg F. Histamine and mucosal mast cells in interstitial cystitis. Agents Actions. 1989;27:113. doi: 10.1007/BF02222214. [DOI] [PubMed] [Google Scholar]
- 18.Zhang CO, Li ZL, Kong CZ. APF, HB-EGF, and EGF biomarkers in patients with ulcerative vs non-ulcerative interstitial cystitis. BMC Urology. 2005;5:7. doi: 10.1186/1471-2490-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rofeim O, Hom D, Freid RM, Moldwin RM. Use of the neodymium: YAG laser for interstitial cystitis: a prospective study. J Urol. 2001;166:134. [PubMed] [Google Scholar]
- 20.Soucy F, Gregoire M. Efficacy of prednisone for severe refractory ulcerative interstitial cystitis. J Urol. 2005;173:841. doi: 10.1097/01.ju.0000153612.14639.19. [DOI] [PubMed] [Google Scholar]


