Abstract
The field of therapeutic cancer vaccines is currently in a state of active preclinical and clinical investigation, and certain novel therapies involving tumor immunotherapy have recently come to the forefront of prostate cancer research. While no therapeutic cancer vaccine has yet been approved by the US FDA, recent findings have demonstrated that new paradigms of combination therapies involving vaccines, employed in clinical trials with appropriate design and end points, may ultimately lead to cancer vaccines being used to treat various malignancies. Several characteristics of prostate cancer make it an ideal target for immunotherapy. Its relative indolence allows sufficient time to generate immune responses, which usually take weeks or months to mount. In addition, prostate cancer-associated antigens direct the immune response to prostate cancer cells, thus sparing normal tissue. This review focuses on the future of promising new vaccines and novel perspectives in the treatment of prostate cancer.
Keywords: clinical trials, dendritic cells, immunology, poxviral vector, prostate cancer, vaccines
Therapeutic cancer vaccines have been extensively investigated in preclinical and clinical settings. Although no therapeutic cancer vaccine has yet been approved by the US FDA, several new milestones in vaccine development have been reached in the areas of tumor-associated antigens (TAAs) as vaccine targets, novel vaccine delivery systems, costimulation, and combination therapy strategies [1].
Prostate cancer, the second leading cause of cancer death among men in the USA [2], is an attractive model for cancer vaccine research. It is generally considered a slow-growing tumor, which may allow adequate time for a vaccine to activate the immune system [3]. Indeed, the median overall survival in patients with rising prostate-specific antigen (PSA) after surgery is 14 years [4]. Furthermore, prostate cancer has many well-described TAAs. In general, TAAs are ideal targets for immunotherapy because they are specific to the cancer and are either not expressed or are minimally expressed in normal tissue and essential organs. The majority of prostate cancers, as well as epithelial cells lining the acini and ducts of the prostate gland, express PSA [5]. Other TAAs include prostatic acid phosphatase (PAP) and prostate-specific membrane antigen. These TAAs are potential targets for prostate cancer immunotherapy. Since the prostate is not an essential organ, targeting PSAs does not generally cause significant morbidity and is not associated with significant systemic side effects [6]. In addition, aside from hormonal manipulations, few systemic therapies have been proven beneficial for patients with prostate cancer. A further advantage of prostate cancer as a model for immunotherapy is the use of PSA for early detection of recurrent disease, which allows vaccine immunotherapy to be initiated while tumor burden is still minimal [7].
Immune enhancement
Since cancer can develop and progress in the presence of an intact immune system, TAAs are by definition weakly immunogenic. Cancer vaccines are designed to break immune tolerance. Strong antigen presentation by antigen-presenting cells (APCs) is required to sufficiently activate cytotoxic T cells (CTLs). Dendritic cells (DCs) are the most potent APCs and are key elements in T-cell activation. APCs internalize the antigen, process it, and express it on their surface as a peptide bound to major histocompatibility complex (MHC). T cells only recognize the TAA peptide–MHC complex on the surface of APCs; they cannot recognize the antigen as a protein. Thus, any antigen made by the tumor (and not just a membrane-bound protein) can be used as a target for a T-cell-mediated attack.
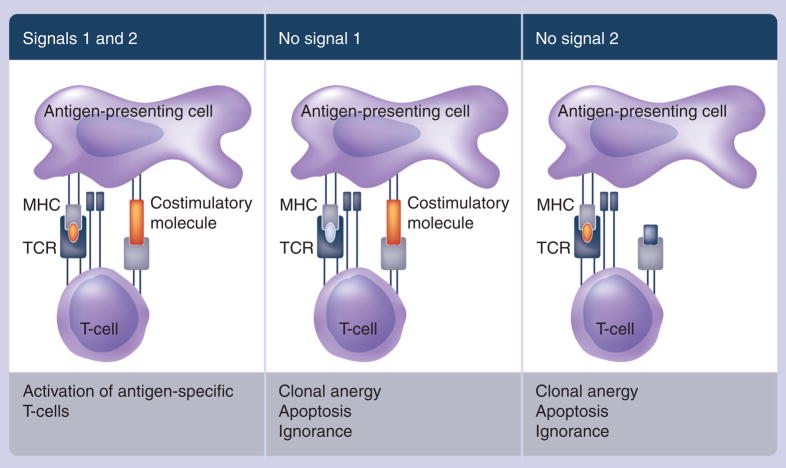
Activating APCs that are able to properly process and present the antigen is pivotal to activating the adaptive immune system. T cells are activated in a dual-signal process. The first signal binds the peptide–MHC complex on the surface of the APC to the T-cell receptor (TCR) on the surface of the T cell. The second signal involves the interaction of T-cell costimulatory molecules on the surface of the APC with their corresponding ligands on the surface of the T cell, which leads to T-cell proliferation (Figure 1). A weak first or second signal can produce suboptimal T-cell activation and an ineffective immune response [8]. Several T-cell costimulatory molecules have been identified, including B7.1, lymphocyte function-associated antigen (LFA)-3 and intercellular-adhesion molecule (ICAM)-1 [8]. Genes from these costimulatory molecules have been engineered into vaccine vectors such as poxviruses, resulting in improved antigen-specific T-cell responses [9,10].
Figure 1. Two-signal model of T-cell dependence on costimulation.
The second (costimulatory) signal is required for T-cell cytokine production and proliferation.
MHC: Major histocompatibility complex; TCR: T-cell receptor.
Reprinted with permission from [66].
Strategies to enhance DC and CTL activation and function have employed various cytokines. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is frequently used as an adjuvant to vaccine therapy [11]. GM-CSF injected at the vaccination site has been shown to induce the growth and maturation of DCs and to increase their infiltration to regional lymph nodes [12,13], while enhancing antigen-specific T-cell responses and delayed-type hypersensitivity reactions [11]. Transduction of GM-CSF genes into whole tumor cells has shown similar effects. Another cytokine, IL-2, is known to cause activation and proliferation of T cells. It has activity as a single agent in melanoma and renal cell carcinoma and as an adjuvant to peptide vaccines for melanoma [14,15]. Metronomic-dose IL-2 as an adjuvant to vaccine therapy has produced immune responses similar to standard-dose IL-2, with a much better safety profile [16]. Other cytokines with anti-tumor and immunogenic activity, such as IL-7, IL-12 and IL-15, are currently under investigation [17–19].
Bacillus Calmette–Guerin (BCG) and pox-viral vaccines, originally designed for infectious diseases, have also been shown to generate strong inflammatory and immunostimulatory responses. They activate DCs through Toll-like receptors, which enhance production of inflammatory chemokines and engage immune-effector cells [20–22]. Cancer vaccine strategies using these approaches are currently being employed in clinical trials in patients with prostate cancer.
Autologous whole-tumor-cell vaccines
Autologous whole-tumor-cell vaccines are derived from the patient’s own tumor cells in a time-consuming process that requires a significant amount of tumor tissue. The advantage of autologous whole-tumor-cell vaccines is that they target the patient’s own TAAs and preclude the need for antigen preselection. However, the use of autologous tumor cells may do little to stimulate an immune response that has already failed against antigens expressed by the tumor. Furthermore, the TAAs could be diluted by other, normal cellular components. In a Phase I clinical study, eight immunocompetent prostate cancer patients were treated with autologous, GM-CSF-secreting, irradiated tumor vaccines prepared from ex vivo retroviral transduction of surgically harvested cells. Expansion of primary cultures of autologous vaccine cells successfully met trial specifications in eight out of 11 cases; however, yields of the primary cell culture limited the number of courses of vaccination [23]. Other disadvantages include the lack of standardization measures and the difficulty of evaluating antigen-specific immune responses.
Allogeneic whole-tumor-cell vaccines
Allogeneic whole-tumor-cell vaccines are derived from various tumor cell lines and are easier to prepare. Whole tumor cells are rendered replication-defective by radiation and are frequently combined with nonspecific immunostimulants [1,24,25]. Two allogeneic whole-tumor-cell vaccines – GVAX and ONY-P1 (described below) – are currently being investigated in clinical trials.
GVAX
GVAX (Cell Genesys, Inc.) consists of two prostate cancer cell lines, LNCaP and PC-3, transfected with a human GM-CSF gene. Phase I and II studies were performed, demonstrating both safety and clinical activity of this vaccine [11,25]. Based on these results, Cell Genesys launched two Phase III trials of GVAX. VITAL-1 was a multicenter, randomized, controlled Phase III trial designed to compare GVAX to the standard of care (docetaxel plus prednisone) in asymptomatic metastatic prostate cancer patients. The primary end point of VITAL-1 was overall survival. VITAL-2 was a two-arm, randomized Phase III study of GVAX in symptomatic metastatic prostate cancer patients. One arm combined GVAX with docetaxel and the other arm employed docetaxel and prednisone. VITAL-2 opened in May 2005 and was halted on August 27, 2008 following increased deaths in the GVAX combination arm versus the control arm (67 vs 47, respectively) [101]. On October 16, 2008 Cell Genesys announced the termination of VITAL-1. The trial was fully enrolled in 2007 with 626 patients, but was terminated based on the results of a previously unplanned futility analysis conducted by the study’s Independent Data Monitoring Committee (IDMC), which indicated that the trial had a less than 30% chance of meeting its predefined primary end point of improved overall survival [102].
ONY-P1
ONY-P1 (Onyvax, Ltd.) consists of three irradiated prostate cancer cell lines (LNCaP, P4E6, and OnyCap-23). In a Phase II study, 26 patients with nonmetastatic castration-resistant prostate cancer (CRPC) received ONY-P1 intradermally, once a month for up to 12 months. The first two injections were given biweekly in combination with BCG [26]. In total, 11 of the 26 patients receiving ONY-P1 demonstrated a decrease in PSA doubling time. A randomized, double-blind, placebo-controlled Phase II trial of ONY-P1, designed to evaluate whether the vaccine can prolong time to metastatic disease, has recently completed accrual in patients with nonmetastatic CRPC. Another randomized, double-blind, placebo-controlled Phase II study in patients with biochemical failure following local therapy is currently ongoing at the National Cancer Institute (NCI) in Bethesda, MD, USA.
Antigen-presenting cell vaccines
Dendritic cells play a crucial role in the activation of naive CD4 and CD8 T cells as well as NK cells, and thus bridge the innate to adaptive immune response. Researchers in cancer immunotherapy have focused a tremendous amount of interest on DC physiology, activation, maturation and antigen presentation. In an effort to enhance their activity, DCs have been loaded with peptides, proteins and tumor lysates, infected with viral vectors, TAAs and mRNAs, or fused with tumor cells [27,28]. However, the disadvantage of autologous DC-based vaccines is that their production is costly and labor-intensive. Large amounts of peripheral-blood mono-nuclear cells must be cultured in the presence of several cytokines before being engineered and reintroduced into patients.
Sipuleucel-T (Provenge®; Dendreon, Inc.) is an autologous APC vaccine pulsed ex vivo with PA2024, a recombinant fusion protein of human PAP and GM-CSF. Two Phase II trials of sipuleucel-T in patients with CRPC showed a greater than 25% decline in PSA in 30 and 15% of patients, respectively. Immune responses correlated with improved time to progression [29,30]. Subsequently, two Phase III placebo-controlled studies were conducted in patients with meta-static CRPC, with a primary end point of time to progression. Both studies allowed for crossover upon progression. In the first trial there was a trend toward improved time-to-progression in patients receiving sipuleucel-T (11.7 vs 10.0 weeks; p = 0.052). Interestingly, the trial demonstrated an overall survival benefit favoring the treatment arm (median survival: 25.9 vs 21.4 months; p = 0.01). Additional overall survival benefit was observed on longer follow-up. A total of 34% of patients in the sipuleucel-T arm and 11% in the placebo arm were alive at 36 months (p = 0.005). In the placebo arm, 34 patients (75.5%) crossed over to the sipuleucel-T arm. However, 36 months was not prespecified as the primary efficacy end point and results should therefore be interpreted cautiously. Based on the trial’s failure to meet its primary end point, the FDA declined to approve sipuleucel-T and called for further study. A Phase III trial with a primary end point of overall survival has completed accrual of 512 patients [31]. On October 6, 2008, Dendreon announced interim data from their Provenge IMPACT study. Although the company still remains blinded to the data, the IDMC reported to Dendreon a 20% reduction in the risk of death in the Provenge arm relative to placebo (hazard ratio: 0.80; 95% CI: 0.610–1.051). The IDMC observed no safety concerns and recommended that the study continue to its final analysis [104], anticipated in mid-2009.
Vector-based vaccines
Vectors such as viruses, bacteria or yeasts are a convenient vehicle for vaccine delivery. The advantages of using vectors are that:
Some have large genomes that allow for insertion of multiple genes for TAAs, costimulatory molecules and cytokines;
Some vectors cause an inflammatory response at the injection site, instigating migration of APCs to the site;
Many vectors can infect the APCs, allowing for better antigen processing;
Compared with DC-based and autologous whole-tumor cell vaccines, vector-based vaccines are easy and inexpensive to produce.
The major disadvantage of some vectors is that host-induced antibodies can neutralize the vector and limit its efficacy with repeated use.
Poxviruses have been extensively studied as potential vaccine vectors. Vaccinia virus, used as a vector in many vaccines, induces strong immunostimulation at the injection site. Its large genome can integrate many transducible genes and it has an excellent safety profile. Vaccinia has been administered to more than a billion people since the WHO’s 1967 launch of the Global Smallpox Eradication Program [32]. There was an initial concern that vaccinia-immune individuals would not be able to mount as strong an immune response as vaccinia-naive individuals. Further investigation demonstrated that with higher doses of recombinant vaccinia viruses, even vaccinia-immune patients can generate a potent immune response. However, it has been demonstrated that after one or two injections of vaccinia, immune response declines owing to the effect of neutralizing antibodies [33]. This has led to the development of a diversified prime-and-boost strategy, whereby the immune system is primed by replication-competent vaccinia virus, then boosted with a replication-defective avipox virus such as fowlpox, which is not associated with significant neutralizing antibodies [34–36]. Replication-defective vectors have the advantage of superior safety, since they cannot replicate in the human body. They can, however, infect human cells and express their encoded transgenes for 2–3 weeks before cell death.
Poxviruses have been employed as vehicles for prostate cancer vaccines in various combinations. In a randomized Phase II study in non-metastatic CRPC, 42 patients were randomized to second-line hormonal therapy with nilutamide (an androgen-receptor antagonist) versus vaccine. The priming vaccine consisted of recombinant vaccinia (rV)-PSA and rV-B7.1, followed by monthly boosts of recombinant fowlpox (rF)-PSA. Patients on both arms were permitted to add the treatment of the other arm at PSA progression, if their disease had not metastasized. Time-to-treatment failure was similar in both arms (9.9 vs 7.6 months) [37]. The study demonstrated an additional survival benefit in the 12 patients who received vaccine first followed by nilutamide, compared with those who received nilutamide first followed by vaccine (median overall survival: 6.2 vs 3.7 years; p = 0.045) [37,38].
PSA-TRICOM is a poxvirus-based vaccine expressing PSA and three costimulatory molecules (B7.1, ICAM-1, and LFA-3). A Phase II study in patients with metastatic CRPC evaluated 32 patients treated with PSA-TRICOM on a prime-and-boost schedule. In total, 38% of patients experienced a PSA decline from baseline; the median overall survival for all patients was 26.6 months, which exceeded the Halabi predicted survival of less than 18 months. For patients with more favorable prognostic factors (Halabi median predicted overall survival of 20.9 months), median overall survival has not been reached at 44.6 months [39].
Results from a multicenter, randomized, placebo-controlled Phase II study of 125 patients with advanced prostate cancer demonstrated that PSA-TRICOM extended median overall survival by 8.5 months (p = 0.015) and had a favorable safety and tolerability profile [105]. Based on these promising results, a confirmatory overall survival Phase III study is planned.
Combination therapies
Studies suggest synergistic effects with the combination of immunotherapy and conventional treatments for prostate cancer such as radiation, androgen-deprivation therapy (ADT), and certain chemotherapies.
Radiation plus vaccine
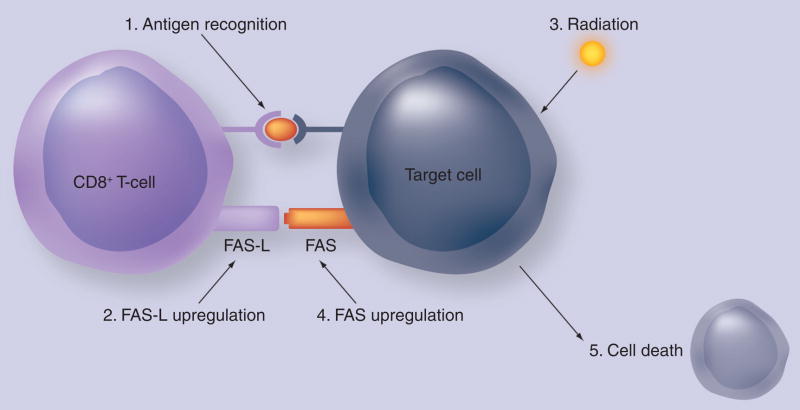
Radiation can alter tumor-cell phenotype and upregulate the expression of some TAAs, costimulatory molecules and cytokine receptors (Figure 2). MHC class I and Fas are among the genes that can be upregulated by radiation [40–43]. A randomized Phase II trial evaluated immune responses in patients undergoing definitive radiotherapy for prostate cancer. A total of 30 patients were randomized to receive radiation alone or in combination with a poxviral-PSA vaccine. In total, 13 out of 17 patients in the combination arm who received all eight scheduled vaccinations had a threefold or greater increase in PSA-specific T cells, compared with no increase in the radiotherapy-only arm (p < 0.0005) [44]. Indeed, in patients on vaccine for 3 months prior to radiation therapy, there was evidence of immune-mediated tumor killing, with the formation of de novo immune responses to prostate-associated antigen not found in the vaccine.
Figure 2. Local radiation of tumor cells, at doses insufficient to kill a tumor, can modulate numerous classes of genes (e.g., Fas).
This, in turn, may render these tumor cells more susceptible to killing by cytotoxic T lymphocytes.
Reprinted with permission from [67].
ADT plus vaccine
Androgen-deprivation therapy has been shown to potentiate immune responses and mitigate immune tolerance to prostate-cancer antigens [45,46]. Preclinical studies have shown that castration in aged male mice can cause regeneration of the thymus, with normalization of the thymic microenvironment and MHC class II expression in thymocytes increased to young-adult levels by 4 weeks postcastration [47]. In one clinical study, T-cell infiltration of the prostate was observed after 1 to 3 weeks of ADT. These T cells were predominantly CD4+ and demonstrated an oligoclonal pattern of TCR restriction [48]. Another study demonstrated an increase in tumor-associated autoantibody responses in patients receiving neoadjuvant ADT for prostate cancer [49]. A study in patients with androgen-dependent prostate cancer and rising PSA after prostatectomy randomized patients to sipuleucel-T or placebo following 3 months of ADT. There was a trend toward prolonged time-to-biochemical failure favoring the treatment arm [50]. An ongoing randomized study at the NCI is examining whether there is a clinical benefit to combining antiandrogen therapy (flutamide) with vaccine (PSA-TRICOM) compared with antiandrogen therapy alone. Another ongoing study at the NCI randomized prostate-cancer patients with rising PSA after definitive therapy to receive ONY-P1 or placebo following 3 months of ADT.
Chemotherapy plus vaccine
There are increasing data suggesting that the long-held belief that chemotherapy will blunt the ability of a vaccine to activate an immune response may not be completely accurate. Certain chemotherapy agents have been shown to positively modulate the immune response through different mechanisms such as upregulation of TAAs and MHC class I, depletion of regulatory T cells (Tregs), and increased cytokine production [51–53]. A randomized Phase II study of patients with metastatic CRPC compared a poxviral-PSA vaccine with or without weekly docetaxel and demonstrated no difference in PSA-specific T-cell response [54]. However, it should be noted that in patients heavily pretreated with chemotherapy, the immune system could be impaired, so that combining chemotherapy with vaccine in these patients may not produce a potent immune response. Moreover, careful selection of the chemotherapy agent and close attention to vaccine/chemotherapy scheduling and dosing are critical considerations.
Monoclonal antibodies plus vaccine
Monoclonal antibodies have been combined with vaccines for the treatment of various tumor types. In prostate cancer, a human cytotoxic T-lymphocyte antigen-4 (CTLA-4) mono-clonal antibody has been tested in combination with vaccines. CTLA-4 is a T-cell surface glycoprotein that is upregulated following T-cell activation to inhibit the immune response. Its main function is to prevent autoimmunity by regulating the body’s immune activity. T cells express two counteracting receptors on their cell surface – CD28 and CTLA-4. Both bind to the same ligands or costimulatory molecules on the surface of APCs (B7.1 and B7.2, also known as CD80 and CD86). Binding of these costimulatory molecules to CD28 activates T cells, while interacting with CTLA-4 inhibits T-cell stimulation. Blocking CTLA-4 with a neutralizing antibody has been shown to sustain and potentiate immune responses [55–58]. A Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, and GVAX in 16 patients with metastatic prostate cancer suggested a correlation between immune-related adverse events and immune response [59]. Another Phase I trial, which recently closed to accrual, combined ipilimumab and PSA-TRICOM vaccine in meta-static prostate cancer patients. An interim analysis demonstrated a significant increase in PSA doubling time (from 2.6 months pre-study to 11.4 months post-study; p = 0.01) in 11 patients at a dose of 10 mg/kg [106].
Conclusion
Vaccine strategies for the treatment of prostate cancer are under active investigation, using a wide variety of approaches including whole-tumor-cell vaccines, DC approaches and vector-driven delivery of TAAs. Preclinical and clinical results of studies employing these various vaccine approaches have demonstrated immunologic responses that, in some instances, correlate with clinical activity of these vaccines. Larger clinical studies are needed to validate these findings.
Novel vaccine strategies are also being implemented, including the addition of costimulatory molecules to help activate and proliferate T-cell responses, and combination therapies with cytokines, antibodies, hormones and chemotherapy that may help reduce regulatory Tregs and initiate an antigen cascade that could allow additional tumor antigens to be targeted by the immune system.
Future perspective
In the treatment of cancer, it is becoming more evident that traditional response criteria, which focus strictly on reduction of tumor size, may not be the best criteria for determining the efficacy of vaccine therapy. Tumors treated with vaccines may grow initially before shrinking, or may stabilize or grow at a slower rate. Therefore, overall survival may be a better end point than disease-free survival for evaluating vaccines in clinical trials. Furthermore, clinical and preclinical data suggest that vaccines are more effective in patients with small tumor volume and less aggressive disease [31,60]. There are several possible reasons for this:
Activation of an immune response outpaces tumor growth;
In a large tumor, T-cell infiltration and penetrance is incomplete;
Immunosuppression in patients heavily pre-treated with cytotoxic chemotherapy and radiation impedes optimal immune stimulation;
Immunosuppressive cytokines produced by tumor cells and/or suppressive cells in the microenvironment of a large tumor further hinder immune response.
Based on these considerations, vaccine therapies may lead to better outcomes in adjuvant settings than in metastatic settings, while tumor burden is still relatively low. As previously discussed, sipuleucel-T demonstrated an overall survival advantage without a significant benefit in time-to-progression [31].
Better understanding of APCs and the mechanisms of T-cell activation have led to the development of a new generation of vaccines that incorporate costimulatory molecules. One finding on the physiology of adoptive cell-mediated immune response is that T-cell quality and avidity are more important than quantity. Use of costimulatory molecules and cytokine adjuvants can increase the function and cytolytic activity of T cells [52,61].
Another advancement in our understanding of the mechanism of immune response regulation is the identification of Tregs. A subtype of CD4+ T cells – Tregs – are responsible for down-regulating host immune activation, thereby reducing the risk of autoimmunity. Tregs may play an important role in immune tolerance and anergy to TAAs; higher levels of Tregs correlate with negative clinical outcomes [62,63]. Types of regulatory cells include CD4+CD25highFoxP3+ Tregs, immature macrophages and CD4+NKT+ cells [64,65]. Ongoing research is focusing on ways to inhibit these cells and thus increase the efficacy of tumor vaccines.
Executive summary.
Immune enhancement
Tumor-associated antigens (TAAs) are, by definition, weakly immunogenic.
Strong antigen presentation by antigen-presenting cells (APCs) is required to sufficiently activate cytotoxic T cells (CTLs).
T cells are activated in a dual-signal process: the first signal binds the peptide–MHC complex on the surface of the APC to the T-cell receptor (TCR); the second signal involves the interaction of T-cell costimulatory molecules on the surface of the APC with their corresponding ligands on the surface of the T cell.
Strategies to enhance dendritic cell (DC) and CTL activation and function have employed various cytokines including granulocyte- macrophage colony-stimulating factor (GM-CSF), IL-2, IL-7 and IL-15.
Prostate cancer vaccines
Allogeneic whole-tumor-cell vaccines:
Allogeneic whole-tumor-cell vaccines are derived from various tumor cell lines and are easier to prepare.
Whole tumor cells are rendered replication-defective by radiation, frequently combined with nonspecific immunostimulants, GVAX and ONY-P1 – investigated in clinical trials.
APC vaccines:
DCs play a crucial role in the activation of naive CD4 and CD8 T cells.
DCs have been loaded with peptides, proteins and tumor lysates, infected with viral vectors, TAAs and mRNAs, or fused with tumor cells.
Sipuleucel-T (Provenge; Dendreon, Inc.) – autologous APC vaccine pulsed ex vivo with PA2024, a recombinant fusion protein of human prostatic acid phosphatase (PAP) and GM-CSF. The final Phase III analysis is scheduled in mid 2009.
Vector-based vaccines:
Vector-based vaccines allow for insertion of multiple genes for TAAs and costimulatory molecules and cytokines.
Some vectors cause an inflammatory response at the injection site, instigating migration of APCs to the site.
Many vectors can infect the APCs, allowing for better antigen processing.
PSA-TRICOM, a poxvirus-based vaccine expressing PSA and three costimulatory molecules (B7.1, ICAM-1 and LFA-3), has shown promising Phase II results.
Combination therapies
Radiation plus vaccine:
Radiation can alter tumor-cell phenotype and upregulate the expression of some TAAs, costimulatory molecules and cytokine receptors.
Androgen-deprivation therapy (ADT) plus vaccine:
ADT has been shown to potentiate immune responses and mitigate immune tolerance to prostate cancer antigens.
Chemotherapy plus vaccine:
Certain chemotherapy agents have been shown to positively modulate the immune response.
Mechanisms include upregulation of TAAs and MHC class I, depletion of regulatory T cells (Tregs), and increased cytokine production.
Monoclonal antibodies plus vaccine:
Blocking CTLA-4 with a neutralizing antibody has been shown to sustain and potentiate immune responses.
Acknowledgments
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Philip M Arlen, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, Bethesda, MD, 20892, USA.
Mahsa Mohebtash, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, Bethesda, MD, 20892, USA.
Ravi A Madan, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, Bethesda, MD, 20892, USA.
James L Gulley, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, 10 Center, Drive, Building 10, Room 8B09, MSC, 1750, Bethesda, MD 20892-1750, USA, Tel.: +1 301 435 2956, Fax: +1 301 480 5094, gulleyj@mail.nih.gov.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Coffey DS, Isaacs JT. Prostate tumor biology and cell kinetics – theory. Urology. 1981;17:40–53. [PubMed] [Google Scholar]
- 4.Freedland S, Humphreys E, Mangold L, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 5.Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907–923. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427–4433. [PubMed] [Google Scholar]
- 7.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Wingren AG, Parra E, Varga M, et al. T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit Rev Immunol. 1995;15:235–253. doi: 10.1615/critrevimmunol.v15.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 9.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 10.Hodge JW, Greiner JW, Tsang KY, et al. Costimulatory molecules as adjuvants for immunotherapy. Front Biosci. 2006;11:788–803. doi: 10.2741/1837. [DOI] [PubMed] [Google Scholar]
- 11.Disis ML, Bernhard H, Shiota FM, et al. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 12.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor – secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 13.Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–214. [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley M, Rosenberg S. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 18.Fry TJ, Moniuszko M, Creekmore S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 19.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 20.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 21.Prud’homme GJ. DNA vaccination against tumors. J Gene Med. 2005;7:3–17. doi: 10.1002/jgm.669. [DOI] [PubMed] [Google Scholar]
- 22.Wooldridge JE, Weiner GJ. CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response. Curr Opin Oncol. 2003;15:440–445. doi: 10.1097/00001622-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 24.Tani K, Azuma M, Nakazaki Y, et al. Phase I study of autologous tumor vaccines transduced with the GM-CSF gene in four patients with stage IV renal cell cancer in Japan: clinical and immunological findings. Mol Ther. 2004;10:799–816. doi: 10.1016/j.ymthe.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Hobeika AC, Clay TM, Mosca PJ, Lyerly HK, Morse MA. Quantitating therapeutically relevant T-cell responses to cancer vaccines. Crit Rev Immunol. 2001;21:287–297. [PubMed] [Google Scholar]
- 26.Michael A, Ball G, Quatan N, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005;11:4469–4478. doi: 10.1158/1078-0432.CCR-04-2337. [DOI] [PubMed] [Google Scholar]
- 27.Kiessling A, Fussel S, Wehner R, et al. Advances in specific immunotherapy for prostate cancer. Eur Urol. 2008;53:694–708. doi: 10.1016/j.eururo.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Koido S, Chen D, Gendler SJ, Kufe D, Gong J. Vaccination with allogeneic dendritic cells fused to carcinoma cells induces antitumor immunity in MUC1 transgenic mice. Clin Immunol. 2001;101:192–200. doi: 10.1006/clim.2001.5112. [DOI] [PubMed] [Google Scholar]
- 29.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 30.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 31▪▪.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled Phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. Placebo-controlled Phase III study involving 127 men with asymptomatic hormone-refractory prostate cancer (HRPC) who received Provenge or placebo. Median overall survival was 25.9 months for those patients randomized to the vaccine versus 21.4 months for those patients receiving placebo control (p = 0.02) [DOI] [PubMed] [Google Scholar]
- 32.Fenner F, Arita I, Jezek A, Ladnyi I. Smallpox and its eradication. World Health Organization; Geneva, Switzerland: 1988. [Google Scholar]
- 33.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 34.Cooney EL, Collier AC, Greenberg PD, et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 36▪.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. Patients were randomized to receive recombinant poxvirus vectors with the gene for prostate-specific antigen (PSA). There was a substantial difference in PSA progression-free survival favoring the use of vaccinia priming and avipox vector boosting. [DOI] [PubMed] [Google Scholar]
- 37.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 38▪▪.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. HRPC patients with no measurable disease on scans were randomized to receive a pox-vector PSA vaccine versus the androgen-receptor antagonist nilutamide. The results demonstrate an overall survival benefit for patients randomized to receive vaccine therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madan R, Gulley J, Dahut W, et al. Overall survival (OS) analysis of a Phase II study using a pox viral-based vaccine, PSA-TRICOM, in the treatment of metastatic, castrate-resistant prostate cancer (mCRPC): implications for clinical trial design. J Clin Oncol. 2008;16(20S) (Abstract 3005) [Google Scholar]
- 40.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–7994. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 41.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 43.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology (Williston Park) 2008;22:1064–1070. discussion 1075, 1080–1081, 1084. [PMC free article] [PubMed] [Google Scholar]
- 44.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 45.Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aragon-Ching J, Williams K, Gulley J. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 48.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesslinger NJ, Sahota RA, Stone B, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- 50.Beer T, Bernstein G, Corman J, et al. Randomized trial of active cellular immunotherapy with sipuleucel-T in androgen dependent prostate cancer (ADPC) [abstract] J Clin Oncol. 2007;25(18S):5059. doi: 10.1158/1078-0432.CCR-10-3223. [DOI] [PubMed] [Google Scholar]
- 51.Terando A, Mule JJ. On combining antineoplastic drugs with tumor vaccines. Cancer Immunol Immunother. 2003;52:680–685. doi: 10.1007/s00262-003-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 53.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arlen PM, Gulley JL, Parker C, et al. A randomized Phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 56.Langer LF, Clay TM, Morse MA. Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7:1245–1256. doi: 10.1517/14712598.7.8.1245. [DOI] [PubMed] [Google Scholar]
- 57.O’Mahony D, Morris JC, Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 58.Gulley JL, Dahut WL. Future directions in tumor immunotherapy: CTLA4 blockade. Nat Clin Pract Oncol. 2007;4:136–137. doi: 10.1038/ncponc0749. [DOI] [PubMed] [Google Scholar]
- 59.Gerritsen W, van den Eertwegh A, de Gruijl T, et al. Expanded Phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC) [abstract] J Clin Oncol. 2008;26:5146. [Google Scholar]
- 60.Sin JI, Hong SH, Park YJ, Park JB, Choi YS, Kim MS. Antitumor therapeutic effects of e7 subunit and DNA vaccines in an animal cervical cancer model: antitumor efficacy of e7 therapeutic vaccines is dependent on tumor sizes, vaccine doses, and vaccine delivery routes. DNA Cell Biol. 2006;25:277–286. doi: 10.1089/dna.2006.25.277. [DOI] [PubMed] [Google Scholar]
- 61.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 62.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 63.Kawaida H, Kono K, Takahashi A, et al. Distribution of CD4+CD25 high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. doi: 10.1016/j.jss.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Serafini P, De Santo C, Marigo I, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hodge JW, Grosenbach DW, Schlom J. Vector-based delivery of tumor-associated antigens and T-cell co-stimulatory molecules in the induction of immune responses and anti-tumor immunity. Cancer Detect Prev. 2002;26:275–291. doi: 10.1016/s0361-090x(02)00095-8. [DOI] [PubMed] [Google Scholar]
- 67.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol. 2007;27:451–462. doi: 10.1615/critrevimmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
Websites
- 101▪▪.Cell Genesys halts VITAL-2 GVAX trial in advanced prostate cancer. 2008 http://phx.corporate-ir.net/phoenix.zhtml?c=98399&p=irol-newsArticle&ID=1191052 Showed an increased death rate in vaccine and chemotherapy combination arm in metastatic castration-resistant prostate cancer patients and may have an impact on future vaccine trials design.
- 102.Cell Genesys announces termination of VITAL-1 Phase 3 trial of GVAX immunotherapy for prostate cancer. 2008 www.drugs.com/news/cell-genesys-announces-termination-vital-1-phase-3-trial-gvax-immunotherapy-prostate-cancer-14159.html.
- 103.Cellular, Tissue and Gene Therapies Advisory Committee: Sipuleucel-t briefing document. 2007 www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4291B1_01.pdf.
- 104▪▪.Dendreon announces interim data from Phase 3 PROVENGE IMPACT trial. 2008 http://investor.dendreon.com/releasedetail.cfm?ReleaseID=338495 Independent data monitoring committee reported to Dendreon a 20% reduction in the risk of death in the Provenge arm relative to placebo.
- 105▪▪.Prostvac™. 2008 www.bavarian-nordic.com/prostvac Randomized Phase II study of Prostvac™ versus placebo, showing an improvement of 8 months in overall survival in vaccinated patients.
- 106.Gulley J. Phase I trial of a targeted therapy with a PSA-based vaccine and ipilimumab in patients with metastatic castration-resistant prostate cancer (CRPC) Targeted Anticancer Therapies 2008 [presentation] 2008 www.mccm.nl/images/pp4%20james%20gulley.pdf.