Abstract
Background
Childhood Crohn’s disease (CD) is associated with poor growth and decreased body mass index (BMI); however, lean mass (LM) and fat mass (FM) deficits prior to therapy have not been characterized.
Objectives
To quantify LM and FM in incident pediatric CD subjects and controls, and to identify determinants of LM and FM deficits.
Methods
Whole body LM and FM were assessed using DXA in 78 CD subjects and 669 healthy controls, ages 5–21 yr. Gender specific z-scores for LM (LM-Ht) and FM (FM-Ht) relative to height were derived using log linear regression models in the controls. Multivariate linear regression models adjusted for potential confounders.
Results
CD was associated with significantly lower height and BMI for age. Within CD subjects, FM-Ht and LM-Ht were significantly lower in females compared with males (FM-Ht z: −0.66 ± 0.83 vs. −0.08 ± 0.95, p < 0.01; LM-Ht z: −1.12 ± 1.12 vs. −0.57 ± 0.99, p < 0.05). In females, CD was associated with significantly lower LM-Ht (p < 0.001) and FM-Ht (p < 0.001), adjusted for age, race and Tanner stage, compared with controls. LM and FM deficits were significantly greater in older females with CD; 47% of adolescent females had LM-Ht ≤ 5th percentile. In non-black males, CD was also associated with lower LM-Ht (p < 0.02); FM-Ht deficits were not significant.
Conclusions
Incident CD was associated with significant LM deficits in males and females, and FM deficits in females. Future studies are needed to identify etiologies for the age and gender differences and to evaluate therapies for these deficits.
Keywords: Inflammatory bowel disease, Crohn’s disease, growth failure, body composition, lean mass, fat mass, cachexia
Crohn’s disease (CD) is an idiopathic, destructive, chronic inflammatory condition of the gastrointestinal tract. In children with CD, reduced caloric intake, malabsorption, micronutrient deficiencies, delayed puberty, decreased physical activity, glucocorticoid therapy, and increased production of inflammatory cytokines may contribute to poor growth and alterations in body composition. Manifestations include poor weight gain, linear growth, and bone accrual, as well as alterations in fat mass and lean mass. Decreased body mass index (BMI, kg/m2) is a recognized complication of CD. However, decreased BMI does not distinguish between deficits in lean mass and fat mass, which are distinct markers of protein and energy reserves.1,2 Growth and development are characterized by age-, gender-, maturation-, and race-specific increases in lean mass and fat mass. In adults, lean mass deficits are associated with demonstrable morbidity, including poor physical functioning, altered energy metabolism, and increased susceptibility to infections.3 In children and adolescents, muscle forces are a key determinant of bone mass accrual.4 We recently reported that deficits in lean mass were strongly associated with deficits in whole-body bone mineral content in a prevalent cohort of children and young adults with CD compared with healthy controls.5
Previous studies of lean mass and fat mass in children and adults with inflammatory bowel disease (IBD) were performed in small numbers of patients and produced conflicting results. 6–14 More recently, we reported the results of a cross-sectional study of growth and body composition in 104 children and young adults with CD, evaluated an average of 4 years after disease diagnosis.5 CD was associated with significant deficits in BMI and height for age compared with healthy controls. Whole-body lean mass was significantly lower in subjects with CD, adjusted for age, height, and maturation; however, fat mass was preserved, consistent with inflammatory cachexia.15–17 This earlier study was the first to describe inflammatory cachexia in pediatric CD. Given the variable disease duration in these patients, and the fact that the majority had received glucocorticoid therapy, it was not possible to distinguish between disease and treatment effects on lean mass and fat mass in this prevalent cohort. The aims of the present study were to assess lean mass and fat mass in children and adolescents with CD at the time of disease diagnosis compared to healthy controls, and to identify determinants of lean mass and fat mass deficits in this incident cohort.
MATERIALS AND METHODS
Study Subjects
Incident CD subjects between the ages of 5 and 21 years were recruited from the Inflammatory Bowel Diseases Center of the Children’s Hospital of Philadelphia. CD subjects known to have any other chronic illnesses or medications potentially affecting growth and development were excluded. Healthy control subjects were recruited from general pediatric clinics in the greater Philadelphia area. Control subjects were excluded for height or BMI below the third percentile for age and for any chronic illness or medications that may affect growth and development.
Ethical Considerations
The study protocol was approved by the Committee for the Protection of Human Subjects of the Institutional Review Board at the Children’s Hospital of Philadelphia. Informed consent was obtained from young adult subjects and the parents or guardians of subjects under the age of 18 years. Assent was obtained from subjects between 7 and 18 years of age.
CD Characteristics and Definitions
CD subjects were enrolled within 2 weeks of diagnosis; diagnosis was confirmed by radiographic, endoscopic, histologic, and clinical parameters. All 78 subjects were evaluated by colonoscopy and 75 also underwent esophagoduodenos-copy. Upper gastrointestinal tract disease was defined as disease proximal to the colon, including the terminal ileum. CD activity was assessed at the time of the study visit using the Pediatric CD Activity Index (PCDAI), which is based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%).18 The widely used PCDAI defines growth failure as either involuntary stable weight, weight loss of 1%–9%, or at least a one channel decrease in height on the growth chart. PCDAI scores range from 0–100, and were categorized as follows: no disease activity (0–10), mild disease activity (11–30), and moderate to severe disease activity (>30). Data on weight and height prior to diagnosis were obtained from the general pediatric medical record. At the time of the study visits, data were collected on duration of symptoms, extraintestinal manifestations, dietary intake, medications, and nutritional supplements. Positive family history was defined as the identification of any biologically related family member with CD.
Anthropometry and Pubertal Development
All study visits were conducted within 2 weeks of the diagnosis of CD. Weight and height were measured using a digital scale to the nearest 0.1 kg (Scaltronix, White Plains, NY) and a wall-mounted stadiometer to the nearest 0.1 cm (Holtain, Croswell, Crymych, UK), respectively. Pubertal status was assessed with a validated self-assessment questionnaire in subjects greater than 8 years of age19 and classified according the method of Tanner.20
Body Composition Measures
Whole-body lean mass (kg) and fat mass (kg) were assessed by dual energy x-ray absorptiometry (DXA) using a Hologic Delphi densitometer (Bedford, MA) with a fan beam in the array mode (software v. 12.4). Lean mass was calculated as fat-free mass minus bone mineral content. Measurements of whole-body lean mass and fat mass did not include the head.15,21 DXA is a precise (CV 1%–4%)22 method that has been used extensively to describe age-, gender-, race-, and pubertal maturation-related variability in body composition compartments.23–27 The instrument was calibrated daily using a hydroxyapatite phantom and weekly with a whole-body phantom.
Laboratory Studies
Laboratory studies included serum hematocrit (mg/dL), erythrocyte sedimentation rate (mm/h), and albumin (g/dL) in the CD subjects; assays were performed using standard techniques in the Clinical Laboratories at the Children’s Hospital of Philadelphia. Anemia was defined using age- and gender-specific reference values. In addition, serum tumor necrosis factor-α (TNF-α) (pg/mL) and interleukin-6 (IL-6) (pg/mL) levels were measured by enzyme-linked immunosorbent assay (ELISA; Quest Diagnostics, San Juan Capistrano, CA) in the subjects with CD and a subset of healthy controls.
Statistical Analysis
Analyses were conducted using STATA 9.0 (Stata, College Station, TX). A two-tailed P-value <0.05 was the criterion for statistical significance. Differences in means were assessed using Student’s t-test for normally distributed variables and Wilcoxon’s rank sum test for non-normally distributed variables, respectively. Correlations between body composition z-scores and continuous variables were assessed by Pearson product moment correlations or Spearman rank correlations, where appropriate.
Height and BMI were converted to age- and gender-specific standard deviation scores (z-scores) in the CD subjects and controls using National Center for Health Statistics 2000 Centers for Disease Control reference data.28 CD was associated with significantly lower height for age z-scores compared with controls, and body composition variables were highly correlated with height (lean mass: R = 0.96, P < 0.001; fat mass: R = 0.59, P < 0.001). Therefore, whole-body lean mass and fat mass were assessed as gender-specific z-scores relative to height, as previously described.15,21 The distribution of lean mass and fat mass relative to height demonstrated significant heteroscedasticity, as assessed by the Cook–Weisburg test, and fat mass was skewed, as assessed by graphical checks and the skewness test for normality (sktest) in Stata 9. Therefore, lean mass for height (LM-Ht) and fat mass for height (FM-Ht) z-scores were generated in CD subjects and controls based on the control population utilizing the LMS method to account for the variably skewed distribution of body composition data.29 Height- and gender-specific estimates of the distribution median (M), coefficient of variation (S), and degree of skewness (L) in lean mass and fat mass were obtained by a maximum-likelihood curve-fitting technique.
Subsequent comparisons of LM-Ht and FM-Ht z-scores in CD and controls were adjusted for covariates that may confound this comparison in a sequential fashion in order to identify variables that may explain the group differences. Due to the known differences in body composition in blacks and whites, and given the racial distribution of our sample, an indicator variable was constructed that compared blacks to nonblacks. Because of delays in the onset and progression through puberty in children with CD, Tanner stage of pubertal maturation (with indicator variables for each Tanner stage given a value of 0 or 1 if absent or present, respectively) was entered in the model next. Age was also entered into the model to determine whether there were effects of age on LM-Ht and FM-Ht z-scores, independent of height and Tanner stage. We previously reported that greater FM-Ht z-scores were associated with greater LM-Ht z-scores in healthy children and adolescents5; therefore, FM-Ht z-score was subsequently included in the models for LM-Ht z-scores in order to determine if the lean mass was appropriate relative to the fat mass. After the sequential addition of the covariates discussed above, the effect of multiplicative interaction terms was used to determine whether the impact of CD varied as a function of subject age, subject Tanner stage, and subject race. Finally, the model for LM-Ht z-score evaluated whether the impact of CD varied as a function of FM-Ht z-score via the addition of a CD by FM-Ht z-score interaction term.
RESULTS
Subject Characteristics
Table 1 summarizes subject characteristics in CD and controls. A total of 78 subjects with incident CD and 669 healthy controls were enrolled in the study. The predominance of whites and males among the CD subjects was consistent with the demographic distribution of the disease.30 Within the CD cohort, 83% of patients were white, 10% were black, and 6% identified as mixed race. The availability of 245 black subjects within the control group allows for adjustment for race in the multivariate analyses. The proportions of subjects that were prepubertal were comparable in CD subjects and controls. However, the subjects with CD had significantly greater age for Tanner stage compared with controls, consistent with delayed puberty (P < 0.001). The subjects with CD had significantly lower height and BMI z-scores compared with controls. Within the subjects with CD, there were no gender differences in height and BMI z-scores. Of note, within the 44 male subjects with CD, the three black subjects had markedly higher height z-scores (1.24 ± 0.26 versus −0.32 ± 1.01, P = 0.012) and BMI z-scores (1.97 ± 0.24 versus −0.47 ± 0.96, P < 0.001) compared with the 41 nonblack subjects. There were no racial differences in height and BMI z-scores in the female subjects with CD.
TABLE 1.
Subject Characteristics in Incident Crohn’s Disease and Controls
| Crohn’s Disease N = 78 |
Controls N = 669 |
P | |
|---|---|---|---|
| Age* | 12.7 ± 2.8 | 12.1 ± 3.8 | NS |
| Gender (% Male) | 56% | 48% | NS |
| Race (% Black) | 10% | 37% | <0.001 |
| Prepubertal (%) | |||
| (Tanner stage 1–2) | 54% | 43% | NS |
| Height z-score* | |||
| Male | −0.21 ± 1.06 | 0.15 ± 0.90 | 0.01 |
| Female | −0.32 ± 1.07 | 0.23 ± 0.86 | <0.001 |
| BMI z-score* | |||
| Male | −0.35 ± 1.21 | 0.18 ± .97 | <0.01 |
| Female | −0.78 ± 1.02 | 0.26 ± .89 | <0.001 |
NS, not significant; BMI, body mass index.
Mean ± SD.
Disease Characteristics
Disease characteristics, laboratory values, and therapies are summarized in Table 2. Data on each of the variables other than serum cytokines were available in at least 95% of the subjects; cytokine data were available in 73% of subjects. Over-all, the majority of subjects had moderate to severe CD activity, growth failure, anemia, and extraintestinal manifestations such as fever, apthous ulcers, joint pain, and erythema nodosum. Male subjects were more likely to have a family history of CD (40% versus 17%, P < 0.05), which was not associated with duration of symptoms, PCDAI, laboratory values, or clinical symptoms. Black CD subjects had a significantly higher rate of hospitalization prior to diagnosis than white subjects (75% versus 30%, P < 0.05) and were more likely to present with hematochezia (88% versus 41%, P < 0.05). There were no gender and racial differences in the remaining laboratory values, medical therapies, or patient symptoms, including extraintestinal manifestations. Among the 35% of CD subjects (n = 27, 59% male) with duration of symptoms of 12 months or longer prior to diagnosis, the following symptoms were reported with the greatest frequency: abdominal pain (85%), diarrhea (48%), arthralgia (44%), apthous ulcers (44%), hematochezia (41%), and fever (26%).
TABLE 2.
Crohn’s Disease Characteristics
| Variable | Value |
|---|---|
| Age at diagnosis, (yr), Mean ± SD | 12.7 ± 2.8 |
| Duration of symptoms prior to diagnosis (months) | Median: 7.1 Range: 0.5−52.6 |
| Family history of Crohn’s disease, n (%) | 22 (30%) |
| History of growth failure | 62 (84%) |
| PCDAI | 38.4 ± 18.0 |
| No active disease (≤10) | 3 (4%) |
| Mild (11– 30) | 28 (38%) |
| Moderate to Severe (>30) | 43 (58%) |
| Symptoms | |
| Diarrhea | 49 (63%) |
| Hematochezia | 36 (46%) |
| Abdominal pain | 66 (84%) |
| Extraintestinal manifestations | 55 (71%) |
| Fever | 35 (45%) |
| Oral ulcers | 25 (32%) |
| Joint symptoms | 29 (37%) |
| Erythema nodosum | 2 (3%) |
| Albumin (g/dL) | 3.41 ± 0.65 |
| Erythrocyte sedimentation rate (mm/h) | 26.7 ± 21.4 |
| Median: 21 Range: 0–128 | |
| ESR > 20 | 41 (53%) |
| Hematocrit (%) | 34.7 ± 4.6 |
| Anemia | 60 (77%) |
| Cytokines | |
| TNF-α (normal range: 1.2-15.3 pg/ml) | 2.3 ± 1.1 |
| Median: 2.0 Range: 0.9−5.1 | |
| IL-6 (normal range: 0.3−5.0 pg/mL) | 11.2 ± 29.8 |
| Median: 4.2 Range: 0.3−221.5 | |
| Site of disease | |
| Upper GI tract disease | 66 (88%) |
| Isolated upper GI tract disease | 5 (7%) |
| Colonic disease | 71 (91%) |
| Isolated Colonic disease | 7 (9%) |
| Perianal involvement | 28 (36%) |
| Granulomas | 37 (47%) |
| Esopagheal | 1(1%) |
| Gastric | 10 (13%) |
| Duodenal | 4 (5%) |
| Ileal | 14 (20%) |
| Colonic | 27 (35%) |
| Crohn’s disease related hospitalizations prior to diagnosis | 27 (35%) |
| History of surgery | |
| Indication for total of 13 procedures in 9 subjects | 9 (12%) |
| Abscess drainage | 3 (23%) |
| Fistulotomy | 7 (54%) |
| Bowel resection | 1 (8%) |
| Nephrolithiasis | 1 (8%) |
| Appendicitis | 1 (8%) |
| Medications at time of study visit | |
| Oral corticosteroids (excluding budesonide) | 34 (44%) |
| Budesonide | 4 (5%) |
| Aminosalicylates | 72 (92%) |
| Mesalamine | 69 (88%) |
| Balsalazide | 3 (4%) |
| 6-mercaptopurine/Azathioprine | 8 (10%) |
| Methotrexate | 2 (3%) |
| Antibiotics | 24 (31%) |
| Histamine-2 blockers | 6 (8%) |
| Proton pump inhibitors | 33 (44%) |
| Iron supplements | 5 (6%) |
| Folic acid | 4 (5%) |
PCDAI, Pediatric CD Activity Index; GI, gastrointestinal. Continuous data are presented as mean ± SD, or median and range, where appropriate. Categorical data are presented as n (%).
Fat Mass
Unadjusted gender-specific FM-Ht z-scores in the CD subjects and controls are presented in Table 3, stratified by gender and race. Black and nonblack females with CD had significantly lower FM-Ht z-scores compared with controls (P < 0.01). Within the males, FM-Ht z-scores were marginally lower in the nonblack subjects with CD compared with controls (P = 0.07). The three black male subjects with CD had significantly higher FM-Ht z-scores compared with controls (P < 0.05); however, this is of uncertain clinical significance given the small number of subjects. Within the subjects with CD, FM-Ht z-scores were significantly lower in females compared to males (P < 0.005). Within female CD subjects, FM-Ht z-scores were significantly lower in blacks (P < 0.05), and within males subjects, FM-Ht z-scores were significantly greater in blacks (P < 0.001).
TABLE 3.
Body Composition Z-scores in Subjects with Crohn’s Disease and Controls, Stratified by Gender and Race
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Crohn’s Disease n = 34 29 Nonblack 5 Black |
Controls N = 347 228 Nonblack 119 Black |
P | Crohn’s Disease n = 44 41 Nonblack 3 Black |
Controls N = 322 196 Nonblack 126 Black |
P | |
| FM-Ht* z-score | ||||||
| Nonblack | −0.52 ± 0.73 | 0.05 ± 0.97 | <0.01 | −0.22 ± 0.81 | 0.07 ± 0.93 | 0.07 |
| Black | −1.47 ± 0.98 | −0.09 ± 1.10 | <0.01 | 1.88 ± 0.29 | −0.11 ± 1.10 | <0.01 |
| LM-Ht** z-score | ||||||
| Nonblack | −1.11 ± 1.16 | −0.14 ± 0.94 | <0.001 | −0.71 ± 0.89 | −0.10 ± 0.98 | <0.001 |
| Black | −1.17 ± 1.02 | 0.26 ± 1.06 | <0.01 | 1.23 ± 0.21 | 0.14 ± 1.03 | 0.07 |
FM-Ht* Fat mass for height.
LM-Ht** Lean mass for height.
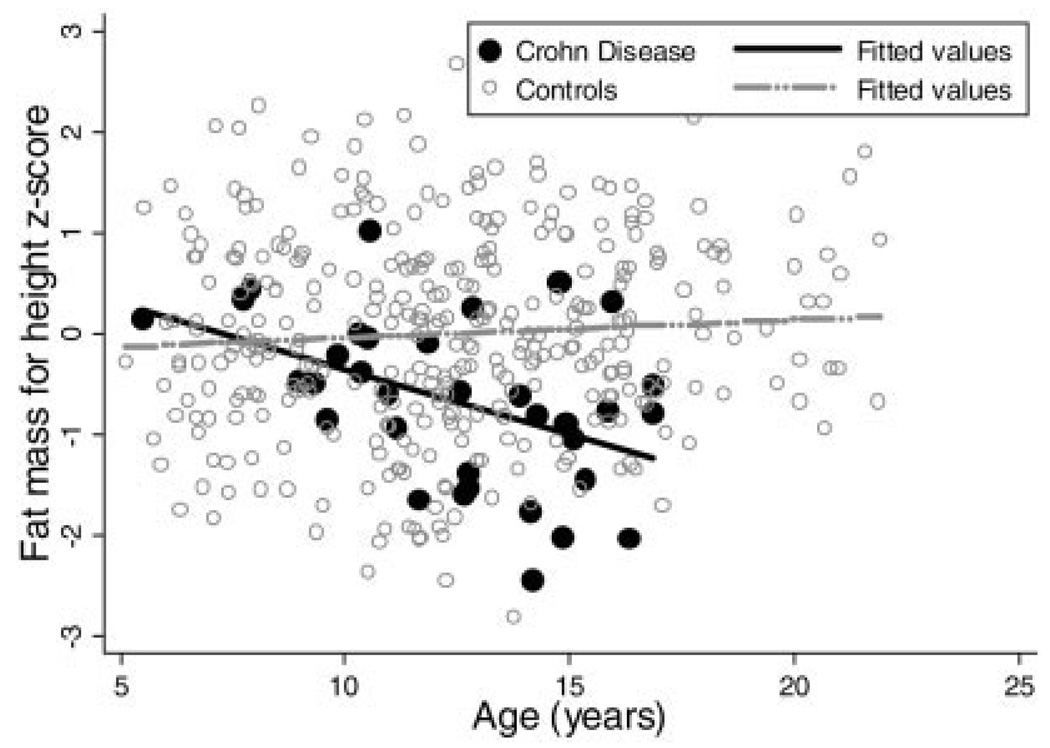
Table 4 illustrates the results of the sequential multivariate analyses for FM-Ht z-score in males and females. Within the females, adjustment for race, Tanner stage, and age attenuated the effect of CD on FM-Ht z-scores, but the mean z-score deficits remained significant at −0.60 (95% confidence interval [CI]: −0.95, −0.24; P < 0.001) compared with controls. The addition of multiplicative interaction terms revealed that the impact of CD on FM-Ht z-scores in females did not vary as a function of subject race or Tanner stage. However, there was a significant interaction between CD status and age in female subjects (P < 0.05). That is, FM-Ht z-scores were lower among the older female subjects with CD, while FM-Ht z-scores were unrelated to age in the female controls. Figure 1 illustrates these relations.
TABLE 4.
Sequential Linear Regression Models of the Independent Effect of Crohn’s Disease on Fat Mass for Height Z-score in Females
| Covariates | Group Difference in Z-score (95% CI) | P | |
|---|---|---|---|
| Female | Unadjusted model | −0.66 (−1.01,−0.31) | <0.001 |
| Race* | −0.70 (−1.04,−0.35) | <0.001 | |
| Race,* Tanner | −0.65 (−1.00,−0.30) | <0.001 | |
| Race, Tanner, Age | −0.60 (−0.95,−0.24) | 0.001 | |
| Male | Unadjusted model | −0.08 (−0.39, 0.24) | >0.2 |
| Race* | −0.11 (−0.43, 0.13) | >0.2 | |
| Race,* Tanner* | −0.11 (−0.43, 0.21) | >0.2 | |
| Race,* Tanner,* Age* | −0.10 (−0.44, 0.23) | >0.2 |
Group difference represents the effect of Crohn’s disease on FM-Ht z-score, adjusted for covariates.
Not significant in the multivariate model.
FIGURE 1.
Fat mass for height z-score versus age in female Crohn’s disease subjects and controls.
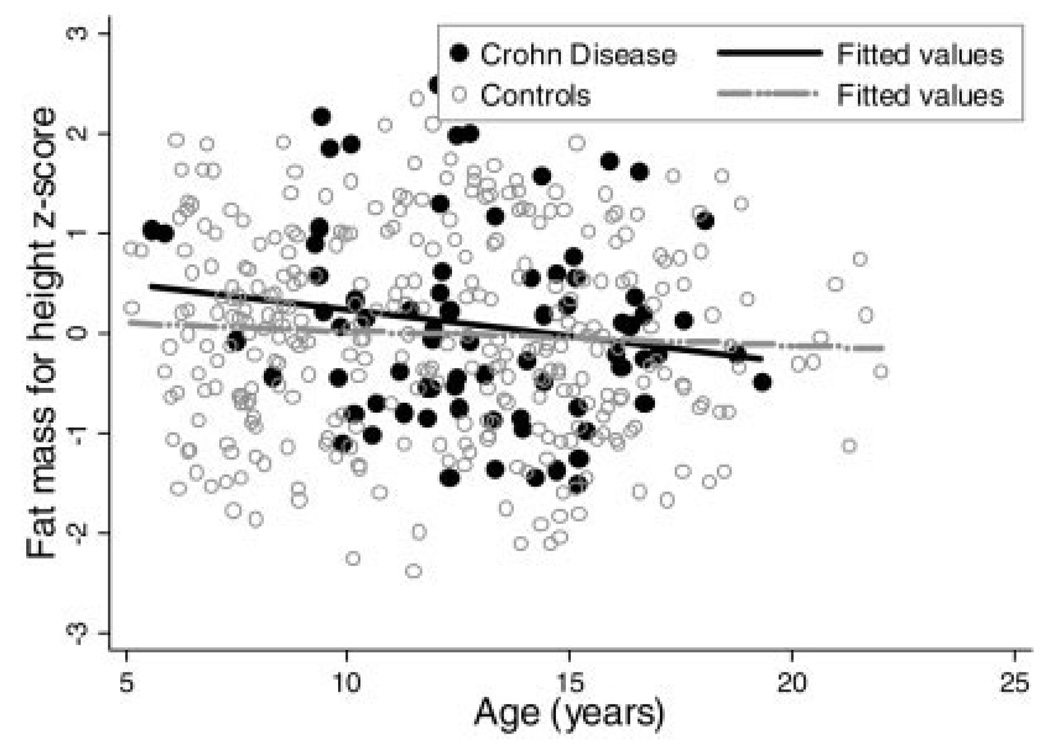
Among males, no significant deficits were detected in FM-Ht z-scores in CD compared with controls in the unadjusted model, or after adjustment for race, Tanner stage, and age. Similarly, there was no evidence of interactions between CD and age within males, illustrated in Figure 2.
FIGURE 2.
Fat mass for height z-score versus age in male Crohn’s disease subjects and controls.
Lean Mass
Unadjusted gender-specific LM-Ht z-scores in the CD subjects and controls are presented in Table 3, stratified by gender and race. The racial differences in LM-Ht z-scores within the control sample are consistent with prior reports that blacks have greater lean mass compared with nonblacks.31 LM-Ht z-scores were substantially lower in nonblack (P < 0.001) and black (P < 0.01) females with CD compared with controls. Within the males, LM-Ht z-scores were significantly lower in the nonblack subjects with CD compared with controls (P < 0.001) and marginally greater in the three black subjects with CD compared with controls (P = 0.07). Within subjects with CD, LM-Ht z-scores were significantly lower in females compared with males (P < 0.05).
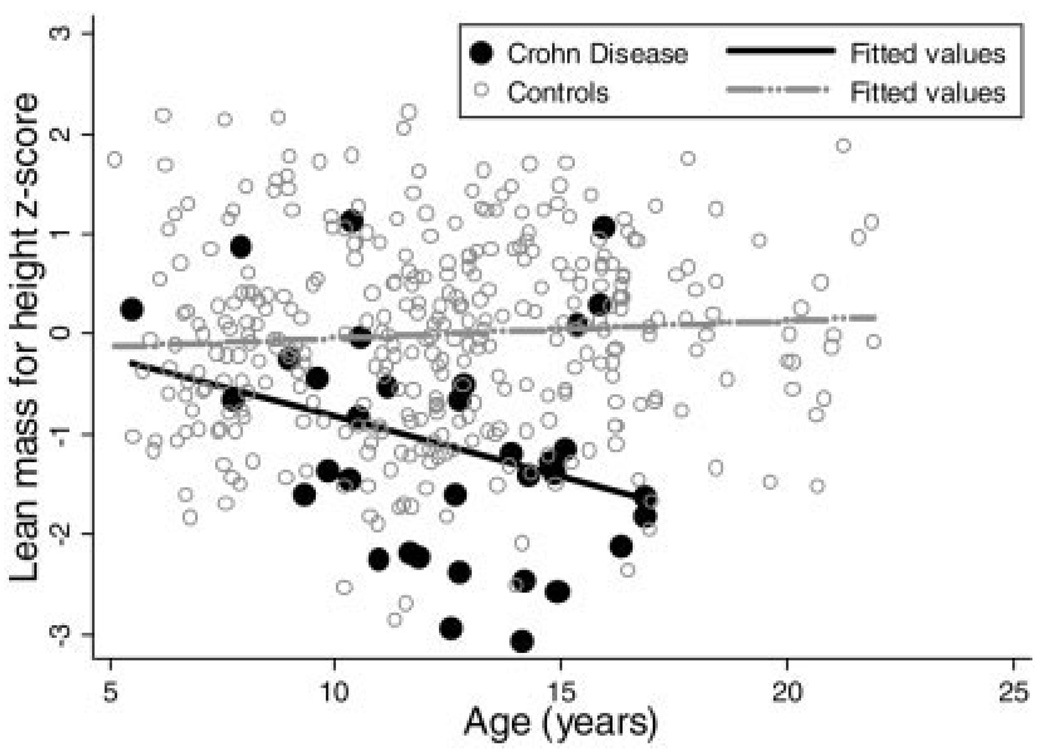
Sequential multivariate linear regression models were used to compare LM-Ht z-scores in subjects with CD and controls, adjusting for race, Tanner stage, age, and FM-Ht z-score within the males and females (Table 5). The sequential multivariate analyses in the females revealed that the significant deficits in LM-Ht z-scores in the subjects with CD compared with controls persisted after adjustment for race, Tanner stage, age, and FM-Ht z-score, averaging −0.67 (95% CI: −1.31, −0.95; P < 0.001). Tests for interaction demonstrated that the effect of CD on lean mass did not vary as a function of race, Tanner stage, or FM-Ht z-score. However, there was a significant interaction between CD status and age in females, indicating that the LM-Ht z-scores were lower in the older females with CD compared with younger subjects, while the LM-Ht z-scores varied less with age in the controls (Fig. 3).
TABLE 5.
Sequential Linear Regression Models of the Independent Effect of Crohn’s Disease on Lean Mass for Height Z-score
| Covariates | Group Difference in z-score (95% CI) | P | |
|---|---|---|---|
| Males | Unadjusted model | −0.57 (−0.88,−0.25) | <0.001 |
| Race | −0.47 (−0.79,−0.15) | <0.005 | |
| Race, Tanner | −0.41 (−0.73,−0.09) | <0.02 | |
| Race, Tanner,* Age* | −0.42 (−0.74,−0.90) | <0.02 | |
| Race, Tanner,* Age,* Ft-Ht Z | −0.38 (−0.68,−0.07) | <0.02 | |
| Females | Unadjusted model | −1.11 (−1.47,−0.76) | <0.001 |
| Race | −1.04 (−1.40,−0.69) | <0.001 | |
| Race, Tanner | −1.00 (−1.36,−0.64) | 0.001 | |
| Race, Tanner, Age* | −0.95 (−1.31,−0.59) | <0.001 | |
| Race, Tanner,* Age,* Ft-Ht Z | −0.67 (−1.00,−0.34) | <0.001 |
Group difference represents the effect of Crohn’s disease on LM-Ht z-score, adjusted for covariates.
Not significant in the multivariate model.
FIGURE 3.
Lean mass for height z-score versus age in female Crohn’s disease subjects and controls.
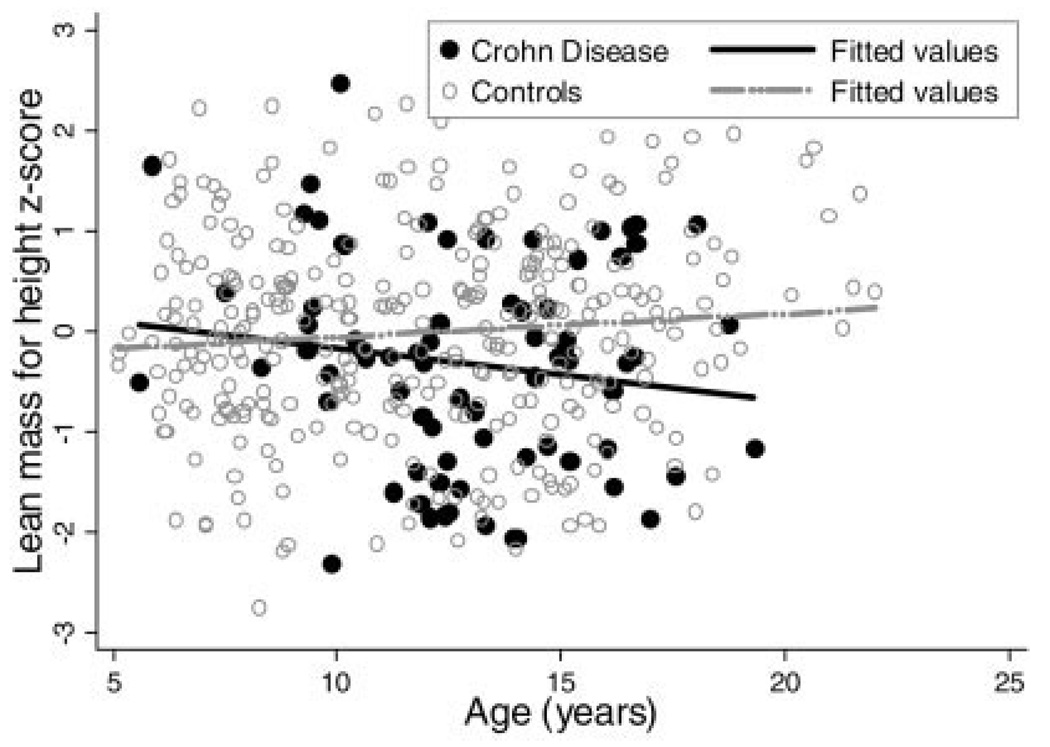
Within the males the sequential multivariate analyses revealed that adjustment for race, Tanner stage, age, and FM-Ht z-score attenuated the effect of CD on LM-Ht z-score; however, the lean mass deficits remained significant, averaging −0.38 (95% CI: −0.68, −0.07; P < 0.02). Tests for interaction did not demonstrate that the impact of CD on LM-Ht z-scores in males varied as a function of subject age (Fig. 4), Tanner stage, or FM-Ht z-score. The test for interaction between CD and race was significant, indicating that the black males with CD had significantly higher LM-Ht z-scores compared with nonblack males with CD; however, this difference was explained by the markedly greater FM-Ht z-scores within the three black males with CD.
FIGURE 4.
Lean mass for height z-score versus age in male Crohn’s disease subjects and controls.
CD-Specific Factors and Body Composition
LM-Ht and FM-Ht z-scores in subjects with CD were not associated with PCDAI, site of disease, duration of symptoms, or TNF-α levels. FM-Ht z-scores were positively associated with serum albumin (r = 0.43, P < 0.001) and hematocrit (r = 0.23, P < 0.05) and negatively associated with erythrocyte sedimentation rate (ESR) (r = −0.23, P < 0.05). LM-Ht z-scores were positively associated with serum albumin (r = 0.27, P < 0.05) and hematocrit (r = 0.23, P < 0.05), and negatively associated with serum IL-6 levels (r = −0.31, P < 0.05).
DISCUSSION
This study of an incident cohort of 78 children and adolescents with CD demonstrated significant alterations in growth, pubertal development, and body composition compared with a robust contemporary control sample. The female subjects with CD demonstrated decreased fat mass and lean mass relative to height, age, race, and Tanner stage, and the deficits were most pronounced in the females that were diagnosed with CD during adolescence. The LM-Ht z-score in the females (black and nonblack combined) averaged –1.12, which is equivalent to the 13th percentile. Furthermore, the LM-Ht was below the 5th percentile (z-score < −1.64) in 10 (29%) of the 34 female subjects with CD, demonstrating the significant downward shift of the lean mass distribution in females with CD. Of even greater concern, among the female subjects greater than 12 years of age at diagnosis the lean mass was at the 5th percentile or below in 47% of these subjects. The profound lean mass deficits in the adolescent females are demonstrated in Figure 2. In contrast, in the males the fat mass was essentially preserved, the lean mass deficits were less pronounced, and the lean mass deficits did not vary as a function of age at diagnosis. Although the body composition deficits were not associated with CD activity (as measured by the PCDAI), both fat mass and lean mass were both positively correlated with serum albumin and hematocrit. The availability of a contemporary, large, racially diverse control sample allowed for adjustment for the potential confounding effects of height, race, age, and Tanner stage, and is a significant strength of this study.
Previous studies on body composition deficits in CD were performed in prevalent samples of patients with variable disease duration, treatment regimens, and cumulative corticosteroid exposure.6,10 –13,15,32 For example, we recently examined whole-body lean mass and fat mass in a cross-sectional sample of 104 children and young adults with CD with an average disease duration of 4 years.15 The study demonstrated significant deficits in lean mass in males and females after adjustment for height, age, race, and Tanner stage; however, no deficits in fat mass were observed in males or females. Ninety-five percent of these subjects had been treated with glucocorticoids. We hypothesized that the glucocorticoid therapy potentially contributed to the lean mass deficits and preserved fat mass. However, such studies conducted in subjects with prevalent disease were unable to distinguish between the effects of CD and its therapies on body composition.
Both glucocorticoids and inflammatory cytokines have adverse effects on muscle cells. Glucocorticoids may cause muscular atrophy by increasing the expression of myostatin, a negative regulator of skeletal muscle mass.33 Glucocorticoids also suppress glucose and amino acid muscle uptake by inhibiting cellular transporters.34 Inflammatory cytokines that are elevated in CD, such as IL-6 and TNF-α, promote protein degradation35 and inhibit skeletal muscle differentiation by suppressing MyoD mRNA at the posttranscriptional level.36 Furthermore, malnutrition and cytokines result in alterations in the growth hormone–insulin-like growth factor (IGF) axis. 37 IGF-1 increases muscle protein synthesis,38 and alterations in IGF-1 have been reported in children with incident CD.39–41
To our knowledge, our study is the first to examine body composition in an incident cohort, documenting CD effects on growth, maturation, and body composition, prior to the initiation of glucocorticoid therapy and nutritional supplementation. Therefore, the fat mass and lean mass deficits seen in the CD subjects in this study reflect the cumulative effects of inflammation, malnutrition/malabsorption, and pubertal delay. Fat mass was inversely correlated with serum ESR, and lean mass was inversely correlated with serum IL-6, suggesting a role for inflammation. TNF-α was not correlated with fat mass nor lean mass deficits; however, studies have suggested that intestinal mucosal, rather than serum, levels of TNF-α levels provide better surrogates of inflammatory activity in CD.42 Additionally, it has been proposed that intestinal inflammation in CD may stimulate the peripheral circulation of activated T cells, which in turn cause the local secretion of proinflammatory cytokines such as TNF-α, resulting in end-organ musculoskeletal deficits observed in CD.43
Despite the absence of gender differences within CD subjects in height and BMI z-scores at the time of diagnosis, gender-specific fat mass and lean mass deficits were significantly greater in females compared with males. Previous cross-sectional studies performed in prevalent CD samples have shown conflicting results in terms of gender differences in anthropometric and body composition deficits, suggesting no gender differences15 or greater deficits in males.15,32,44,45 The male CD subjects reported here suffered lean mass deficits in the absence of significant fat mass deficits; this pattern has been termed inflammatory cachexia.17 Female CD subjects at diagnosis presented with both lean mass and fat mass deficits, exhibiting a pattern consistent with wasting, likely representing inadequate calories as well as elevated cytokines. Future studies are needed that incorporate measures of resting energy expenditure, physical activity, dietary intake, and the growth hormone–IGF-1 axis in order to identify potential etiologies for the age and gender differences observed here.
The goals of therapy in pediatric CD are to induce and maintain clinical remission, prevent disease-related complications, and promote normal growth and development; recognition of the extent of body composition deficits at diagnosis is central to these goals. The clinical significance of lean mass deficits in adults is well recognized.46 Cachexia frequently complicates cardiac disease, rheumatoid arthritis, malignancy, chronic pulmonary disease, HIV, and chronic renal disease, and is associated with markedly increased morbidity and mortality.47 The clinical significance of lean mass deficits in children with CD is not known; however, lean mass deficits may be associated with poor physical functioning and greater infection risk during childhood and compromised peak bone mass by young adulthood. Mechanical forces on the skeleton arise primarily from muscle contraction and are a critical determinant of bone accrual. The capacity of bone to respond to these forces is greatest during growth, particularly prior to the attainment of puberty.48 Decreased bone accrual may result in increased fractures in childhood, while suboptimal peak bone mass in young adults may result in early onset of osteoporosis due to the inexorable decline in bone mass that occurs with aging. We have reported vertebral fractures in a series of children with CD and others have demonstrated that CD is associated with increased hip and vertebral fractures in adults.49,50
In summary, pediatric CD at diagnosis is associated with significant body composition deficits, presenting with cachexia in males and wasting in females. Therapeutic approaches to controlling inflammation and providing nutritional support may address these deficits; however, our data in a prevalent cohort suggest that the lean mass deficits persist.15 Longitudinal studies of the response of bone and body composition to the new generation of biologic agents used for the treatment of CD are warranted.
REFERENCES
- 1.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–680. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 2.Bechard L, Puig M. Body composition and growth. In: Walker W, Watkins J, Duggan C, editors. Nutrition in pediatrics. London: BC Decker; 2003. pp. 32–51. [Google Scholar]
- 3.Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49:163–175. doi: 10.1111/j.1753-4887.1991.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 4.Schonau E, Werhahn E, Schiedermaier U, et al. Influence of muscle strength on bone strength during childhood and adolescence. Horm Res. 1996;45 Suppl 1:63–66. doi: 10.1159/000184834. [DOI] [PubMed] [Google Scholar]
- 5.Burnham JM, Shults J, Semeao E, et al. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19:1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 6.Boot AM, Bouquet J, Krenning EP, et al. Bone mineral density and nutritional status in children with chronic inflammatory bowel disease. Gut. 1998;42:188–194. doi: 10.1136/gut.42.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capristo E, Addolorato G, Mingrone G, et al. Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease. Am J Gastroenterol. 1998;93:2411–2419. doi: 10.1111/j.1572-0241.1998.00696.x. [DOI] [PubMed] [Google Scholar]
- 8.Capristo E, Mingrone G, Addolorato G, et al. Metabolic features of inflammatory bowel disease in a remission phase of the disease activity. J Intern Med. 1998;243:339–347. doi: 10.1046/j.1365-2796.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 9.Mingrone G, Benedetti G, Capristo E, et al. Twenty-four-hour energy balance in Crohn disease patients: metabolic implications of steroid treatment. Am J Clin Nutr. 1998;67:118–123. doi: 10.1093/ajcn/67.1.118. [DOI] [PubMed] [Google Scholar]
- 10.Haderslev KV, Jeppesen PB, Sorensen HA, et al. Body composition measured by dual-energy X-ray absorptiometry in patients who have undergone small-intestinal resection. Am J Clin Nutr. 2003;78:78–83. doi: 10.1093/ajcn/78.1.78. [DOI] [PubMed] [Google Scholar]
- 11.Azcue M, Rashid M, Griffiths A, et al. Energy expenditure and body composition in children with Crohn’s disease: effect of enteral nutrition and treatment with prednisolone. Gut. 1997;41:203–208. doi: 10.1136/gut.41.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahnsen J, Falch JA, Mowinckel P, et al. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556–1562. doi: 10.1111/j.1572-0241.2003.07520.x. [DOI] [PubMed] [Google Scholar]
- 13.Geerling BJ, Lichtenbelt WD, Stockbrugger RW, et al. Gender specific alterations of body composition in patients with inflammatory bowel disease compared with controls. Eur J Clin Nutr. 1999;53:479–485. doi: 10.1038/sj.ejcn.1600780. [DOI] [PubMed] [Google Scholar]
- 14.Schneeweiss B, Lochs H, Zauner C, et al. Energy and substrate metabolism in patients with active Crohn’s disease. J Nutr. 1999;129:844–848. doi: 10.1093/jn/129.4.844. [DOI] [PubMed] [Google Scholar]
- 15.Burnham JM, Shults J, Semeao E, et al. Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr. 2005;82:413–420. doi: 10.1093/ajcn.82.2.413. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R, Roubenoff RA, Ward LM, et al. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol. 1992;19:1505–1510. [PubMed] [Google Scholar]
- 17.Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxf) 2004;43:1219–1223. doi: 10.1093/rheumatology/keh321. [DOI] [PubMed] [Google Scholar]
- 18.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 19.Morris NN, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 20.Tanner JM. Growth at adolescence. London: Blackwell Scientific; 1962. [Google Scholar]
- 21.Foster BJ, Shults J, Zemel BS, et al. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr. 2004;80:1334–1341. doi: 10.1093/ajcn/80.5.1334. [DOI] [PubMed] [Google Scholar]
- 22.Ellis KJ, Shypailo RJ, Abrams SA, et al. The reference child and adolescent models of body composition. A contemporary comparison. Ann N Y Acad Sci. 2000;904:374–382. doi: 10.1111/j.1749-6632.2000.tb06486.x. [DOI] [PubMed] [Google Scholar]
- 23.Ellis KJ. Body composition of a young, multiethnic, male population. Am J Clin Nutr. 1997;66:1323–1331. doi: 10.1093/ajcn/66.6.1323. [DOI] [PubMed] [Google Scholar]
- 24.Ellis KJ, Abrams SA, Wong WW. Body composition reference data for a young multiethnic female population. Appl Radiat Isot. 1998;49:587–588. doi: 10.1016/s0969-8043(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 25.Goulding A, Taylor RW, Gold E, et al. Regional body fat distribution in relation to pubertal stage: a dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr. 1996;64:546–551. doi: 10.1093/ajcn/64.4.546. [DOI] [PubMed] [Google Scholar]
- 26.Ogle GD, Allen JR, Humphries IR, et al. Body-composition assessment by dual-energy x-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr. 1995;61:746–753. doi: 10.1093/ajcn/61.4.746. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd T, Chinchilli VM, Eggli DF, et al. Body composition development of adolescent white females: the Penn State Young Women’s Health Study. Arch Pediatr Adolesc Med. 1998;152:998–1002. doi: 10.1001/archpedi.152.10.998. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 30.Andres PG, Friedman LS. Epidemiology and the natural course of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281. doi: 10.1016/s0889-8553(05)70056-x. [DOI] [PubMed] [Google Scholar]
- 31.Schutte JE, Townsend EJ, Hugg J, et al. Density of lean body mass is greater in blacks than in whites. J Appl Physiol Resp Environ Exerc Physiol. 1984;56:1647–1649. doi: 10.1152/jappl.1984.56.6.1647. [DOI] [PubMed] [Google Scholar]
- 32.Sentongo TA, Semeao EJ, Piccoli DA, et al. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:33–40. doi: 10.1097/00005176-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Ma K, Mallidis C, Bhasin S, et al. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285:E363–E371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 34.Dimitriadis G, Leighton B, Parry-Billings M, et al. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J. 1997;321(Pt 3):707–712. doi: 10.1042/bj3210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clowes GH, Jr, George BC, Villee CA, Jr, et al. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. 1983. Nutrition. 1995;11:775. discussion 774, 776–777. [PubMed] [Google Scholar]
- 36.Guttridge DC, Mayo MW, Madrid LV, et al. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 37.Fan J, Char D, Bagby GJ, et al. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204–R1212. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- 38.Tirapegui J. Effect of insulin-like growth factor-1 (IGF-1) on muscle and bone growth in experimental models. Int J Food Sci Nutr. 1999;50:231–236. doi: 10.1080/096374899101102. [DOI] [PubMed] [Google Scholar]
- 39.Kirschner BS, Sutton MM. Somatomedin-C levels in growth-impaired children and adolescents with chronic inflammatory bowel disease. Gastroenterology. 1986;91:830–836. doi: 10.1016/0016-5085(86)90683-9. [DOI] [PubMed] [Google Scholar]
- 40.Corkins MR, Gohil AD, Fitzgerald JF. The insulin-like growth factor axis in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2003;36:228–234. doi: 10.1097/00005176-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Thomas AG, Holly JM, Taylor F, et al. Insulin like growth factor-I, insulin like growth factor binding protein-1, and insulin in childhood Crohn’s disease. Gut. 1993;34:944–947. doi: 10.1136/gut.34.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plevy SE, Landers CJ, Prehn J, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J Immunol. 1997;159:6276–6282. [PubMed] [Google Scholar]
- 43.Sylvester FA, Davis PM, Wyzga N, et al. Are activated T cells regulators of bone metabolism in children with Crohn disease? J Pediatr. 2006;148:461–466. doi: 10.1016/j.jpeds.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Markowitz J, Grancher K, Rosa J, et al. Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993;16:373–380. doi: 10.1097/00005176-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths A, Nguyen P, Smith C, et al. Growth and clinical course of children with Crohn’s disease. Gut. 1993;34:939–943. doi: 10.1136/gut.34.7.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 47.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 48.Parfitt AM. The two faces of growth: benefits and risks to bone integrity. Osteoporos Int. 1994;4:382–398. doi: 10.1007/BF01622201. [DOI] [PubMed] [Google Scholar]
- 49.Semeao EJ, Stallings VA, Peck SN, et al. Vertebral compression fractures in pediatric patients with Crohn’s disease. Gastroenterology. 1997;112:1710–1713. doi: 10.1016/s0016-5085(97)70055-6. [DOI] [PubMed] [Google Scholar]
- 50.Sylvester FA. Cracking the risk of fractures in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38:113–114. doi: 10.1097/00005176-200401000-00028. [DOI] [PubMed] [Google Scholar]