Abstract
The thyroid develops within the pharyngeal apparatus from endodermally-derived cells. The many derivatives of the pharyngeal apparatus develop at similar times and sometimes from common cell types, explaining why many syndromic disorders express multiple birth defects affecting different structures that share a common pharyngeal origin. Thus, different derivatives may share common genetic networks during their development. Tbx1, the major gene associated with DiGeorge syndrome, is a key player in the global development of the pharyngeal apparatus, being required for virtually all its derivatives, including the thyroid. Here we show that Tbx1 regulates the size of the early thyroid primordium through its expression in the adjacent mesoderm. Because Tbx1 regulates the expression of Fgf8 in the mesoderm, we postulated that Fgf8 mediates critical Tbx1-dependent interactions between mesodermal cells and endodermal thyrocyte progenitors. Indeed, conditional ablation of Fgf8 in Tbx1-expressing cells caused an early thyroid phenotype similar to that of Tbx1 mutant mice. In addition, expression of an Fgf8 cDNA in the Tbx1 domain rescued the early size defect of the thyroid primordium in Tbx1 mutants. Thus, we have established that a Tbx1->Fgf8 pathway in the pharyngeal mesoderm is a key size regulator of mammalian thyroid.
INTRODUCTION
The thyroid is an endocrine gland that secretes two types of hormones, thyroxin and calcitonin, which are produced by two distinct cell types, the thyroid follicular cells (TFC) and the para-follicular or C-cells, respectively. These two cell types derive from distinct regions of the pharyngeal endoderm, specifically the ventral the pharyngeal endoderm provides the TFC progenitors, while the endoderm of the 4th pharyngeal pouches provides the C-cell progenitors. The TFC progenitor population constitutes the early primordium at approximately embryonic day (E) 8.5, when it appears as thickened epithelium on the ventral-medial wall of the pharynx, just caudal to the 1st pharyngeal arch. The early primordium then grows without further cell proliferation (Fagman et al., 2006) presumably by recruiting cells from the adjacent endoderm, and forms a pit by invaginating into the pharyngeal mesenchyme. Between E11.5 and E13.5, the primordium grows deeper into the mesenchyme, detaches from the pharyngeal endoderm, and begins to expand laterally. Later, the thyroid primordium reaches the trachea where it fuses with the 4th pouch-derived ultimobranchial bodies, which provide the C-cells of the mature organ. At around E15–16, the thyroid gland acquires its final shape, i.e. two lobes connected by a narrow isthmus. From this stage, the organ grows and begins to express functional markers such as thyroglobulin (tg), thyroperoxidase (TPO) and Tshr (De Felice, 2004).
The signals that induce the initial events of specification and migration of thyroid precursor cells are still unknown. It has been postulated that the invagination process involves epithelial-mesenchyme transition. However, recently it has been demonstrated that the epithelial phenotype is maintained by thyroid progenitor cells throughout organogenesis (Fagman et al., 2003). Indeed, thyroid precursors express high levels of E-cadherin, which is epithelial cell-specific, throughout development (Fagman et al., 2003), suggesting that the invagination is not associated with transition and active migration, but rather it might be due to passive movement of the primordium along with remodeling of the surrounding mesenchyme. In addition, it has been shown a cell autonomous role of Foxe2 in the migration process (De Felice et al., 1998). The disruption of thyroid morphogenesis associated with mutations of genes expressed in tissues surrounding the primordium, e.g. Shh and Hoxa5, demonstrates the importance of tissue interactions during thyroid development (Fagman et al., 2004; Meunier et al., 2003).
DiGeorge syndrome (DGS) is associated with developmental defects of the derivatives of the pharyngeal apparatus that result in many birth defects, including cardiovascular, craniofacial and ear defects (Scambler, 1993; Shprintzen et al., 2005). DGS patients also show developmental abnormalities of pharyngeal-derived glands such as the thymus, parathyroids and, in some cases, thyroid (Bassett and Thakker, 1995; Scuccimarri and Rodd, 1998). Consistent with this, mice mutated for Tbx1, a key gene in the pathogenesis of DGS, recapitulate most, if not all, the above mentioned abnormalities (Baldini, 2005), including thyroid abnormalities (Fagman et al., 2007; Liao et al., 2004).
Tbx1 encodes a T-box transcription factor that is expressed regionally and dynamically in the ectoderm, endoderm and mesoderm of the developing pharyngeal apparatus. The endodermal expression domain encompasses the dorsal and lateral aspects of the pharynx but does not include the thyroid domain, while the mesodermal expression domain is adjacent to and partially surrounds the thyroid domain.
The thyroid primordium and the ultimobranchial bodies, which derive from the 4th pharyngeal pouches, fuse at E13 and contribute to the mature gland. Tbx1-null mutants do not have 4th pharyngeal pouches, thus thyroid hypoplasia in these mutants could be explained in part by the absence of the ultiomobranchial bodies-derived component of the mature organ (Liao et al., 2004). However, loss of Tbx1 already affects thyroid development at E11.5 (Fagman et al., 2007), indicating that loss of the 4th pharyngeal pouch is insufficient to explain the phenotype.
In this study, we show that Tbx1-dependent signals from the mesoderm are required to ensure that the proper number of endodermally-derived thyroid precursors populate the primordium. We provide genetic evidence that Fgf8 is a critical intermediary of this function because 1) Tbx1 positively regulates Fgf8 expression in the mesoderm, 2) removal of Fgf8 from Tbx1-expressing cells causes thyroid hypoplasia, 3) forced expression of Fgf8 from the endogenous Tbx1 locus partially rescues the thyroid phenotype in Tbx1 mutants.
It has been shown that Fgf10 (also expressed in mesoderm) and its receptor Fgfr2 are required for thyroid development (Celli et al., 1998; De Felice, 2004; Ohuchi et al., 2000). However, Fgf8 has not been implicated in mammalian thyroid morphogenesis until now. Our data support a Tbx1-dependent role for Fgf8 in thyroid development, and they are consistent with a recent report showing a critical role of this gene in Zebrafish thyroid development (Wendl et al., 2007). In addition, our data show that pharyngeal mesoderm (including the cardiogenic mesoderm of the secondary heart field) supports thyroid development, thus providing a possible explanation for the frequent association between congenital heart disease and thyroid dysmorphogenesis (Olivieri et al., 2002).
MATERIALS AND METHODS
Mouse lines
The following mouse lines have been described: Tbx1+/− (Lindsay et al., 2001), Tbx1Cre/+ (Huynh et al., 2007), Tbx1flox/flox (Xu et al., 2004), Tbx1 ΔE5/+ (a null allele, (Xu et al., 2004)), Tbx1Fgf8/+ (Vitelli et al., 2006), Mesp1Cre/+ (Saga et al., 1999), Fgf8flox/flox (Meyers et al., 1998), Tie2Cre (Kisanuki et al., 2001), Foxa2mcm/+ (Park et al., 2008), Fgfr1flox/flox (Xu et al., 2002), Fgfr2flox/flox (Yu et al., 2003), and R26R (Soriano, 1999). All lines were backcrossed into the C57Bl/6 genetic background for at least two generations. PCR strategies for mouse genotyping have been described in the original reports. For ablation of FgfR1 and FgfR2 in the pharyngeal endoderm by E8.0 , pregnant females were orally gavaged with 0.05mg/gm body weight tamoxifen at E6.75 as described in (Park et al., 2008). We have examined at least 3–5 embryos per experimental point and developmental stage.
Immunohistochemistry and in situ hybridizatiion
Mouse embryos or dissected thyroids were collected in phosphate buffered saline (PBS), and fixed in 4% paraformaldehyde overnight. Following fixation, embryos were dehydrated through graded ethanols, embedded in paraffin wax and sectioned (7µm). Sections were dewaxed by standard techniques, and heat-treated for the antigen retrieval. To quench endogenous peroxidases, sections were treated with hydrogen peroxide in methanol at room temperature. Sections were incubated for 1 hr at room temperature with blocking solution (3% BSA, 5% goat serum, 20 mM MgCl2, 0.3% Tween 20 in PBS) and then with primary antibodies overnight at 4°C. Staining procedures and chromogenic reactions were carried out according to the protocols of the Vectastain ABC kit protocol (Vector Laboratories). The primary antibodies used were: anti Titf1/Nkx2.1 (kindly provided by Dr R. Di Lauro), anti-human Thyroglobulin (Tg, Dako), anti phosphorylated histone H3 (Upstate), anti-Fgf8 (MAB323, R&D Systems), and anti-Fgfr4 (H-121, Santa Cruz). For morphological analyses, thyroid sections were dewaxed and stained with Hematoxylin and Eosin. Digital images from serial histological sections were used for 3-dimensional reconstructions using the softtware WinSURF (provided by SurfDriver, http://www.surfdriver.com/).
Cell counts of thyroid primordia (E9.0-E11.0) were carried out using high magnification microscopy on consecutive tissue sections and counting all the cells of the primordia.
Non radioactive in situ hybridization experiments were carried out using standard methods and probes for Tbx1, Fgf8, Fgfr1, Fgfr2, Fgfr3 and Fgfr4.
Beta-galactosidase detection
Mouse embryos were fixed in 4% paraformaldehyde and processed for X-gal staining according to standard procedure. Embryos were embedded in paraffin wax and sectioned (10µm). Sections were counterstained with nuclear fast red.
RESULTS
Tbx1 is required in early thyroid development
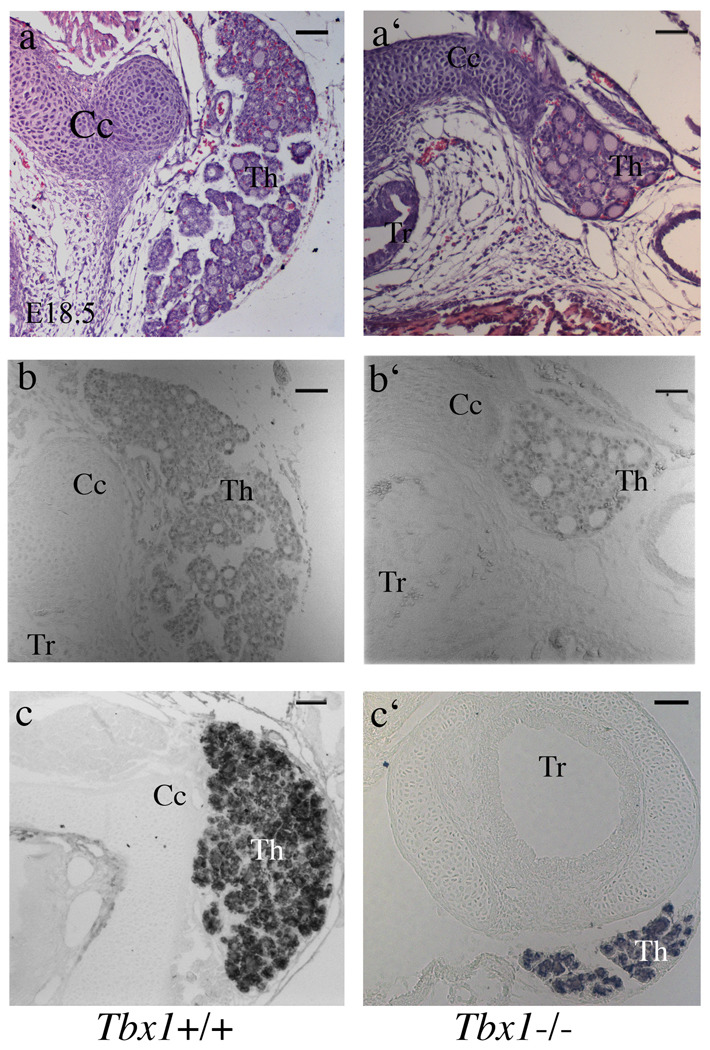
The thyroid of E17.5 Tbx1−/− embryos is hypoplastic (Fagman et al., 2007; Liao et al., 2004). However, the primary developmental defects of thyroidogenesis caused by Tbx1 loss of function are unknown. To define the mutant phenotype during development, we carried out a morphological analysis of the thyroid and thyroid primordium at different embryonic stages. Consistent with previously reported data (Fagman et al., 2007), we found that at E18.5, Tbx1−/− embryos had hypoplastic thyroid that was sometimes correctly located close to the cricoid cartilage, and was formed by very small left and right lobes (Fig. 1a–a’) or, in most cases, by a single lobe (Fig. 5b’). In addition, we observed that the lumen of thyroid follicles of mutant embryos appeared slightly enlarged (Fig. 1a’,b’). Immunohistochemistry revealed that Nkx2-1 is normally expressed and that thyroglobulin is normally produced by mutant follicles (Fig. 1c–c’).
Figure 1. Hypoplasia and dysmorphogenesis of the thyroid in Tbx1−/− embryos at E18.5.
Hematoxylin and eosin staining of histological sections from wild type and mutant thyroids (a-a’). Immunohistochemistry with anti-Nkx2-1(b-b’) and anti-Tg (c-c’) antibodies demonstrates the presence of differentiated and functional thyroid follicular cells. Cc cricoid cartilage, Th thyroid, Tr trachea. The scale bar is 50µm.
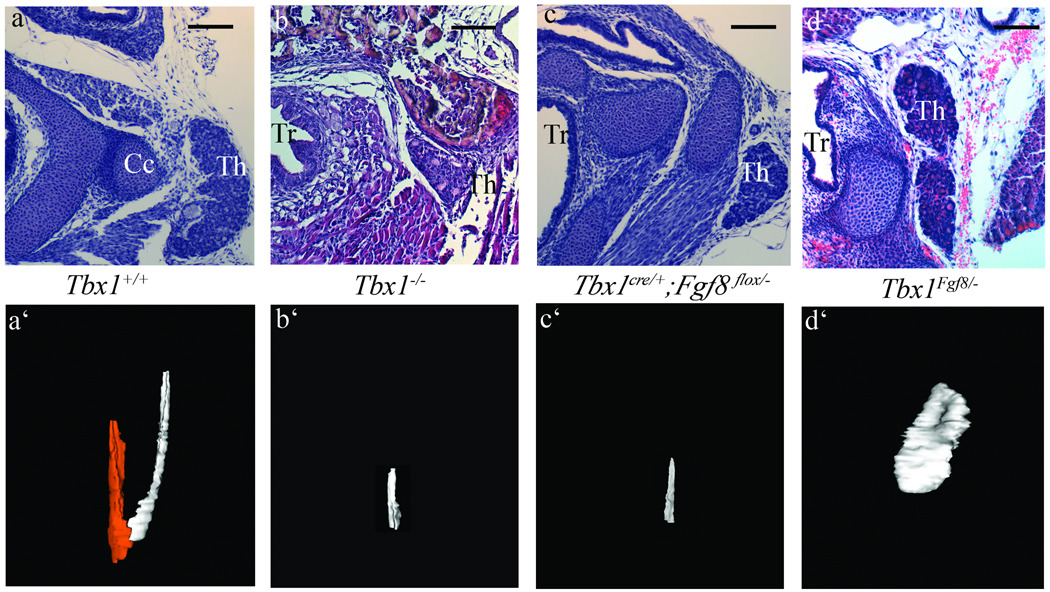
Figure 5. Fgf8 expression in the Tbx1 domain is able to rescue the Tbx1−/− thyroid phenotype.
Histological analyses and corresponding 3D reconstructions of wild type (a,a’), Tbx1−/− (b,b’), Tbx1cre/+ ;Fgf8flox/− (c,c’) and Tbx1Fgf8/− thyroids at E18.5. The two colors in the a’ panel indicate the left (white) and right (red) lobes. Note the increased size but abnormal shape of the rescued thyroid of Tbx1 mutant embryos expressing Fgf8 from the Tbx1 domain (d’). The scale bar is 50µm.
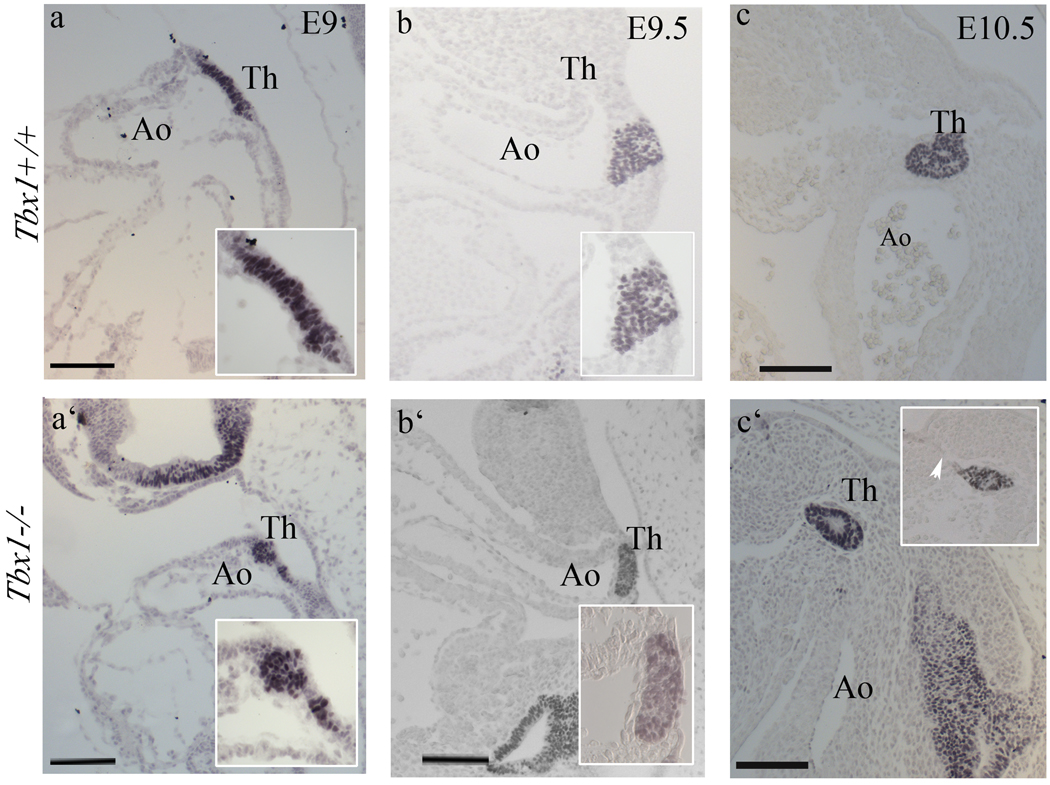
The thyroid primordium was clearly identifiable from E9.0, when it appeared as a well-organized bi-layer of cells in the ventral endoderm of the primitive pharynx, that stained with anti Nkx2-1 immunohistochemistry (Fig. 2a). In Tbx1−/− embryos, we observed that Nkx2-1+ cells are not organized in a cell bi-layer, but appeared clustered (Fig. 2a’). At E9.5, thyroid precursor cells of wild type embryos invaded the surrounding mesenchyme to form the thyroid primordium. In Tbx1−/− embryos, the thyroid primordium was flatter than in controls (Fig. 2b’). At E10, the migration of the thyroid primordium toward the aortic sac was delayed in mutants (Fig. 2c’), and we observed persistence of the thyroglossal duct, which constitutes a continuity between the primordium and the pharyngeal endoderm (arrowhead in Fig. 2c’). Because the mutant primordium was smaller at all stages observed, we quantified its size by counting the number of Nkx2-1+ cells at E9.0-E10.5 (14–40 somites). Results showed a significant reduction of Nkx2-1+ cells at all stages tested (Tab. 1).
Figure 2. Early abnormalities of the thyroid primordium in Tbx1 mutants.
(a–c’) Nkx2-1immunohistochemistry on sagittal sections of wild type (a–c) and Tbx1-null (a’-c’) embryos at E9, E9.5 and E10.5. The mutant thyroid primordium (14 somites) has lost the characteristic bi-layer cell morphology (compare insets in a’ and a). At E9.5, the mutant thyroid primordium is flatter than in wild type (b,b’), while at E10.5 it is clearly smaller than wild type and shows a persistent thyroglossal duct (white arrow). Ao aortic arch. The scale bar is 100µm.
Table 1.
| Tbx1+/+ | Tbx1−/− | Tbx1Cre/+ ;Fgf8fl/− | ||||
|---|---|---|---|---|---|---|
| Age | Som. | n cells | Som. | n cells | Som. | n cells |
| E9 | 16 s | 369 | 14 s | 152 | ||
| 14 s | 360 | 14 s | 144 | |||
| P=0.001 | ||||||
| E9.5 | 24 s | 438 | 25 s | 127 | 24–26 s | 271 |
| 22 s | 515 | 21 s | 306 | 250 | ||
| 22 s | 615 | 22 s | 392 | 170 | ||
| P=0.008 | P=0.03 | |||||
| E10.5-11 | 40 s | 540 | 40 s | 479 | ||
| 39 s | 600 | 39 s | 390 | |||
| 39 s | 635 | 39 s | 320 | |||
| 38 s | 696 | 38 s | 190 | |||
| P=0.03 | ||||||
Cell count (n cells) of thyroid primordia in embryos with the genotype and developmental stages indicated. P values are referred to comparisons with wild type counts. Som: somite stage.
These data show that the mutant thyroid primordium is abnormal from the earliest stages of its development (14 somites), in terms of morphology and cell number, but known differentiation markers of the primordium, such as Tg, Nkx2-1, Foxe1, Pax8, and Hhex1 were expressed normally (Fig. 2 and supplementary Fig. 1). In some experiments (for example see supplementary Fig. 1), Foxe1 immunostaining appeared slightly increased in the Tbx1−/− samples. However, repeated experiments could not confirm this difference.
Mesodermal expression of Tbx1 regulates the size of the thyroid primordium
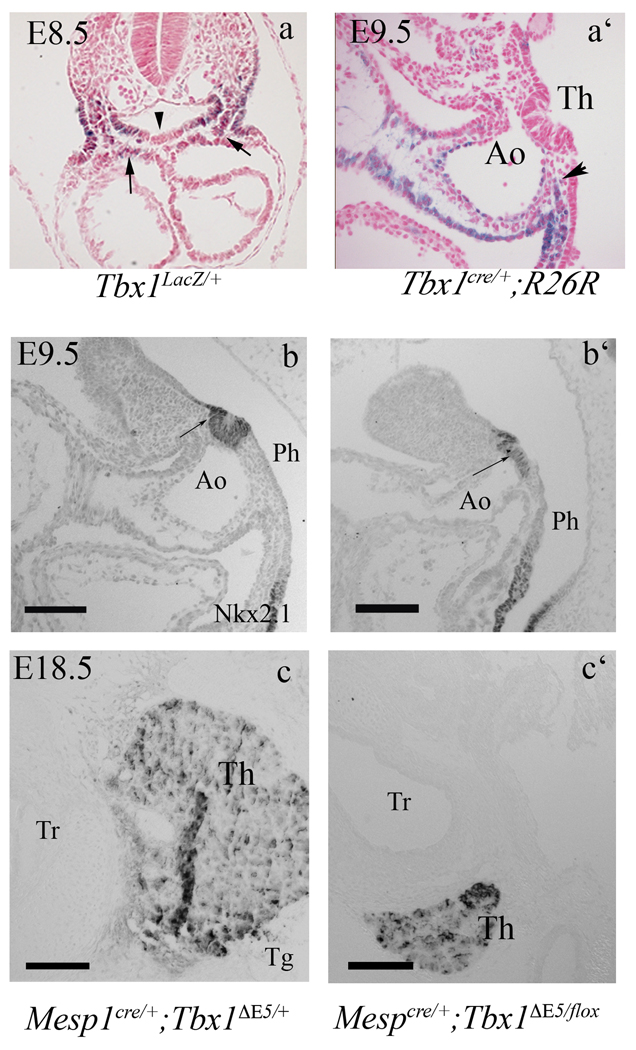
Tbx1 is not expressed in the thyroid primordium (Fig. 3a) but it is strongly expressed in the surrounding mesoderm and in the pharyngeal endoderm lateral to the primordium. In order to test whether Tbx1 is expressed in endodermal precursors of the thyroid primordium, we used a Tbx1Cre knock-in line (Huynh et al., 2007) in combination with the R26R reporter (Soriano, 1999), to label the progeny of Tbx1-expressing cells. We examined E9.5 Tbx1Cre/+;R26R embryos and found very few (1–3) β-gal+ cells in the thyroid primordium (Fig. 3a’), indicating that Tbx1-expressing cells do not represent an important source of thyroid cells. Thus, the role of Tbx1 in the thyroid primordium must be cell non-autonomous. Because Tbx1 is expressed in neighboring mesodermal cells ((Fagman et al., 2007) and Fig. 3a), we hypothesized that there may be a Tbx1-dependent signal in this tissue. To address this hypothesis, we eliminated Tbx1 in mesodermal cells using the Mesp1Cre driver (Saga et al., 1999). The ability of Mesp1cre to delete Tbx1 exclusively in the mesoderm has been demonstrated previously (Zhang et al., 2006). We examined the thyroid size in Mesp1cre/+;Tbx1ΔE5/fl embryos (M-ko mutants) at two embryonic stage, E9.5–10 and E18.5, using Nkx2-1 as a marker. At both stages, we found a strong reduction of organ size, similar to Tbx1−/− embryos (Fig. 3b’,c’). Thus, mesodermal Tbx1 is required for early thyroid development. We postulated that Tbx1 may have a positive, cell non-autonomous effect on endodermal cell proliferation, thus indirectly controlling the number of endodermal cells destined to become thyroid cells. To test this, we evaluated cell proliferation in the ventral endoderm of M-ko mutants at E8.5. Results showed a significant reduction of cell proliferation in M-ko embryos (Tab. 2, an example of data is shown on supplementary Fig. 2) suggesting that the reduced size of the primordium may be due to a reduced number of thyroid precursors or to their reduced proliferation at early stages of development.
Figure 3. Thyroid development requires Tbx1 expression in the mesoderm.
(a) Transverse section of an X-gal stained, Tbx1lacZ/+ E8.5 embryo showing Tbx1 expression in the mesenchyme adjacent to the thyroid primordium (arrows) but not in the primordium itself (arrowhead). (a’) cell fate analysis in Tbx1Cre/+ ; R26R mice. A group of β-gal+ cells surrounds the thyroid primordium (black arrow) but no labeling is visible within the primordium. (b–c) The thyroid phenotype in Mesp1cre/+;Tbx1flox/− embryos at E9.5 and E18.5. Nkx2-1immunohistochemistry on sagittal sections of mutant embryos at E9.5 (b’) reveals a differentiated thyroid primordium that is smaller and flatter than in controls (b). (c–c’) Tg immunohistochemistry of Mesp1Cre/+ ;Tbx1flox/− mutant embryos at E18.5. Ao aortic arch, Tr trachea. The scale bar is 100µm.
Table 2.
| Mesp1cre/+; Tbx1ΔE5/+ | Mesp1cre/+ ;Tbx1ΔE5/flox | ||
|---|---|---|---|
| Total cell number | Mitotic index | Total cell number | Mitotic index |
| 828 | 0.11 | 644 | 0.08 |
| 542 | 0.09 | 680 | 0.03 |
| 839 | 0.11 | 737 | 0.07 |
| P<0.05 | |||
Mitotic index (number of PH3+ cells over the total) in the ventral region of the pharyngeal endoderm of E8.5 embryos (3 controls and 3 M-ko, somite-matched).
Thyroid morphogenesis requires expression of Fgf8 in Tbx1-expressing cells
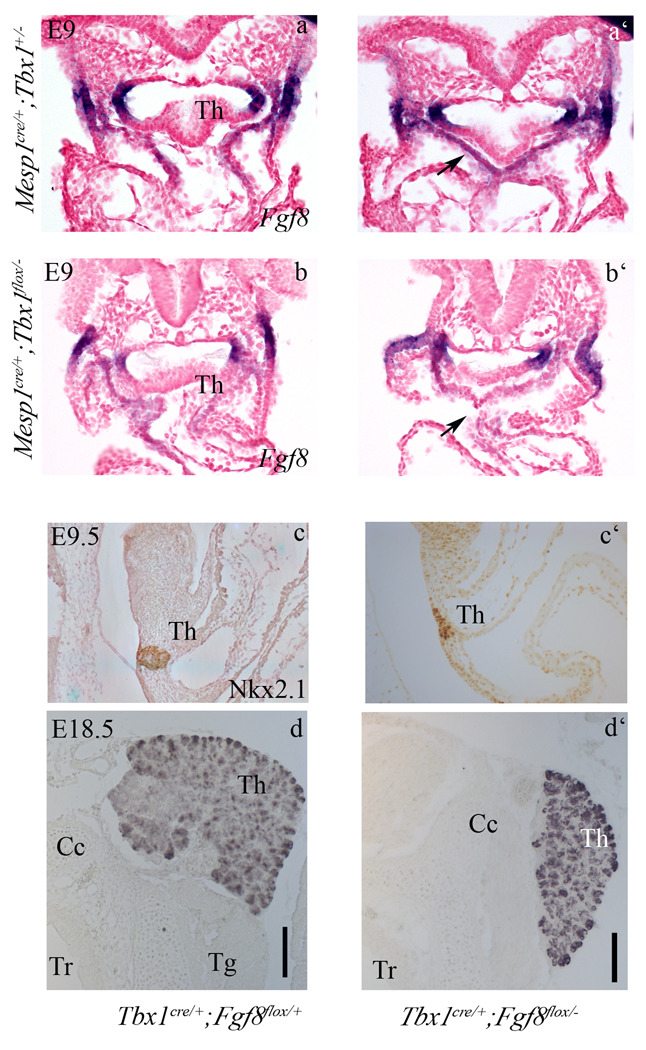
Tbx1 regulates Fgf8 expression in the secondary heart field (Hu et al., 2004; Zhang et al., 2006), a mesodermal population located lateral and ventral to the pharyngeal endoderm. This expression domain is located just caudal to the thyroid primordium at E9.0 (Fig. 4a–a’) and is abolished in M-ko embryos (Fig. 4b–b’). At later stages we could not identify any mesenchymal expression domain near the thyroid primordium (stages tested: E10.5, E11.5, and E14.5, data not shown). It has been shown that mesodermal Fgf8 is important for endodermal cell proliferation (Park et al., 2006). Therefore, we tested whether Fgf8 may function as the Tbx1-dependent extra cellular signal critical for thyroid growth. To establish whether Fgf8 expression in Tbx1-expressing cells is important for thyroid development, we conditionally deleted the gene using a Tbx1Cre driver with a conditional (floxed) allele of Fgf8 indicated as Fgf8fl (Meyers et al., 1998). We observed that the thyroid of Tbx1Cre/+;Fgf8fl/− embryos at E9.5 (Fig. 4c–c’) and E18.5 (Fig. 4d,d’) is smaller than in controls. Complete cell counts of thyroid primordia confirmed the significant reduction of primordium size at E9.5 (Tab. 1). Three dimensional reconstruction of histological sections from E18.5 organs illustrates these differences (compare Fig. 5a’, b’ and c’).
Figure 4. Ablation of Fgf8 in Tbx1-expressing cells causes thyroid hypoplasia.
Transverse sections of E9.0 embryos show Fgf8 expression in the mesoderm (black arrow in a’) caudal to the thyroid primordium, indicated as Th in the more cranial sections, a and b, in control and Mko embryos (a,a’, and b, b’, respectively). Note the down regulation of the mesodermal domain in the M-ko embryo.
Nkx2.1 immunohistochemistry in control (c) and Tbx1Cre/+;Fgf8flox/− E9.5 embryos (c’) reveals a differentiated but smaller thyroid in embryos with conditional ablation of Fgf8. Similarly, at E18.5, Tg immunohistochemistry reveals a small thyroid in a Tbx1Cre/+;Fgf8flox/− embryo (d’) compared to wild type (d). The scale bar is 50µm
Thus, Tbx1-expressing cells represent a source of Fgf8 important for thyroid morphogenesis.
Fgf8 expression in the Tbx1 domain partially rescues the Tbx1−/− thyroid phenotype
If loss of Fgf8 expression in the Tbx1 domain is part of the pathogenetic mechanism leading to thyroid hypoplasia in Tbx1−/− embryos, then forced expression of Fgf8 in the Tbx1 domain should ameliorate the Tbx1−/− thyroid phenotype. To test this idea, we used the Tbx1fgf8 allele in which an Fgf8 cDNA has been knocked into the Tbx1 locus (Vitelli et al., 2006). In these animals, the Fgf8 cDNA is expressed in the Tbx1 domain and the Tbx1 gene in which the cDNA has been inserted is not functional. Thus, Tbx1Fgf8/− embryos are null for Tbx1, but express Fgf8. We harvested Tbx1Fgf8/− and Tbx1Fgf8/Fgf8 embryos at E10.5 and carried out cell counts of thyroid primordia. Results showed that the cell number of knock-in embryos was not statistically different from those of wild type embryos (Tab. 3). We also carried out histological analyses of Tbx1Fgf8/− E18.5 embryos. Results indicated that Tbx1Fgf8/− thyroids are substantially larger than in Tbx1−/− embryos (compare Fig. 5 b–b’ and d–d’), although they have abnormal morphology, mostly limited to a large lobe (compare with control, Fig. 5a’). These data demonstrate a partial rescue of the Tbx1−/− thyroid size phenotype and support a pathogenetic role of Fgf8 dosage reduction in the thyroid phenotype of Tbx1−/− embryos.
Table 3.
| Tbx1+/+ | Tbx1Fgf8/− | Tbx1Fgf8/Fgf8 | ||||
|---|---|---|---|---|---|---|
| Age | Som. | n cells | Som. | n cells | Som. | n cells |
| E10 | 34 s | 395 | 34 s | 539 | 34 s | 434 |
| 34 s | 417 | 34 s | 331 | 34 s | 564 | |
| 34 s | 303 | |||||
| P=0.42 | P=0.17 | |||||
Cell count of thyroid primordia of Fgf8-rescued embryos compared to wild type. P values indicate that the size of primordia in rescued embryos is not significantly different from that of wild type embryos.
Next, we addressed the question as to which FGF receptor may mediate the response of the pharyngeal endoderm/thyroid primordium to Fgf8. Because it has been shown that Fgfr1 and Fgfr2 are expressed in this tissue, and because conditional alleles are available for these genes, we have deleted both these genes in the pharyngeal endoderm using the Foxa2mcm cre driver (Park et al., 2008). To this end, we have examined the thyroid gland in Fgfr1flox/−;Fgfr2flox/−;Foxa2mcm/+ E18.5 embryos. Results showed that the thyroids of these mutants were not significantly different from those of control littermates (Supplementary Fig. 3). Thus, the expression of these two receptor genes in the pharyngeal endoderm appears to be dispensable for thyroid development. Next, we have tested the pharyngeal endoderm expression of the other two receptors, Fgfr3 (by in situ hybridization) and Fgfr4 (by in situ hybridization and immunohistochemistry). Results showed that at E8.5 and E9.5, Fgfr3 is very weakly expressed (Supplementary Fig. 4) while Fgfr4 is not expressed at this stage (although it has been shown to be expressed in the pharyngeal endoderm at earlier stages (Serls et al., 2005)) (data not shown).
DISCUSSION
Thyroid organogenesis is associated with the expression of a set of transcription factor-encoding genes, Nkx2-1, Foxe1, Pax8 and Hhex1 (Parlato et al., 2004). Although these factors are also expressed in other embryonic tissues, they are co-expressed only in the endodermal cells fated to become TFC. Mouse knockout data have demonstrated their key role in thyroid organogenesis. In Nkx2-1−/− or Pax8−/− embryos, the thyroid primordium forms but by E11.5 it degenerates (Mansouri et al., 1998; Minoo et al., 1999). In Hhex1−/− embryos, the thyroid primordium forms and expresses Nkx2-1, Foxe1 and Pax8, but at later stages their expression is downregulated and the thyroid is small (Parlato et al., 2004). In Foxe1−/− embryos, the thyroid primordium forms but does not migrate properly. Thus, none of these transcription factors is individually required for thyroid specification or primordium formation. There are also examples of genes that are not expressed in the thyroid but that affect its development. For example, Hoxa3−/− and Eya1−/− mutants have thyroid hypoplasia. In both cases, thyroid defects have been interpreted as being secondary to failed development of the ultimobranchial bodies (Manley and Capecchi, 1995; Xu et al., 2002)).
Congenital hypothyroidism has been reported in several cases of 22q11DS patients, although it is not a common feature of the syndrome (Weinzimer et al., 1998). TBX1 is the major candidate gene in this syndrome, and Tbx1 mouse mutants present with a small thyroid, suggesting that the gene contributes, directly or indirectly, to thyroid development (Liao et al., 2004). Recently, it has been suggested that the bilobation defect of the thyroid in Tbx1-null mice could be due to failure of the thyroid primordium to establish a contact with the aortic sac, which normally occurs at around E11.5–12 (Fagman et al., 2007), possibly through failed signaling between endothelial cells and primordium. Our data presented here revealed that Tbx1 has a much earlier role in thyroid size, as the primordium is smaller from E9. In order to exclude a role of Tbx1 in the endothelium adjacent to the primordium, we ablated the gene in endothelial cells using the Tie2-Cre driver. Results showed that the thyroid of Tie2-Cre; Tbx1flox/− embryos at E18.5 was of normal size (Supplementary Fig. 5).
The role of Tbx1 in the thyroid appears to be limited to regulating the number of cells of the primordium and does not appear to be involved in differentiation of thyroid precursors, as the expression of thyroid specific markers was not affected in Tbx1 mutants. In addition, the mutant thyroid primordium does not regress and is still capable of growing, although it will never reach the normal, final size. Suggesting that the primary defect is at or precedes primordium formation. Conditional ablation of Tbx1 in the mesoderm is sufficient to recapitulate the null thyroid phenotype, thus indicating that the mesoderm plays a critical role in signaling to the developing thyroid or their progenitors. We show that loss of Tbx1 in the mesoderm is associated with reduced cell proliferation in the pharyngeal endoderm of early embryos, thus providing a possible explanation as to why the primordium is small. We have tested the hypothesis that one of the possible Tbx1-dependent signals critical for thyroid growth is Fgf8. Indeed, conditional ablation of Fgf8 in Tbx1-expressing cells causes severe early and late thyroid hypoplasia, similarly to Tbx1 ablation. Conversely, forced expression of Fgf8 in the Tbx1 expression domain, partially rescues the early and late Tbx1−/− thyroid phenotype. Endogenous Fgf8 expression in the mesoderm near the thyroid primordium is only detectable in early embryogenesis (up to approx. E9.0), consistent with the early appearance of the Tbx1 mutant phenotype. In an attempt to determine whether the tissue targeted by Fgf8 is the pharyngeal endoderm, we have tested the requirement of Fgfr1 and Fgfr2 in this tissue using conditional deletion. Fgfr1 and Fgfr2 are expressed in the pharyngeal endoderm (Moon et al., 2006; Wright et al., 2003). Results indicated that compound deletion of these two receptors does not cause thyroid abnormalities suggesting that the relevant receptors may be Fgfr3, weakly expressed in the pharyngeal endoderm, and/or Fgfr4, expressed in the pharyngeal endoderm at the 7 somite stage (Serls et al., 2005) but not at E9.0. An alternative possibility is that the target tissue is a non-endodermal tissue that, in turn, signals to thyroid precursors.
The Fgf8-rescued thyroids are misshapen as they do not show distinct bilobation. This may be due to at least four reasons. First, Tbx1 ablation affects a number of genes, including other members of the FGF family, most notably Fgf10, which is also required for thyroid development (Ohuchi et al., 2000) and is expressed in the mesoderm. Because deletion of Fgf8 is sufficient to cause thyroid hypoplasia, it is possible that the two ligands have distinct roles in thyroid development. Second, forced expression of Fgf8 from the Tbx1 locus does not rescue any of the major Tbx1−/− phenotypic findings in Tbx1Fgf8/− embryos (Vitelli et al., 2006), including the profound dysmorphogenesis of the pharyngeal apparatus as a whole. Thus, the “rescued” thyroid grows within a highly abnormal anatomical environment, which could explain the abnormal shape. Third, Tbx1Fgf8/− embryos, like Tbx1−/−embryos, do not develop 4th pharyngeal pouches, thus the rescued thyroid would not have the ultimobranchial body-derived component. Fourth, size and bilobation of the thyroid may be regulated by different mechanisms. For example, bilobation may be achieved through physical interactions with arteries (which are abnormal in Tbx1−/− embryos and not rescued by forced expression of Fgf8) , as previously suggested (Alt et al., 2006; Fagman et al., 2007; Liao et al., 2004).
Our data demonstrate an important role for Fgf8 in mammalian thyroid development and are consistent with recent findings in the Zebrafish model (Wendl et al., 2007). In addition, we show that Tbx1 is a critical regulator of Fgf8 expression relevant to thyroid development. A Tbx1-responsive enhancer has been previously demonstrated in the Fgf8 gene (Hu et al., 2004). Thus, Tbx1 regulates, via Fgf8, the size of the early thyroid primordium. We hypothesize that this function is operated by regulating the proliferation of endodermal progenitors of thyrocytes. Direct demonstration of this mechanism will require positive identification of these progenitors, which is not currently possible.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank Drs. R. Di Lauro, G. Martin, E. Meyers, Y. Saga, D. Ornitz, C. Deng and M. Yanagisawa for making available reagents and mouse mutant lines. We thank Dr. Elizabeth Illingworth for critical reading of the manuscript. This work was supported by grants from the NIH (HL064832), the EU (AnEUploidy project), and the Telethon Foundation (to AB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alt B, Elsalini OA, Schrumpf P, Haufs N, Lawson ND, Schwabe GC, Mundlos S, Gruters A, Krude H, Rohr KB. Arteries define the position of the thyroid gland during its developmental relocalisation. Development. 2006;133:3797–3804. doi: 10.1242/dev.02550. [DOI] [PubMed] [Google Scholar]
- Baldini A. Dissecting contiguous gene defects: TBX1. Curr Opin Genet Dev. 2005;15:279–284. doi: 10.1016/j.gde.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Bassett JH, Thakker RV. Molecular genetics of disorders of calcium homeostasis. Baillieres Clin Endocrinol Metab. 1995;9:581–608. doi: 10.1016/s0950-351x(95)80621-0. [DOI] [PubMed] [Google Scholar]
- Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. Embo J. 1998;17:1642–1655. doi: 10.1093/emboj/17.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice DL. Thyroid development and its disorders: genetics and molecular mechanisms. Endocrine review. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- De Felice M, Ovitt C, Biffali E, Rodriguez-Mallon A, Arra C, Anastassiadis K, Macchia PE, Mattei MG, Mariano A, Scholer H, Macchia V, Di Lauro R. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet. 1998;19:395–398. doi: 10.1038/1289. [DOI] [PubMed] [Google Scholar]
- Fagman H, Andersson L, Nilsson M. The developing mouse thyroid: embryonic vessel contacts and parenchymal growth pattern during specification, budding, migration, and lobulation. Dev Dyn. 2006;235:444–455. doi: 10.1002/dvdy.20653. [DOI] [PubMed] [Google Scholar]
- Fagman H, Grande M, Edsbagge J, Semb H, Nilsson M. Expression of classical cadherins in thyroid development: maintenance of an epithelial phenotype throughout organogenesis. Endocrinology. 2003;144:3618–3624. doi: 10.1210/en.2003-0393. [DOI] [PubMed] [Google Scholar]
- Fagman H, Grande M, Gritli-Linde A, Nilsson M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am J Pathol. 2004;164:1865–1872. doi: 10.1016/S0002-9440(10)63745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagman H, Liao J, Westerlund J, Andersson L, Morrow BE, Nilsson M. The 22q11 deletion syndrome candidate gene Tbx1 determines thyroid size and positioning. Hum Mol Genet. 2007;16:276–285. doi: 10.1093/hmg/ddl455. [DOI] [PubMed] [Google Scholar]
- Hu T, Yamagishi H, Maeda J, McAnally J, Yamagishi C, Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131:5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- Huynh T, Chen L, Terrell P, Baldini A. A fate map of Tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis. 2007;45:470–475. doi: 10.1002/dvg.20317. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Liao J, Kochilas L, Nowotschin S, Arnold JS, Aggarwal VS, Epstein JA, Brown MC, Adams J, Morrow BE. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum Mol Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- Meunier D, Aubin J, Jeannotte L. Perturbed thyroid morphology and transient hypothyroidism symptoms in Hoxa5 mutant mice. Dev Dyn. 2003;227:367–378. doi: 10.1002/dvdy.10325. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo JH, Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, De Angelis S, Grandolfo ME, Taruscio D, Cordeddu V, Sorcini M. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991–1998) J Clin Endocrinol Metab. 2002;87:557–562. doi: 10.1210/jcem.87.2.8235. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Sun X, Nichol P, Saijoh Y, Martin JF, Moon AM. System for tamoxifen-inducible expression of cre-recombinase from the Foxa2 locus in mice. Dev Dyn. 2008;237:447–453. doi: 10.1002/dvdy.21415. [DOI] [PubMed] [Google Scholar]
- Parlato R, Rosica A, Rodriguez-Mallon A, Affuso A, Postiglione MP, Arra C, Mansouri A, Kimura S, Di Lauro R, De Felice M. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev Biol. 2004;276:464–475. doi: 10.1016/j.ydbio.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. Deletions of human chromosome 22 and associated birth defects. Curr Opin Genet Dev. 1993;3:432–437. doi: 10.1016/0959-437x(93)90117-8. [DOI] [PubMed] [Google Scholar]
- Scuccimarri R, Rodd C. Thyroid abnormalities as a feature of DiGeorge syndrome: a patient report and review of the literature. J Pediatr Endocrinol Metab. 1998;11:273–276. doi: 10.1515/jpem.1998.11.2.273. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Higgins AM, Antshel K, Fremont W, Roizen N, Kates W. Velo-cardio-facial syndrome. Curr Opin Pediatr. 2005;17:725–730. doi: 10.1097/01.mop.0000184465.73833.0b. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain [letter] Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Zhang Z, Huynh T, Sobotka A, Mupo A, Baldini A. Fgf8 expression in the Tbx1 domain causes skeletal abnormalities and modifies the aortic arch but not the outflow tract phenotype of Tbx1 mutants. Dev Biol. 2006;295:559–570. doi: 10.1016/j.ydbio.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinzimer SA, McDonald-McGinn DM, Driscoll DA, Emanuel BS, Zackai EH, Moshang T., Jr Growth hormone deficiency in patients with 22q11.2 deletion: expanding the phenotype. Pediatrics. 1998;101:929–932. doi: 10.1542/peds.101.5.929. [DOI] [PubMed] [Google Scholar]
- Wendl T, Adzic D, Schoenebeck JJ, Scholpp S, Brand M, Yelon D, Rohr KB. Early developmental specification of the thyroid gland depends on hanexpressing surrounding tissue and on FGF signals. Development. 2007;134:2871–2879. doi: 10.1242/dev.02872. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Hatch EP, Karabagli H, Karabagli P, Schoenwolf GC, Mansour SL. Expression of mouse fibroblast growth factor and fibroblast growth factor receptor genes during early inner ear development. Dev Dyn. 2003;228:267–272. doi: 10.1002/dvdy.10362. [DOI] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129:3033–3044. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.