Abstract
Background & Aims
The impact of childhood Crohn’s disease (CD) on volumetric bone mineral density (vBMD), bone structure, and muscle mass have not been established. The objective of this longitudinal study was to assess musculoskeletal outcomes in an incident cohort of children with CD using peripheral quantitative computed tomography (pQCT).
Methods
Tibia pQCT was performed in 78 CD subjects (ages, 5–18 years) at diagnosis and in 67 over the subsequent year. pQCT outcomes were converted to sex- and race-specific z scores based on reference data in over 650 controls. Multivariable linear regression models identified factors associated with changes in bone outcomes.
Results
At diagnosis, CD subjects had significant deficits in trabecular vBMD (z score, −1.32 ± 1.32; P < .001), cortical section modulus (a measure of bone geometry and strength) (z score, −0.44 ± 1.11; P < .01), and muscle (z score, −0.96 ± 1.02; P < .001) compared with controls. Over the first 6 months, trabecular vBMD and muscle z scores improved significantly (both, P < .001); however, section modulus worsened (P = .0001), and all 3 parameters remained low after 1 year. Increases in muscle z scores were associated with less severe declines in cortical section modulus z scores. Improvements in trabecular vBMD z scores were greater in prepubertal subjects. Glucocorticoids were associated with increases in cortical vBMD.
Conclusions
Substantial deficits in trabecular vBMD, cortical bone geometry, and muscle were observed at CD diagnosis. Trabecular vBMD improved incompletely; however, cortical deficits progressed despite improvements in muscle. Glucocorticoids were not associated with bone loss. Therapies to improve bone accrual in childhood CD are needed.
Children with Crohn’s disease (CD) have multiple risk factors for impaired bone accrual, including poor growth, delayed maturation, malnutrition, decreased physical activity, chronic inflammation, and glucocorticoid (GC) therapy. The impact of these threats to bone health may be immediate, resulting in fragility fractures during childhood,1 or delayed because of sub-optimal bone accrual. Multiple investigators have documented decreased bone mass in children2–4 and adults5,6 with CD as well as significantly increased fracture rates in adults.7,8 However, the structural basis for decreased bone strength in CD has not been established.
Inflammatory cytokines and GCs have adverse effects on bone metabolism. Tumor necrosis factor α and inter-leukin-6 impair bone formation through inhibition of osteoblast differentiation and function and promotion of osteoblast apoptosis.9,10 These cytokines also accelerate bone resorption by osteoclasts.11 GCs have similar adverse effects on osteoblasts and osteoclasts.12 GCs may also impair osteoclast adherence and function,13 potentially resulting in a state of low bone turnover. The growing skeleton may be particularly vulnerable to the detrimental effects of chronic inflammation and GCs on bone formation.
We previously used dual energy x-ray absorptiometry (DXA) to examine bone outcomes in children with CD.3,4 These studies demonstrated significant deficits in whole body bone mineral content and femoral shaft dimensions. Bone deficits were strongly associated with muscle deficits, consistent with the observation that the growing skeleton is especially sensitive to biomechanical loading. 14 However, DXA is a 2-dimensional projection technique that does not provide discrete information about trabecular and cortical volumetric bone mineral density (vBMD) or geometry; therefore, the impact of CD on these characteristics of bone strength has not been established. Furthermore, these cross-sectional studies in prevalent, heterogeneous samples did not allow us to distinguish between the effects of CD and its therapies on bone.
Peripheral quantitative computed tomography (pQCT) provides 3-dimensional estimates of cortical and trabecular vBMD and geometry in the appendicular skeleton that are highly correlated with fracture load.15 PQCT also provides measures of muscle and fat cross-sectional area (CSA). We established an incident cohort of children and adolescents at the time of CD diagnosis to determine the effects of CD on pQCT measures of tibia bone vBMD, structure, and strength prior to GC therapy to determine the relations between bone and muscle deficits and to identify determinants of changes in these outcomes over the first year following diagnosis.
Patients and Methods
Study Subjects
Incident CD subjects, ages 5 to 21 years, were eligible for enrollment and were recruited from the Inflammatory Bowel Diseases Center of the Children’s Hospital of Philadelphia and gastroenterologists in the community. Baseline study visits were completed within 2 weeks of diagnosis, and follow-up visits were completed 6 and 12 months later. CD diagnosis was confirmed by radiographic, endoscopic, histologic, and clinical parameters. CD subjects known to have other chronic illnesses or medications potentially affecting growth and nutrition were excluded. Subjects were enrolled between February 2002 and January 2005. The baseline DXA measures of whole body lean mass and fat mass were recently described in this cohort,16 demonstrating significant muscle deficits in males and females and greater fat deficits in females. A total of 98 eligible subjects were identified, and 78 (80%) enrolled. The demographic characteristics (age, sex, and race) did not differ between eligible subjects who declined participation and those who enrolled.
A cross-sectional sample of healthy control subjects of similar age was recruited from general pediatric clinics in the greater Philadelphia area and through newspaper advertisements. Control subjects were excluded for height or body mass index (BMI [kg/m2]) below the third percentile for age and sex or for a history of illnesses or medications that may affect growth or nutritional status. Scanning protocols in the controls were modified over the study interval. Therefore, pQCT measures of muscle and fat CSA, trabecular bone, and cortical bone were available for comparison with the CD subjects in 865, 652, and 676 controls, respectively. The distributions of age, sex, race, pubertal maturation, and anthropometrics did not differ across these subsets of controls.
The study protocol was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. Informed consent was obtained from young adult subjects and the parents or guardians of subjects under the age of 18 years. Assent was obtained from subjects between 7 and 18 years of age.
CD Characteristics and Definitions
CD activity was assessed using the Pediatric Crohn’s Disease Activity Index (PCDAI) based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%).17 PCDAI scores range from 0 to 100, and disease activity was categorized as none (0–10), mild (11–30), and moderate to severe (>30). Upper tract disease was defined as disease proximal to the colon, including the terminal ileum. Medical charts were reviewed, and participants were interviewed for date of diagnosis, prior therapies, symptom duration prior to diagnosis, extraintestinal manifestations, and current medications. Participants were provided with GC diaries at the baseline visit; the diaries were reviewed at 6 and 12 months and compared with the medical records. Intravenous methylprednisolone doses were converted to prednisone equivalents, and the mean GC dose in each subject (mg/kg/day) was tabulated over each 6-month interval.
Anthropometry and Pubertal Development
Weight and height were measured using a digital scale to the nearest 0.1 kg (Scaltronix, White Plains, NY) and a stadiometer to the nearest 0.1 cm (Holtain Ltd, Croswell, Crymych, United Kingdom), respectively. Pubertal status was assessed with a validated self-assessment questionnaire18 and classified according the method of Tanner.19
pQCT of the Tibia
Bone and muscle measures in the left tibia were obtained by pQCT using a Stratec XCT2000 device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/seconds. Scans were analyzed with Stratec software version 5.50. A scout view was obtained to place the reference line at the proximal border of the distal tibia growth plate, and measurements were obtained at 3% and 38% of tibia length proximal to the reference line. Trabecular vBMD (TrabBMD; mg/cm3) was measured at the 3% (metaphysis) site. Cortical vBMD (CortBMD; mg/cm3), periosteal circumference (millimeters), endosteal circumference (millimeters), and polar section modulus (Zp; mm3) were measured at the 38% (diaphysis) site. The Zp is a function of the outer (periosteal) and inner (endosteal) dimensions of the cortical shell and provides an estimate of bone strength in bending and torsion.15 Muscle and fat CSA (mm2) were evaluated at the 66% site. The manufacturer’s (Orthometrix) hydroxyapatite phantom was scanned daily for quality assurance. The coefficients of variation ranged from 0.5% to 1.6% for pQCT outcomes in children and adolescents.
Laboratory Studies
Serum hematocrit (mg/dL), erythrocyte sedimentation rate (mm/h), and albumin (g/dL) assays were performed in the CD subjects at each visit using standard techniques in the Clinical Laboratories at the Children’s Hospital of Philadelphia.
Statistical Analysis
Analyses were conducted using STATA 9.2 (Stata Corporation, College Station, TX). A 2-tailed P value of < .05 was the criterion for statistical significance. Differences in means were assessed using Student t test for normally distributed variables or Wilcoxon rank-sum test for nonnormally distributed variables. Correlations between continuous variables were assessed by Pearson product moment correlations or Spearman rank correlations, where appropriate. Differences in proportions were assessed using the χ2 test.
Age- and sex-specific z scores for height and BMI were calculated using National Center for Health Statistics data.20 The pQCT outcomes were converted to sex- and race (black vs all others)-specific z scores using the LMS method in the control subjects.21 The LMS method accounts for the nonlinearity, heteroscedasticity, and skew of bone data in growing children. The pQCT density outcomes (TrabBMD and CortBMD) were assessed relative to age. The remaining pQCT outcomes (endosteal and periosteal circumference, Zp, and muscle and fat CSA) were highly correlated with tibia length (all, P < .0001); therefore, the z scores for these parameters were generated relative to tibia length.
Delayed puberty and muscle deficits may contribute to the bone deficits in children and adolescents with CD compared with controls. Therefore, the effects of Tanner stage and muscle z scores were examined in multivariable models for the baseline outcomes. Tanner stage of pubertal maturation was categorized as prepuberty to early puberty (Tanner stages 1 and 2) vs middle to late puberty (Tanner stages 3–5).
The changes in musculoskeletal parameters over the first year were not linear, with greater changes observed during the first 6 months after diagnosis. Therefore, changes over each of the two 6-month intervals were examined separately using paired t tests and multivariable regression. Given the large number of bone outcomes, the longitudinal regression analyses assessed 3 primary outcomes: TrabBMD, CortBMD, and Zp (the summary measure of cortical geometry and strength). The regression models examined the impact of baseline bone deficits, baseline PCDAI, changes in PCDAI, pubertal maturation, baseline muscle z scores, changes in muscle z score, and medications on the change in bone outcomes over each interval. The mean GC exposure (mg/kg/day) over each interval was categorized as none vs each tertile of GC exposure.
Results
Subject Characteristics
Table 1 summarizes subject characteristics in CD and controls. The predominance of whites and males among the CD subjects was consistent with the demographic distribution of the disease.22 Subjects with CD had delayed puberty (assessed as age relative to Tanner stage and sex, P < .001) and significantly lower height and BMI z scores at disease diagnosis compared with controls.
Table 1.
Characteristics of Subjects With Incident Crohn’s Disease and Healthy Controls
| Variable | Crohn’s disease | Controls | P value |
|---|---|---|---|
| N | 78 | 902 | |
| Age, y | 12.7 ± 2.8 | 11.8 ± 3.9 | .04 |
| Range | 5.5 – 18.0 | 5.0 – 22.0 | |
| Sex, % male | 56 | 48 | .1 |
| Race, % black | 10 | 44 | <.001 |
| Prepubertal to early pubertal, % Tanner 1–2 |
45 | 54 | .3 |
| Height z score | −0.26 ± 1.1 | 0.27 ± 0.9 | <.001 |
| Range | −3.2 to 2.2 | −2.6 to 3.3 | |
| BMI z score | −0.55 ± 1.1 | 0.36 ± 1.0 | <.001 |
| Range | −2.7 to 2.2 | −2.4 to 3.0 |
Baseline Disease Characteristics
Disease characteristics, laboratory values, and therapies at baseline have been reported previously16 and are summarized in Table 2. The majority of subjects had moderate to severe CD activity, growth failure, anemia, and extraintestinal manifestations at diagnosis.
Table 2.
Crohn’s Disease Characteristics at the Baseline Visit
| Variable | Value at baseline |
|---|---|
| Age at diagnosis, y, mean ± SD | 12.7 ± 2.8 |
| Duration of symptoms prior to diagnosis, mo, median (range) |
7.1 (0.5 – 52.6) |
| Family history of Crohn’s disease, n (%) | 22 (30) |
| History of growth failure, n (%) | 62 (84) |
| PCDAI, mean ± SD | 38.4 ± 18.0 |
| No active disease (≤10), n (%) | 3 (4) |
| Mild (11 – 30), n (%) | 28 (38) |
| Moderate to severe (>30), n (%) | 43 (58) |
| Symptoms, n (%) | |
| Diarrhea | 49 (63) |
| Hematochezia | 36 (46) |
| Abdominal pain | 66 (85) |
| None | 12 (16) |
| Mild/brief | 27 (36) |
| Moderate/severe | 36 (48) |
| Extraintestinal manifestations, n (%) | 55 (71) |
| Fever | 35 (45) |
| Joint symptoms | 29 (37) |
| Oral ulcers | 25 (32) |
| Erythema nodosum | 2 (3) |
| Albumin, g/dL, median (range) | 3.5 (1.8 – 4.7) |
| ESR, mm/h, median (range) | 21 (0 – 128) |
| Anemia, n (%) | 60 (77) |
| Site of disease, n (%) | |
| Upper GI tract disease | 66 (88) |
| Isolated upper GI tract disease | 5 (7) |
| Colonic disease | 71 (91) |
| Isolated colonic disease | 7 (9) |
| Perianal involvement | 28 (36) |
| Crohn’s disease related hospitalizations prior to diagnosis, n (%) |
27 (35) |
| History of surgery, n (%) (13 procedures total) | 9 (12) |
PCDAI, Pediatric CD Activity Index; ESR, erythrocyte sedimentation rate; GI, gastrointestinal.
Baseline Bone and Body Composition Deficits
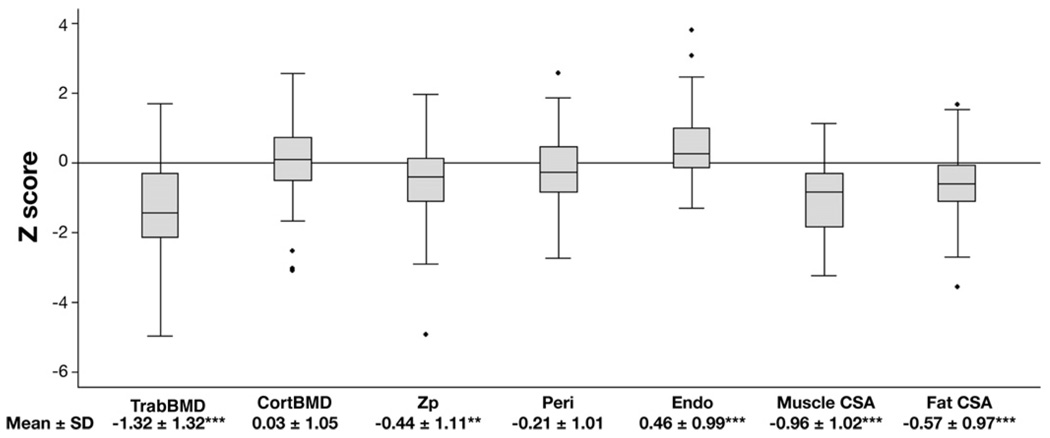
Significant musculoskeletal deficits were observed at the time of diagnosis (Figure 1). TrabBMD was markedly decreased; however, CortBMD did not differ between CD and controls. Rather, cortical deficits were characterized by significant increases in endosteal dimensions (bone loss on the inner cortical surface) and marginal reductions in periosteal dimensions (P = .08) with a consequent significant reduction in Zp z scores compared with controls. The magnitude of these deficits was not associated with the age or height z score at diagnosis. Lower BMI z scores at diagnosis were significantly associated with greater deficits in TrabBMD and cortical geometry z scores (all, P ≤ .01). Lower muscle CSA z scores were significantly associated with lower TrabBMD z scores (R, 0.38; P < .01) and were highly correlated with deficits in periosteal circumference and Zp z scores (R, 0.61– 0.66; P < .0001). Neither BMI z scores nor muscle CSA z scores were associated with CortBMD z scores. Fat CSA z scores were significantly lower in females compared with males (−0.93 ± 0.90 vs −0.30 ± 0.94, respectively, P < .01), consistent with our prior DXA study of body composition in these subjects.16
Figure 1.
PQCT bone and body composition z scores in 78 subjects with Crohn’s disease at diagnosis. The box plots represent the z score distributions. The mean ± SD results are provided below each plot. Peri, periosteal circumference z score; Endo, endosteal circumference z score. *P < .05, **P < .01, ***P < .001.
The z scores for cortical geometry and muscle CSA relative to tibia length were subsequently adjusted for age because CD subjects, on average, were older than controls of comparable tibia length (P < .0001). These adjustments had a nominal effect on group differences, although the periosteal circumference z score point estimate became significantly lower compared with controls (−0.24; 95% confidence interval [CI], −0.48 to −0.004; P < .05). Further adjustment for pubertal maturation did not affect results.
To determine the relations between bone and muscle deficits, the bone outcomes in Figure 1 were adjusted for age, pubertal maturation, and muscle CSA z scores. These adjustments attenuated the CD deficits in TrabBMD z scores to −1.04 (95% CI, −1.29 to −0.80; P < .001) and increased the estimate for endosteal circumference z score to 0.62 (95% CI, 0.38 − 0.66; P < .001). Adjusted periosteal circumference z scores (0.38; 95% CI, 0.19 – 0.57; P < .001) and Zp z scores (0.19; 95% CI, 0.01– 0.38; P < .05) were significantly greater in CD compared with controls.
Associations Between Baseline Musculoskeletal Parameters and CD Characteristics
The correlation between greater PCDAI score and lower TrabBMD z score did not reach significance (R, −0.21; P = .07). However, components of the PCDAI score, serum albumin (R, 0.38; P < .001), and hematocrit (R, 0.23; P < .05) were associated with lower TrabBMD z scores. TrabBMD z scores were lower in subjects with vs without upper tract disease (P = .02). Zp z scores were correlated with serum albumin (R, 0.29; P = .001) and hematocrit (R, 0.27; P < .02). Symptom duration prior to diagnosis was not associated with any of the outcomes.
Disease Activity and Therapies During the First Year After Diagnosis
Of the 78 CD subjects enrolled at baseline, 74 and 67 completed the 6- and 12-month visits, respectively. Sex, race, baseline PCDAI, growth, and pQCT z scores were not significantly different between subjects who did and did not complete all visits. Disease activity, as measured by the PCDAI score, improved significantly. The median PCDAI decreased from 36 at baseline to 13 at 6 months (P < .0001) and to 7 at 12 months (P < .01).
CD therapies over the 1-year interval are summarized in Table 3. The average GC dose among the subjects treated with GC in the first interval was 0.20 mg/kg/day (range, 0.02–0.96) and was 0.18 mg/kg/day (range, 0.05–0.41) in the second interval. Baseline anthropometric and pQCT z scores did not differ in the 46 subjects who were subsequently treated with GCs over the first 6 months compared with the 28 who were not; however, baseline PCDAI scores were significantly greater in those treated with GCs (P = .001).
Table 3.
Glucocorticoid, Immunomodulator, and Biologic Use Over Each 6-Month Interval
| Baseline to 6 months (n = 74) |
6 to 12 months (n = 67) |
|
|---|---|---|
| n (%) | n (%) | |
| Systemic glucocorticoids | 46 (62) | 21 (31) |
| 6-Mercaptopurine | 29 (39) | 33 (49) |
| Azathioprine | 4 (5) | 5 (7) |
| Methotrexate | 4 (5) | 6 (9) |
| Infliximab | 6 (8) | 9 (13) |
Changes in Bone and Muscle Parameters During the First Year After Diagnosis
The z scores for anthropometry and pQCT measures at baseline and 6 and 12 months are summarized in Table 4. The results for each outcome are limited to the subjects with available data at all 3 visits to allow comparisons across visits.
Table 4.
Baseline, 6-Month, and 12-Month Bone and Body Composition Z Scores in CD
| Z score | Baseline | 6 months | 0–6 month P value | 12 months | 6–12 month P value |
|---|---|---|---|---|---|
| TrabBMD (n = 59) | −1.49 ± 1.30 | −1.11 ± 1.26 | .0001 | −1.07 ± 1.26 | .7 |
| (−4.95 to 1.71) | (−4.43 to 1.29) | (−4.52 to 2.05) | |||
| CortBMD (n = 58) | 0.15 ± 1.09 | 0.42 ± 0.98 | .003 | 0.07 ± 1.07 | .0001 |
| (−3.09 to 2.55) | (−2.52 to 2.40) | (−2.56 to 1.90) | |||
| Periosteal (n = 58) | −0.25 ± 1.04 | −0.43 ± 0.93 | <.0001 | −0.43 ± 0.91 | .97 |
| (−2.73 to 2.57) | (−2.88 to 1.78) | (−2.32 to 1.98) | |||
| Endosteal (n = 58) | 0.47 ± 1.04 | 0.49 ± 1.06 | .98 | 0.40 ± 1.06 | .001 |
| (−1.29 to 3.80) | (−1.31 to 3.84) | (−1.44 to 3.75) | |||
| Zp (n = 58) | −0.50 ± 1.14 | −0.71 ± 1.07 | .0001 | −0.66 ± 1.01 | .3 |
| (−4.92 to 1.97) | (−4.89 to 1.70) | (−3.90 to 1.72) | |||
| Muscle CSA (n = 58) | −0.99 ± 1.01 | −0.70 ± 1.08 | <.001 | −0.65 ± 1.08 | .4 |
| (−3.22 to 1.09) | (−2.90 to 2.26) | (−3.12 to 1.86) | |||
| Fat CSA (N = 57) | −0.60 ± 0.81 | −0.27 ± 0.82 | <.0001 | −0.15 ± 0.86 | .07 |
| (−2.70 to 1.35) | (−2.48 to 2.12) | (−2.33 to 2.18) | |||
| Height (n = 63) | −0.34 ± 1.04 | −0.40 ± 1.03 | .02 | −0.35 ± 1.00 | .07 |
| (−3.18 to 1.81) | (−3.02 to 1.66) | (−2.82, 1.79) | |||
| BMI (n = 63) | −0.66 ± 1.02 | −0.15 ± 0.92 | <.0001 | −0.10 ± 0.89 | .4 |
| (−2.75 to 2.18) | (−1.79 to 2.22) | (−2.31 to 1.95) |
NOTE. Data for each z score are limited to subjects who completed the measure at all 3 time points. All values are means ± SD and ranges.
During the first 6-month interval, BMI and fat and muscle z scores improved significantly. Whereas Trab-BMD z scores improved significantly, cortical geometry worsened. Increases in TrabBMD z scores were significantly correlated with improvements in height z scores (R, 0.31; P < .0001). The significant decline in periosteal circumference z scores did not represent a reduction in absolute circumference in any subject. Rather, there was a failure to expand the periosteal circumference relative to the increase in tibia length. The reductions in periosteal circumference z scores resulted in significant reductions in Zp z scores. The Zp z score deficit was attenuated but remained significant (β, −0.23; 95% CI, −0.42 to −0.04; P < .05) after adjustment for muscle CSA z scores in CD compared with controls. CortBMD, which was normal at baseline, increased significantly over the first 6 months.
Over the second 6-month interval, fat CSA z scores continued to increase. TrabBMD z scores did not improve further, and the only significant change in cortical geometry was a significant reduction (improvement) in the endosteal circumference z score. CortBMD z scores declined to normal during the second 6 months.
At the end of the first year following diagnoses, CD subjects had significantly lower TrabBMD, Zp, and muscle z scores compared with controls. Fat CSA z scores were the only deficit observed at baseline that resolved by 12 months.
Determinants of Change in Bone and Body Composition
Multivariable analyses investigated the changes in vBMD (TrabBMD and CortBMD) and the summary measure of cortical geometry (Zp) over the first 6-month interval (Table 5), when the greatest changes were observed. Lower baseline z scores for each of these parameters were associated with significantly greater increases in z scores over the 6 months. Therefore, all models for the changes in z scores over the 6 months were adjusted for the baseline value. Prepubertal and early pubertal subjects demonstrated significantly greater increases in TrabBMD and CortBMD z scores compared with more mature subjects. Neither baseline PCDAI nor change in PCDAI was independently associated with changes in these 3 outcomes; these results did not differ when GC exposure was excluded from the models. Of note, improvements in PCDAI were marginally (P = .08) associated with improvements in TrabBMD z scores in the full model. Interval increases in muscle CSA z scores were associated with significantly smaller declines in Zp z scores. Secondary analyses demonstrated that changes in muscle CSA z scores were positively associated with changes in periosteal circumference z scores (P < .01) but not endosteal circumference z scores. GC doses in the highest tertile were independently associated with increases in CortBMD z scores from baseline to 6 months. Of note, the decline in CortBMD z scores between 6 and 12 months was significantly greater in the subjects who did not receive GC during this interval compared with those who did (change, −0.50 ± 0.56 vs −0.03 ± 0.64, respectively, P < .01). None of the other therapies described in Table 3 were independently associated with changes in bone outcomes.
Table 5.
Multivariable Linear Regression Models of the Changes in Z Scores From Baseline to 6 Months
| ΔTrabBMD β, 95% CI |
ΔCortBMD β, 95% CI |
ΔZp β, 95% CI |
|
|---|---|---|---|
| Baseline z score | −0.16 (−0.31 to −0.01)a | −0.36 (−0.50 to −0.21)b | −0.21 (−0.30 to −0.11)b |
| Tanner stages 1 and 2 vs stages 3–5 | 0.40 (0.03 – 0.77)a | 0.46 (0.15 – 0.76)c | −0.03 (−0.19 to 0.13) |
| PCDAI | |||
| Baseline | −0.01 (−0.03 to 0.01) | 0.01 (−0.00 to 0.03) | 0.00 (−0.01 to 0.01) |
| Change | −0.02 (−0.03 to 0.00) | 0.01 (−0.01 to 0.02) | 0.00 (−0.01 to 0.01) |
| Muscle CSA z score | |||
| Baseline | 0.27 (0.06 – 0.49)c | 0.09 (−0.08 to 0.26) | 0.09 (−0.02 to 0.20) |
| Change | 0.16 (−0.20 to 0.52) | 0.05 (−0.24 to 0.35) | 0.20 (0.04 – 0.36)c |
| Glucocorticoids (mg/kg/day tertiles vs 0) | |||
| First tertile | −0.04 (−0.58 to 0.49) | 0.12 (−0.31 to 0.56) | −0.07 (−0.31 to 0.16) |
| Second tertile | 0.07 (−0.42 to 0.34) | 0.26 (−0.14 to 0.65) | −0.04 (−0.25 to 0.18) |
| Third tertile | −0.20 (−0.74 to 0.34) | 0.47 (0.03 – 0.90)a | 0.00 (−0.23 to 0.23) |
| Adjusted R2 | 0.25 | 0.44 | 0.41 |
Δ, Changes.
P ≤ .05.
P ≤ .001.
P ≤ .01.
Discussion
This study demonstrates significant musculoskeletal deficits in children with CD at diagnosis, with largely persistent deficits over the following year of treatment. The current study advances previous studies in 3 major ways. First, the use of pQCT provides 3-dimensional measures of trabecular and cortical vBMD, as well as measures of cortical dimensions and strength. To our knowledge, this is the first study to use QCT to assess bone outcomes in children or adults with inflammatory bowel disease. DXA is a 2-dimensional projection technique that cannot distinguish between superimposed cortical and trabecular bone and systematically underestimates vBMD in children with reduced height for age.23 Second, the use of an incident cohort allows one to establish the independent musculoskeletal effects of CD prior to initiation of therapy. Three prior studies of children and adolescents with newly diagnosed CD reported decreased DXA measures of lumbar spine or whole body areal BMD24–26; however, these studies were limited by small numbers of controls (less than 50) and did not address the impact of body composition. Third, the robust control population described here constitutes the largest reference population used in the assessment of CD in children or adults, allowing adjustment for age, sex, race, bone length, pubertal maturation, and body composition.
The baseline data illustrate the substantial musculo-skeletal deficits attributable to the underlying inflammation and malnutrition in CD. The mean TrabBMD z score of −1.32 indicates that the average CD subject had a TrabBMD at the 10th percentile for age, sex, and race. These deficits are consistent with cytokine-mediated reductions in bone formation and potential increases in bone resorption. The decreased cortical bone strength (Zp) at the time of diagnosis was a result of significant reductions in the outer (periosteal) dimension coupled with significant expansion of the inner (endosteal) dimensions of the cortical shell in the diaphysis, relative to age and tibia length. This pattern likely represents the combined effects of decreased biomechanical loading by muscle forces and increased inflammatory cytokines. Whereas other studies have reported trabecular bone loss and cortical thinning in patients with disparate inflammatory diseases, those studies were complicated by concurrent GC therapies in subjects of widely varying disease duration.27,28 Finally, the substantial muscle deficits are consistent with prior reports of inflammatory cachexia in children with CD.29
As muscles increase during growth, bones adapt by increasing dimensions and strength. This capacity of bone to respond to mechanical loading with increased bone strength is greatest during childhood.30 Numerous studies have demonstrated that physical activity during childhood promotes cortical bone acquisition, either because of greater periosteal expansion and/or greater endosteal contraction.31,32 These reports are consistent with our finding in this incident CD cohort that greater increases in muscle mass significantly and independently attenuated decreases in Zp and periosteal circumference z scores following diagnosis.
Given the strong associations between muscle mass and cortical bone strength, investigators have advocated a 2-staged algorithm to assess (1) muscle mass relative to body size and (2) bone outcomes relative to muscle mass.14 This “functional muscle-bone unit” approach is intended to distinguish between primary bone disorders (muscle mass is normal and bone mass is low relative to muscle), as opposed to bone disorders that are secondary to muscle deficits (muscle mass is reduced but bone mass is “adequate” for the reduced muscle mass). Whereas bone and muscle deficits are highly correlated, this does not prove a causal relationship. This close association may be mediated by nutritional, hormonal, or inflammatory factors that directly influence both muscle and bone.33
In the CD cohort described here, baseline Zp and periosteal circumference z scores adjusted for age were significantly lower in CD compared with controls. However, when these baseline bone outcomes were adjusted for the low muscle CSA z scores, they were significantly higher in CD compared with controls. Next, mean Zp z scores decreased over the subsequent 6 months while muscle CSA z scores increased, and greater gains in muscle were significantly associated with lesser declines in Zp. At the 6-month visit, Zp z scores adjusted for muscle CSA z scores were significantly lower in CD compared with controls. There are multiple potential explanations for this apparent overcompensation of bone at baseline and undercompensation at 6 months. First, the baseline muscle deficits may have progressed over a short interval prior to diagnosis, such that the bone loss secondary to decreased biomechanical loading lagged behind the muscle loss observed at baseline. Lag time between changes in muscle and bone adaptation to the changes in muscle loading might also explain why Zp z scores decreased over the first 6 months in the setting of increasing muscle CSA z scores. Second, the direct effects of cytokines, malnutrition, and CD therapies on muscle and bone may differ. For example, the progression of Zp deficits, despite improvements in muscle CSA, may have been due to direct GC effects to suppress bone formation, potentially impairing bone acquisition on the periosteal surface. Third, cytokines and glucocorticoids may directly decrease osteocyte function and survival, thereby impairing the bone cells that promote bone formation in response to mechanical strain.34 Finally, muscle CSA may not fully capture muscle forces in the setting of altered muscle composition or metabolism because of CD and its therapies. Measures of muscle force are needed to fully characterize the relations between bone and muscle in CD and controls. These observations illustrate the limitations of the functional muscle bone unit paradigm in chronic inflammatory disease.
TrabBMD z scores improved significantly over the study interval, despite the use of GC therapy in the majority of subjects. Whereas prior studies suggested that GCs were associated with greater bone deficits in CD, disease severity may have confounded the relationship between GC exposure and bone status. Prepubertal and early pubertal subjects demonstrated greater recovery of TrabBMD, compared with middle to late pubertal subjects. This may represent a window of opportunity to improve TrabBMD in these subjects.
Finally, CortBMD z scores were normal at baseline and increased over the first 6 months of therapy. GC exposure was associated with increases in CortBMD z scores over the first 6 months, and absence of GC therapy was associated with subsequent declines in CortBMD in the second 6 months. We recently reported that CortBMD was significantly greater in children and adolescents with juvenile idiopathic arthritis compared with controls.35 We hypothesized that GCs resulted in reduced bone turnover, resulting in reduced intracortical porosity, greater secondary mineralization, and higher CortBMD. Importantly, high CortBMD may not improve bone quality. Thinner, denser cortical bones may be more brittle and susceptible to damage accumulation.36
Our study was limited by the absence of bone biopsy histomorphometry. The impact of CD and its therapies on bone remodeling cannot be definitively addressed in the absence of dynamic measures of bone formation and resorption. However, this study provides important insight into the effects of CD and its therapies on bone vBMD and structure. An additional limitation is the variability in patterns of GC exposure over the study interval. The summary measure of mg/kg/day may not fully capture differences in intermittent GC exposure.
In summary, this study demonstrated that CD is associated with substantial deficits in TrabBMD, cortical dimensions, and muscle mass at diagnosis. Despite improvements in TrabBMD and muscle mass over the study interval, substantial deficits persisted after 1 year. The failure to fully recover TrabBMD and the sustained deficits in cortical dimensions, despite improvements in muscle CSA, suggest that interventions to improve bone acquisition are indicated. Future studies are needed to determine the associations between these bone deficits and fracture risk and to determine the effects of CD and its therapies on dynamic measures of bone remodeling.
Acknowledgments
The authors thank the children and their families who participated in this study for their dedication and enthusiasm and the physicians and nurses in the Division of Gastroenterology at the Children’s Hospital of Philadelphia.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors disclose the following: Supported by grants R01-DK060030 and K24-DK076808 as well as grant number UL1-RR-024134 from the National Center for Research Resources.
Abbreviations used in this paper
- BMD
bone mineral density
- CD
Crohn’s disease
- CortBMD
cortical volumetric bone mineral density
- CSA
cross-sectional area
- DXA
dual energy x-ray absorptiometry
- GC
glucocorticoid
- PCDAI
Pediatric Crohn’s Disease Activity Index
- pQCT
peripheral quantitative computed tomography
- TrabBMD
trabecular volumetric bone mineral densit
- vBMD
volumetric bone mineral density
- Zp
polar section modulus
References
- 1.Semeao EJ, Stallings VA, Peck SN, et al. Vertebral compression fractures in pediatric patients with Crohn’s disease. Gastroenterology. 1997;112:1710–1713. doi: 10.1016/s0016-5085(97)70055-6. [DOI] [PubMed] [Google Scholar]
- 2.Gokhale R, Favus MJ, Karrison T, et al. Bone mineral density assessment in children with inflammatory bowel disease. Gastroenterology. 1998;114:902–911. doi: 10.1016/s0016-5085(98)70309-9. [DOI] [PubMed] [Google Scholar]
- 3.Burnham JM, Shults J, Petit MA, et al. Alterations in proximal femur geometry in children treated with glucocorticoids for Crohn’s disease or nephrotic syndrome: impact of the underlying disease. J Bone Miner Res. 2007;22:551–559. doi: 10.1359/jbmr.070110. [DOI] [PubMed] [Google Scholar]
- 4.Burnham JM, Shults J, Semeao E, et al. Whole body BMC in pediatric Crohn’s disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19:1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff SC, Herrmann A, Goke M, et al. Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1157–1163. [PubMed] [Google Scholar]
- 6.Pollak RD, Karmeli F, Eliakim R, et al. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol. 1998;93:1483–1490. doi: 10.1111/j.1572-0241.1998.468_q.x. [DOI] [PubMed] [Google Scholar]
- 7.van Staa TP, Cooper C, Brusse LS, et al. Inflammatory bowel disease and the risk of fracture. Gastroenterology. 2003;125:1591–1597. doi: 10.1053/j.gastro.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med. 2000;133:795–799. doi: 10.7326/0003-4819-133-10-200011210-00012. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 10.Sylvester FA, Wyzga N, Hyams JS, et al. Effect of Crohn’s disease on bone metabolism in vitro: a role for interleukin-6. J Bone Miner Res. 2002;17:695–702. doi: 10.1359/jbmr.2002.17.4.695. [DOI] [PubMed] [Google Scholar]
- 11.Kudo O, Fujikawa Y, Itonaga I, et al. Proinflammatory cytokine (TNFα/IL-1α) induction of human osteoclast formation. J Pathol. 2002;198:220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 12.Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Zhao H, Kitaura H, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006;116:2152–2160. doi: 10.1172/JCI28084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoenau E, Neu CM, Beck B, et al. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17:1095–1101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Manske SL, Kontulainen SA, et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18:991–997. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 16.Thayu M, Shults J, Burnham JM, et al. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn’s disease. Inflamm Bowel Dis. 2007;13:1121–1128. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 18.Schall JI, Semeao EJ, Stallings VA, et al. Self-assessment of sexual maturity status in children with Crohn’s disease. J Pediatr. 2002;141:223–229. doi: 10.1067/mpd.2002.125907. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM. Growth at adolescence. Oxford, UK: Blackwell Scientific Publication; 1962. [Google Scholar]
- 20.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 22.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 23.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 24.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 25.Harpavat M, Greenspan SL, O’Brien C, et al. Altered bone mass in children at diagnosis of Crohn’s disease: a pilot study. J Pediatr Gastroenterol Nutr. 2005;40:295–300. doi: 10.1097/01.mpg.0000153278.98861.32. [DOI] [PubMed] [Google Scholar]
- 26.Gupta A, Paski S, Issenman R, et al. Lumbar spine bone mineral density at diagnosis and during follow-up in children with IBD. J Clin Densitom. 2004;7:290–295. doi: 10.1385/jcd:7:3:290. [DOI] [PubMed] [Google Scholar]
- 27.Lian KC, Lang TF, Keyak JH, et al. Differences in hip quantitative computed tomography (QCT) measurements of bone mineral density and bone strength between glucocorticoid-treated and glucocorticoid-naive postmenopausal women. Osteoporos Int. 2005;16:642–650. doi: 10.1007/s00198-004-1736-9. [DOI] [PubMed] [Google Scholar]
- 28.Roth J, Palm C, Scheunemann I, et al. Musculoskeletal abnormalities of the forearm in patients with juvenile idiopathic arthritis relate mainly to bone geometry. Arthritis Rheum. 2004;50:1277–1285. doi: 10.1002/art.20128. [DOI] [PubMed] [Google Scholar]
- 29.Burnham JM, Shults J, Semeao E, et al. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413–420. doi: 10.1093/ajcn.82.2.413. [DOI] [PubMed] [Google Scholar]
- 30.Kannus P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123:27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Petit MA, McKay HA, MacKelvie KJ, et al. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 32.Bass SL, Saxon L, Daly RM, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17:2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 33.Roubenoff R, Roubenoff RA, Cannon JG, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–2386. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonewald LF. Osteocyte biology: its implications for osteoporosis. J Musculoskelet Neuronal Interact. 2004;4:101–104. [PubMed] [Google Scholar]
- 35.Burnham JM, Shults J, Dubner S, et al. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58:518–527. doi: 10.1002/art.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tommasini SM, Nasser P, Schaffler MB, et al. Relationship between bone morphology and bone quality in male tibias: implications for stress fracture risk. J Bone Miner Res. 2005;20:1372–1380. doi: 10.1359/JBMR.050326. [DOI] [PubMed] [Google Scholar]