Abstract
Chronic inflammation-induced carcinogenesis is a commonly accepted entity and is frequently seen within the gastrointestinal tract, although the underlying mechanisms remain unclear. Alterations in specific oncogenes and tumor suppressor genes are known to be responsible for malignant transformation. Nevertheless, the inflammatory microenvironment classically affects tumor promotion in its role as an altered stem cell niche and can also affect tumor initiation and tumor progression. The origin of the tumor cells is often attributed to stem cells, a unique subpopulation within tumors that possess the ability to initiate tumor growth and sustain self-renewal, as well as is largely responsible for their metastatic potential. Here, we review the link between inflammation and gastrointestinal carcinogenesis and the relationship between stem cells and cancer stem cells.
Inflammation Leads to Carcinogenesis
The development of cancer in various organs is often associated with chronic inflammation. In 1863, Virchow hypothesized that the origin of cancer was at sites of chronic inflammation (3). Since chronic injury or inflammation can over decades predispose to neoplastic progression, cancer has long been viewed as “the wound that will not heal” (17, 22). Many malignancies are initiated by tissue injury or chronic inflammation, which are often due to known bacterial, viral, or parasitic infections (56). Overall, ~15% of malignancies worldwide (1.2 million/year) can be attributed specifically to chronic infections (52). The most convincing examples of chronic inflammation-induced carcinogenesis are seen within the gastrointestinal tract (Table 1), where the risk for carcinogenesis increases in the presence of chronic inflammatory conditions such as esophagitis, gastritis, colitis, pancreatitis, and hepatitis (57). The bacterium helicobacter pylori, as an example, is one of the main contributing factors to the development of gastric cancer, the second most common cause of cancer-related mortality worldwide (39, 84).
Table 1.
Inflammation-induced cancer
| Tumor | Inflammation | Etiology |
|---|---|---|
| Gastrointestinal | ||
| Hepatocellular carcinoma | Chronic hepatitis | Hepatitis C and B virus |
| Gastric cancer | Chronic gastritis | Helicobacter pylori |
| Colon cancer | Inflammatory bowel disease | Ulcerative colitis and Crohn’s disease |
| Pancreatic cancer | Chronic pancreatitis | Alcohol |
| Gallbladder carcinoma | Chronic cholecystitis | Bile stones, bacterial infections |
| Esophageal adenocarcinoma | Reflux esophagitis | Gastric and bile acids |
| Non-gastrointestinal | ||
| Lung adenocarcinoma | Tuberculosis | Mycobacterium tuberculosis |
| Pleuramesothelioma | Asbestosis | Asbestos |
| Bronchial carcinoma | Chronic bronchitis | Cigarette smoking |
| Melanoma | Skin inflammation | UV light exposure |
| Cervical cancer | Chronic cervitis | Papilloma virus |
| Kaprosi sarcoma | AIDS | HIV |
| Nasopharyngeal carcinoma | Airway infection | Ebstein Barr virus |
| Lymphoma | Mononucleosis | Ebstein Barr virus |
| Bladder cancer | Schistosomiasis (Bilharziose) | Shistosoma hematobium |
Although the link between inflammation and carcinogenesis has been well established, the underlying mechanisms remain unclear (46). Under normal circumstances, the acute inflammatory response is self-limiting. But abnormal cellular alterations accompanying chronic inflammation, such as oxidative stress, gene mutations, epigenetic changes, and inflammatory cytokine-induced cell proliferation, are proposed to be carcinogenic factors. The classical model for inflammation and cancer suggests that chronic inflammation leads to increased oxidative stress. Leukocytes generate reactive oxygen and nitrogen species normally produced to control infection but when present chronically can induce DNA damage in proliferating cells. In addition, chronic inflammation in the intestine appears to promote apoptosis of normal cells that leads to a compensatory, proliferative response by the remaining tissue (36). In contrast to this proliferative response, irreparable DNA damage, including DNA breaks, oxidative lesions, and telomere shortening, can induce senescence, a state of permanent cell cycle arrest. The senescence response can prevent the growth of cells that are potentially oncogenic but can nevertheless be overcome by loss of additional tumor suppressor genes (13).
Cancer development originating from chronic inflammation may be driven by inflammatory cells and a variety of mediators, which together establish an inflammatory microenvironment (36, 47). Chronic inflammation is characterized by leukocyte infiltration in damaged tissue (FIGURE 1 and Table 2). Initially, neutrophils and tissue mast cells are recruited as part of a multifactorial mechanism that coordinates inflammatory cell involvement. B lymphocytes, CD8+ cytotoxic T lymphocytes, and CD4+ T-helper lymphocytes are responsible for an adaptive immune response, following the acute activation of innate immunity, which involves primarily myeloid cells and dendritic cells (42). Premalignant and malignant tissues are associated with suppressed cytotoxic T lymphocyte responses associated with tumor rejection, in combination with enhanced humoral immunity that can promote tumor progression. B lymphocytes, the central component of this humoral immunity, have been found to inhibit Th1-mediated anti-tumor immune responses (91). For example, in a syngeneic mouse xenograft model of colorectal cancer, partial B-cell depletion resulted in significantly reduced tumor burden (4).
Figure 1. Schematic illustration of the relation of cancer development originating from chronic inflammation.
Inflammatory cells and a variety of mediators together establish an inflammatory microenvironment. Neutrophils and tissue mast cells are recruited (2). B lymphocytes, CD8+ cytotoxic T lymphocytes, and CD4+ T-helper lymphocytes are responsible for an adaptive immune response during the acute activation of innate immunity (3). Mast cells release inflammatory mediators that attract migratory inflammatory cells to the site. Then, monocytes migrate to the area, differentiate into macrophages, and become activated in response to local chemokine and cytokine interactions (3). These alterations in the stem cell niche are responsible for the transformation of stem or progenitor cells to tumor stem cells (4a–c). The origin of cancer stem cells and cancer cells could be tissue (4a, 4c) or bone marrow-derived (4b) stem cells. There are two possible origins for tissue progenitor cells, referred to as label-retaining cells (LRCs) or Lgr5 positive crypt base columnar (CBC) cells between Paneth cells.
Table 2.
Inflammatory cells contribute carcinogenesis
| Cell Type | Function |
|---|---|
| Macrophages | |
| M1 “classical activation” | Strong promoters of Th1 immune responses |
| Antiproliferative and cytotoxic activities | |
| M2 “alternative activation” | Anti-inflammatory macrophages |
| Tumor-associated macrophages (TAM) | Promote tumor progression by:
|
| Lymphocytes | |
| T lymphocytes | Anti-tumor response |
| B lymphocytes | Pro-tumor effects in developing neoplasms |
| Inhibit Th1-mediated anti-tumor immune responses | |
| Inflammatory cells | |
| Neutrophiles | First response of innate immunity |
| Mast cells | Disruption of tissue homeostasis |
Furthermore, mast cells especially play an important role in releasing inflammatory mediators that attract migratory inflammatory cells to the site. Then, monocytes migrate to the area, differentiate into macrophages, and become activated in response to local chemokine and cytokine interactions (77). Tumor progression depends, in part, on tumor-associated macrophages (TAMs), since there is a correlation between tumor-associated macrophage abundance and poor prognosis (21). In addition, macrophage-deficient mice display reduced progression of tumors to a more malignant phenotype (70). With the advent of the M1/M2 concept of macrophage activation, TAMs are generally considered as anti-inflammatory M2, characterized by an IL-10 high/IL-12 low cytokine profile and defective NF-κB activation. But it has become clear that inflammatory M1 significantly participates in carcinogenic processes via the secretion of the M1-associated and NF-κB-regulated mediators, such as TNF-α, IL-1β, and MMP-9 (31). It has to be considered that the relative abundance of M1 or M2 markers in TAMs might be related to the phase of tumor progression.
Cytokines Initiate and Promote Carcinogenesis
The cytokine-expression profile of tumor-associated macrophages could be part of the link between inflammation with tumor progression. In general, key proinflammatory cytokines include IL-1, -6, -8, -11, -12, and -18, IFN-γ TNF-α, and macrophage MIF (migration inhibitory factor). Anti-inflammatory cytokines include IL-4 and -10, and IFNα and β (19). Population-based studies by El-Omar et al. (23, 24) indicate that IL-1β is one of the essential proinflammatory cytokines modulated during H. pylori infection that directs the mucosa toward atrophy, metaplasia, and neoplastic transformation. Interestingly, high expressing gene-cluster polymorphisms in the IL-1 locus have been found in patients with stomach cancer (23, 24). Recent research from our laboratory has demonstrated that transgenic overexpression of the cytokine IL-1<beta in the gastric mucosa is sufficient to induce gastric cancer in uninfected mice (92).
IL-6 family members, such as IL-11, also are crucial cytokines promoting chronic gastric inflammation and are associated with tumorigenesis mediated by excessive activation of STAT3 and STAT1 (25, 43). MIF and IL-6 are both known to ameliorate p53 function, which favors cell survival. IL-6 also induces other anti-apoptotic genes including Bcl-2 and Bcl-XL. The role of IL-6 has become increasingly apparent with respect to colon carcinoma progression (7). IL-6 can inhibit dendritic cell maturation and, together with the NF-κB-activating cytokines IL-1 and TNF, can promote tumor progression (44, 65). Cytokines also affect cell death and cell cycle pathways, and IL-2 and TNF-α are able to induce apoptosis in colon cancer cells. IL-10 is secreted by tumor cells as well as macrophages, and among other effects, it inhibits cytotoxic T-cells and thus aids in suppressing the immune response against the tumor (59). The profile of cytokines existing at an inflammatory site seems to define outcome of chronic inflammation and carcinogenesis. For example, TNF-α, which is produced mainly by macrophages but also by tumor cells, is associated with tissue destruction and plays a role in destroying tumor blood supply (46). However, when chronically produced, it can act as a tumor promoter by contributing to tissue remodeling and stromal development. In many preneoplastic conditions, an inflammatory cell infiltrate is already well established and drives pro-tumor effects (96). Consequently, persistent activation of tumor-associated macrophages can result in continued tissue damage through the stimulation of local tissue remodeling, cellular proliferation, and angiogenesis. This helps to potentiate neoplastic progression and influences cancer cells and, interestingly, bone marrow stem cells (3, 38). Recently, it was shown that both enhanced Wnt expression and infection by gastric microflora induce submucosal infiltration by macrophages secreting high levels of tumor necrosis factor-α (TNF-α). Binding of TNF-α to TNF receptors on gastric epithelial cells enhanced Akt phosphorylation that in turn induced glycogen synthase kinase 3b (GSK3b) phosphorylation, resulting in stabilization and nuclear accumulation of β-catenin that potentiated gastric carcinogenesis. The study describes an additional tumor-promoting role for macrophages, independent of NF-κB-regulated pathways in epithelia, by providing a link between the proinflammatory cytokine TNF-α and Wnt/β-catenin signaling (72).
Nevertheless, there is an important role for NF-κB in the link between inflammation and cancer (30). The upregulated expression of cytokines and growth factors promotes cancer cell proliferation both directly and indirectly by increasing NF-κB-mediated angiogenesis, tumor invasion, and metastasis, with anti-apoptotic proteins protecting against apoptosis and immune attack. In a mouse model of colitis-associated cancer, a deletion of IKK-β (leading to decreased NF-κB activity) in enterocytes showed that the tumor-promoting activity of NF-κB results from its ability to suppress the apoptosis of chemically transformed premalignant cells. In this model, mice were injected with the procarcinogen azoxymethane (AOM) followed by oral administration of dextran-sulphate sodium salt (DSS), which induces chronic colitis. The exposure of macrophages in the lamina propria to enteric bacteria resulted in the activation of NF-κB, leading to the production and secretion of pro-inflammatory cytokines that activated NF-κB in intestinal epithelial cells. Enterocyte-specific IKK-β-mediated inhibition of NF-κB decreased tumor incidence without affecting progression or initiation, indicating that the IKK-β-dependent NF-κB activation pathway operates during early tumor promotion. NF-κB activation also leads to a suppression of autophagy, which is an alternative cell death pathway that is called in to play when apoptosis is inactivated. Furthermore, NF-κB contributes to drug resistance in cancer cells (8, 57).
Classically, inflammation affects tumor promotion, but according to the NF-κB data and our latest findings that IL-1b overexpression can induce gastric cancer (92), inflammation also affects tumor initiation and tumor progression. For tumor promotion, inflammation triggers the clonal expansion of initiated cells, owing to increased cell proliferation and reduced cell death. Finally, chronic inflammation might lead to invasion and metastasis, as well as an increase in tumor size. During the later stages, additional mutations can be acquired, and this leads to the cancer cell gaining a further growth advantage and acquiring a more malignant phenotype (60, 95).
Interaction of Seed and Soil
Alterations in specific oncogenes and tumor suppressor genes have been identified in a number of cancers and shown to have causal roles in the initiation, maintenance, and progression of tumors (28, 93). This genome-centric view of tumor progression, however, has largely ignored the substantial contribution of the tumor microenvironment to the malignant phenotype (62). Although the “seed and soil” hypothesis of Paget (75) dates back to 1889, the molecular determinants of the “seed” are currently much better delineated than those of the “soil” for either primary or metastatic lesions (26).
Tissue stem or progenitor cells are thought to reside within a “niche” or a group of cells and extracellular substrates that provides an optimal microenvironment for normal differentiation (12). Tissue-restricted stem cells are in general difficult to identify morphologically and are not easily distinguished from other epithelial cells by any recognized set of markers, except for perhaps their ability to proliferate and self-renew (10, 11). Stem cells within a niche are present in relatively small numbers and remain largely quiescent, undergoing division at a very slow rate (82). The stem cells usually divide asymmetrically, producing one identical quiescent daughter cell and one transient amplifying cell, which is responsible for the bulk of cell division. Transient amplifying cells appear to have a limited lifespan and are replaced periodically by descendents of the true stem cell. This mechanism of maintaining the stem cell in a relatively dormant state protects the genome from mutations while delegating the genetically dangerous task of repeat replication to a largely dispensable cell. The stem cell niche is believed to be primarily responsible for this slow cell division, protecting the vulnerable stem cells (and their genetic material) from damage or exhaustion and protecting the host from unregulated stem cell outgrowth. On the other hand, alterations in the stem cell niche might be responsible for the transformation of stem or progenitor cells to tumor stem cells (66, 97). For gastrointestinal tumors, the location of the stem cell giving rise to cancer has in most cases not been identified.
A tumor is usually not a homogeneous mass of cancer cells but can contain up to 60–90% stromal cells. A significant source for some of these heterogeneous stromal cells is the bone marrow. Alpha smooth muscle actin (aSMA) expressing myofibroblasts, many of which are bone marrow derived, contribute to cancer-associated fibroblasts (CAF) that express SDF-1 and which in turn recruit endothelial progenitor cells that enhance angiogenesis. Furthermore, macrophages and other leucocytes are also stromal cells that create the aberrant tissue microenvironment (45, 88). Although stromal cells are vital for the survival and growth of the tumor, they themselves are typically not malignant. Interestingly, myofibroblasts are found in conditions and environments other than cancer, such as the purported stem cell niche of solid organs such as the colon. However, they are found in increased number in chronically inflamed tissues and preneoplastic lesions, where they may contribute to the local production of growth factors and chemokines (73, 74).
Cancer Stem Cells Initiate Tumor Growth
A tumor can be viewed as an aberrant organ initiated by a tumorigenic cancer cell that acquired the capacity for indefinite proliferation through accumulated mutations. The classical theory that epithelial cancers such as gastric carcinoma arise from resident epithelial cells dates back to the 19th century and can be attributed to the work of Waldeyer. The theory that cancer in adult develops from stem cells represents a modern interpretation of the “embryonal rest theory” developed by Julius Cohnheim in 1867 (32). This cancer stem cell hypothesis suggests that cancer arises from resident tissue stem cells or their early descendents (e.g., restricted progenitors) and that the tumor can be viewed as an aberrant but heterogeneous organ, in which only a small subset of cancer cells, the “cancer stem cells,” are capable of extensive proliferation and metastatic spread (82). If one views a tumor as an abnormal organ, then the principles of normal stem cell biology can be applied to understand better how tumors develop. Both normal stem cells and tumorigenic cells give rise to phenotypically heterogeneous cells that exhibit various degrees of differentiation (54). Thus tumorigenic cells can be thought of as cancer stem cells that undergo an abnormal and poorly regulated process of organogenesis analogous to what normal stem cells do (50). Cancer stem cells are defined as the unique subpopulation in the tumors that possess the ability to initiate tumor growth and sustain self-renewal as well as metastatic potential (15, 87). Colon cancer stem cells, for example, were believed to originate from a rare population of putative CD133+ colonic stem cells. Nevertheless, recent findings suggest that, in several human colon tumors, EpCAM and CD44 are perhaps more robust markers of colon cancer stem cells than CD133 because CD44 appeared to be informative in tumors that do not express CD133. Furthermore, in several CRC tumors, including both xenografts and primary tumors, CD166 can be used for further enrichment of colon cancer stem cells within the EpCAMhigh/CD44+ population (18). Since CD44 is a well established, immature differentiation marker in human colonic mucosa, this underlines the hypothesis that cancer stem cells arise from tissue stem cells that, although genetically monoclonal in origin, differ in their functional state of differentiation. Other studies have supported this view that CD133 is not a specific marker and that a subset of cells in colon cancer are negative for CD133, consisting of primarily nontumorigenic stromal and inflammatory cells (37). Although recent findings support the existence of human gastric cancer stem cells, the precise origin and surface markers have yet to be elucidated (90).
Concerning the origin of cancer stem cells and cancer cells, three types of cells have to be taken into account: 1) tissue or bone marrow-derived stem cells, 2) tissue progenitor cells, and 3) normal, differentiated tissue cells. The latter appears to be the most unlikely but has to be considered since reprogramming of fibroblasts and lymphocytes is possible (33, 89). Concerning the stem cell possibilities, resident adult or tissue stem cells may, in a chronically inflamed environment, slowly acquire a series of genetic and epigenetic changes that lead to their emergence as cancer stem cells. Alternatively, the setting of chronic inflammatory stress and injury may lead to loss of the indigenous stem cells from their niches; bone marrow-derived stem cells may then be recruited to and engraft into the gastric epithelium. Such recruited cells would have the potential to contribute to the tumor mass.
Bone Marrow-Derived Cells Can Contribute to Epithelial Cancer
Stem cells are now thought to span a spectrum from cells of the zygote through embryonic stem (ES) cells to more lineage-restricted adult tissue stem cells. Bone marrow-derived stem cells, while not pluripotent like ES cells, possess a wide range of plasticity and tend to migrate through peripheral organs as a result of inflammation and tissue injury (82). The differentiation pattern and growth regulation of these cells may depend largely on local environmental signals and cues (66). Adult somatic stem cells are defined by two major properties: the ability to generate more stem cells (self-renewal) and the ability to generate differentiated cell lineages (multi lineage differentiation). To establish the presence of these two properties, the gold standard is to assess both of them in vivo and in vitro (54). Several studies have shown in vitro that many tissues carry cells capable of self-renewal and of giving rise to differentiated cell types. Recently, the identification of circulating progenitor cells capable of functioning as lineage-specific stem cells (such as endothelial progenitors) raises questions as to whether distinct and unique stem cell populations exist for each organ or tissue or whether a more centralized source of stem cells exists, with the organ-specific niche the ultimate determinant of stem cell function (40, 51, 53).
Bone marrow-derived epithelial cells have been identified in the lung, gastrointestinal tract, and skin of mice after transplantation of a single purified hematopoietic bone marrow-derived stem cell. In the gastrointestinal tract, engrafted cells were present as rare isolated epithelial cells in the esophagus, the small intestinal villi, the colonic crypt, and the gastric pit of the stomach. Research conducted in our laboratory found in a mouse model of gastric cancer that bone marrow-derived cells (BMDC) contribute to at least parts of both the neoplastic glands originated from BMDCs (38) and more recently to carcinoma-associated fibroblasts (unpublished observations). This model might be restricted to cancers that arise after inflammatory tissue destruction, such as after severe gastric ulceration, and it remains unclear how bone marrow-derived cells undergo malignant conversion after arrival at the gastric mucosa. It has been suggested that the apparent stem cell plasticity may be explained by fusion between a bone marrow-derived and a peripheral cell. In 1911, Aichel first proposed that the source of aneuploidy could be fusion of tumor-invading leukocytes with cancer cells. Two recent puplictions elegantly extend previous findings on so-called heterotypic cell fusion (41, 71). Inflammation seems to be a trigger for fusion of myelolymphoid cells with non-hematopoietic cells, including cardiomyocytes, skeletal muscle, hepatocytes, and Purkinje neurons. Both studies also indicate that heterokaryon formation is a slow process occurring over many weeks of chronic inflammation (41, 71, 94). Another potential explanation for cell fusion might be a role for molecular mediators from macrophages in chronic inflammation. It has been shown in the colon that transplanted bone marrow-derived cells fuse with both normal and neoplastic intestinal epithelium (83). Long-term repopulation by donor-derived cells was detected in all principal intestinal epithelial lineages, including enterocytes, goblet cells, Paneth cells, and enteroendocrine cells, suggesting that the fusion partners of the bone marrow-derived cells are long-lived intestinal progenitors or stem cells. Interestingly, fusion of bone marrow-derived cells with neoplastic epithelium did not result in tumor initiation.
Nevertheless, human studies following gender discordant allogeneic stem cell transplantation have provided supporting evidence that epithelial malignancies can arise from donor cells or BMDCs. In a case report, a child developed a de novo metastatic renal cell carcinoma after allogenic liver and bone marrow transplantation, and tumor cell hybridization with donor bone marrow cells was suggested as a possible explanation (14). Furthermore, donor-derived cancers were described in patients who had undergone not bone marrow but kidney transplants (1). Kidneys are known to harbor mesenchymal progenitor cells that show some degree of multipotency and multilineage differentiation (76). Another report of two women with colonic neoplasia after hematopoietic cell transplantation from male donors showed that, with 4% of adenoma epithelial cells, bone marrow-derived cells directly contribute to neoplastic cells in, for example, colonic adenomas and other secondary tumors (16). The most recent study investigated four male patients who developed solid organ cancers 1–7 years following total body irradiation and bone marrow reconstitution from female donors (2). Donor-derived cancer cells were observed in 2.5–6% of the tumor cellularity, indicating a mixture of donor and recipient cells, but with regions that individually were clearly clonal in origin consistent with the emerging view that many tumors begin as polyclonal lesions.
Taken together, evidence is emerging that bone marrow-derived stem cells directly contribute to several human tumors and have a role in solid organ carcinogenesis, where they can contribute directly to the neoplastic lineage. Chronic inflammation appears to increase homing of bone marrow-derived stem cells, macrophages, or myofibroblasts within these peripheral sites and may actually be required for successful engraftment (2, 16).
Furthermore, bone marrow-derived endothelial progenitor cells can contribute directly to angiogenesis in tumor formation (20). Malignant transformation and the continued growth of a malignant cell require a fertile microenvironment. Myofibroblasts and endothelial cells have been shown to derive in part from circulating bone marrow progenitors (74). Inflammatory cells and carcinoma-associated fibroblasts are important cells within the peritumoral stroma, helping to promote an environment permissive of tumor growth, invasion, and angiogenesis. Together with the tumor cells, they release factors responsible for the mobilization of bone marrow-derived endothelial progenitor cells and induce them to migrate and become incorporated into the developing vasculature of the tumor. Karnoub et al. recently reported that bone marrow-derived human mesenchymal stem cells, when mixed with otherwise weakly metastatic human breast carcinoma cells, cause the cancer cells to increase their metastatic potency greatly, through stimulation of de novo secretion of the chemokine CCL5, when this cell mixture is introduced into a subcutaneous site and allowed to form a tumor xenograft (48).
Locating the Stem Cell and Tumor Stem Cell
Stem cells in the gut are located in specific sites, and a realization has emerged that stem cells should be found in the areas of high cell turnover. In the small intestinal crypt, cell migration begins at the base of the crypt, and cells migrate from here, emerging onto the villi, indicating that basal crypt cells could be candidates for stem cells. The microenvironment for a specific cell position is thought to be supportive of the stem cell state (stem cell niche), but stem cells moving up to position 5 or above are induced to commence a differentiation program. Most of their progenitor offspring supply cells to the villus, but Paneth cells and a subset of other cell types migrate back down into the stem cell zone or below it (58). In the gastric glands, cellular proliferation is confined to the middle portion of the tubule, and cells are thought to migrate bidirectionally to supply cells to the gastric surface and the base of the gland (80). In the colon, although the same concept of basally sited stem cells has also been proposed, bidirectional migration may also occur here (97).
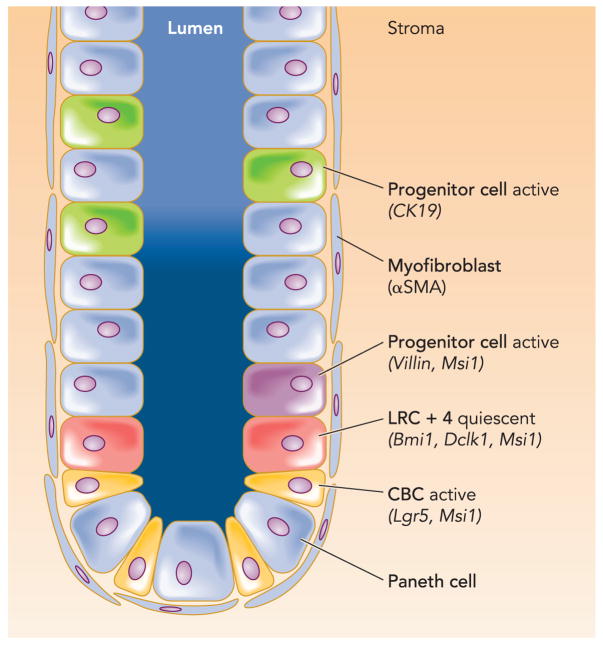
There remains a lack of specific markers to definitively identify stem cells in situ, although some of the characteristics have been inferred from morphological and lineage tracing analyses (9). Additionally, in the stomach, labeling studies with thymidine analogs have identified a highly proliferative zone near the isthmus of the gland, and electron microscopic studies have presumed immature looking cells in this region. Studies from other stem cell systems indicate that adult stem cells in general were either in a prolonged quiescent state or extremely slow cycling (68, 69, 78). Therefore, long-term label retention was developed to assist localization of putative stem cells, referred to as label-retaining cells (LRCs), and LRCs or putative intestinal stem cells to a position of four cells up from the crypt base, directly above the Paneth cell zone (one refers to this cell type as a +4 LRC) (79). Until recently, this slow cycling +4 LRC was generally accepted as the putative intestinal stem cell (FIGURE 2) (58, 80).
Figure 2.
Schematic illustration of the location of putative small intestinal stem cell and progenitor cell markers
Recently, a single marker, Lgr5/GPR49, a leucine-rich orphan G protein-coupled receptor, was identified to specifically label stem cells in the mouse small intestine in the crypt base columnar (CBC) cells between Paneth cells (6). This research has reactivated the still unsolved discussion over the location of intestinal stem cells. Such experiments, which locate stem or progenitor cells in the gut, are done via lineage tracing studies. By engineering mice with a tamoxifen-activated version of Cre recombinase knocked into the specific locus, one can induce an irreversible mark in the DNA of cells that allows genetic tracing of their lineage. This elegant technique allows one to characterize true stem cells (entire crypt will be marked forever) and progenitor cells (crypt loses the marker with complete renewal) but has technical limitations related to the Tamoxifen induction, possible unknown effects of the marker gene on stem cell fate, and a lack of the ability to estimate the asymetric and symmetric cell division.
Studying cell renewal on induction of Cre recombinase activity, Lgr5 cells appeared to be multipotent for all mature intestinal epithelial cells, to undergo self-renewal, to persist for several months, and to be resistant to irradiation. Thus these cells at least possess intestinal stem cell characteristics, proliferate rapidly, and thus stand in contrast to the previously held belief that adult stem cells are slow cycling or maintained in a prolonged quiescent state. Assuming a quiescent intestinal stem cell would explain the intestines’ resistance to radiation, but the fact that the Lgr5 cells are actively proliferating and also resistant to radiation are two characteristics that are still unresolved discrepancies. It is difficult to imagine that stem cells undergo replication so often and would therefore be more likely to develop and accumulate mutations. Nevertheless, when crossed to APCmin mice, Lgr5 expression was restricted to a small number of cells within large adenomas, in contrast with other Wnt target genes, which typically exhibit a uniform high-level expression throughout these tumors. Lgr5 therefore also marks a limited population of cells within colon cancers, which might be cancer stem cells (5).
The more recent identification of another marker for at least a subset of gastric progenitors used a tagged allele of the endogenous villin promoter to visualize single β-galactosidase-positive cells located in the lower third of antral glands (81). Although this rare and quiescent gastric progenitor cell population is most likely not the true stem cell in the antrum, it shows an impressive proliferative response to inflammation (IFNγ injections, radiation) and therefore fits the description for a type of potential progenitor population that could be mobilized to undergo symmetric division to amplify stem cell numbers after noxious insult. In the antrum of adult mice and human beings, the majority of glands are functionally monoclonal (63). The resolution of a mixed gland to a monoclonal state is promoted by gland fission, which is when the gland bifurcates, producing two daughter glands, each with half of the stem cell census of the original (55). Inflammation and/or cell proliferation seem to be responsible for the signal that initiates gland fission, and it has been reported that intestinal crypts divide in response to a doubling of stem cell number. In intestines of mice carrying a conditional phosphatase and tensin homolog (PTEN) deletion, stem cell numbers are increased, and this appears to directly promote crypt budding as well as crypt fission (34). Since the rate of crypt fission is greatly increased with inflammatory states given in Crohn’s disease, ulcerative colitis, and gastritis, representing potentially premalignant conditions, it is intriguing that villin marked progenitor cells increase with inflammation (IFNγ injections) and seem to promote fission of the gland carrying the recently divided villin marked progenitor cells.
In another investigation of gastrointestinal stem cells, Sangiorgi and Capecchi characterized the progeny of crypt Bmi1-positive cells using the same lineage tracing strategy (85). Bmi1 encodes a chromatin remodeling protein of the polycomb group that plays essential roles in self-renewal of hematopoietic and neural stem cells. Activation of Cre recombinase expressed from the Bmi1 locus in this study consistently marks long-lived cell clones (>12 mo) populated by all intestinal lineages and serves as a specific marker of a cell population located at the +4 position of the crypt. Furthermore, ablation of Bmi1+ cells by targeted expression of the diphtheria toxin depletes the epithelium of whole crypt units. Thus expression of Bmi1 also identifies intestinal stem cell candidates. The induction of cancer in adult animals via stabilized β-catenin expression in these cells again indicates that tumors arise from multipotent cells in the intestine.
One other study characterized the progeny of K19-positive epithelial cells using the lineage tracing strategy. The K19 marked cells appeared to distribute randomly all over the intestinal epithelium at early time points after Cre recombinase induction and marked nearly the complete epithelium after longer time period, thus indicating that crypts are at least monoclonal and arise from one progenitor cell that can be marked with K19 (64).
Many studies have identified molecules that could be candidates for specific stem cell markers, for which lineage tracing has not been carried out so far. For example, Musashi-1 expressing cells include +4 LRCs and CBCs (49, 67). Although Musashi-1 functions as a messenger RNA binding protein and inhibits transcription of mNumb, a Notch signaling pathway inhibitor, it most likely does not play a functional role in intestinal stem cell or progenitor cell regulation or intestinal differentiation. sFRP5, a Wnt signaling antagonist known to be expressed in quiescent skin stem cells, is also present at the mRNA level in +4 cells (29). In addition, PTEN and P-Akt, as well as P-β-catenin are predominantly expressed in +4 LRCs (34, 35).
In another study, a promising new putative stem cell marker, doublecortin, and CaM kinase-like-1 (DCAMKL-1), a microtubule-associated kinase that was known to be expressed in neurons, was discovered in gut epithelial progenitors (27) but has not been lineage traced so far. These cells were retrieved by laser capture microdissection of cryosections prepared from the corpus of the stomachs of germ-free transgenic mice with an engineered, attenuated diphtheria toxin A fragment-mediated ablation of their parietal cells. Dcamkl1 marks single cells adjacent to the isthmal stem cell niche of gastric units (+4 LRC). Solitary Dcamkl1-positive cells do not express biomarkers associated with differentiating members of the enteroendocrine parietal or pit cell lineages but co-express glycansrecognized by the neck cell-specific lectin GSII. Moreover, the fractional representation of Dcamkl1-positive cells was increased in parietal cell-deficient mice (Atpb4-tox176 mice), which shows an increased proliferation of gastric epithelial lineage progenitors. Dcamkl1-positive cells were juxtaposed to rapidly cycling BrdUrd-positive progenitors but were quiescent themselves. Another recent study also identified DCAMKL-1 in the intestinal stem cell zone (+4 LRC) and observed stem cell apoptosis and mitotic DCAMKL-1-expressing cells 24 h after irradiation (61). Moreover, in APC/min mice, DCAMKL-1-expressing cells were not among the proliferating cells, and nuclear translocation of β-catenin distinguished normal and adenoma DCAMKL-1 positive cells.
It is indeed confusing that different markers characterize different types of intestinal stem cell candidates, which all fulfill some of the criteria of stemness. Lineage tracing studies so far have identified one intestinal stem cell candidate with the quiescent feature of adult stem cells at the +4 LRCs region (Bmi1), whereas other studies based on functional and genetic evidence have pointed on cells within the crypt base columnar cells (Lgr5) or in the bottom third of the crypts (villin). It is possible that a substantial fraction of Lgr5+ cells may also score positive for Bmi1, villin, and likely K19 expression. Another notable difference is that Lgr5 and villin are expressed throughout the gastrointestinal tract, whereas Bmi1 expression is restricted to the most proximal half of the small intestine.
In a recent review, David Scoville et al. proposed a model of two types of gastrointestinal stem cells: quiescent stem cells at the traditional +4 locations in a prolonged quiescent state, reflecting their inhibitory microenvironment, and the active Lgr5 positive stem cells, representing a population of stem cells more ready to respond to stimulating signals generated from adjacent mesenchymal cells (86). Whether it may be that, in rapidly renewing adult tissues, two stem cell compartments coexist and work coordinately has to be investigated. Nevertheless, the more active stem cell type could serve to maintain the regenerative capacities of these tissues under homeostatic conditions, whereas the other, less affected by environmental stress because of its quiescent state, is held in reserve.
In summary, there has been some progress in identifying gastrointestinal stem or progenitor cells that can contribute to intestinal carcinogenesis. The most important task for future studies will be to find one or multiple markers for the genuine long-lived gastrointestinal stem cell populations and distinguish them from short-lived progenitor cell populations. In addition, further work is needed to clarify the role of bone marrow-derived cells, which have been demonstrated in the stomach to contribute to carcinogenesis arising from chronic inflammation. Nevertheless, the possibility that epithelial and bone marrow-derived stem cells are related or even interchangeable needs to be considered. The link between inflammation and carcinogenesis is not completely understood but seems to be a consistent predisposing factor. Although there has been some progress, there is still a need for further detailed examination of the underlying mechanisms to initiate carcinogenesis and promote tumor progression.
Acknowledgments
T. C. Wang is supported by National Cancer Institute Grants 1U54 CA-126513, RO1 CA-093405, and R01 CA-120979. M. Quante is supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Germany.
References
- 1.Aractingi S, Kanitakis J, Euvrard S, Le Danff C, Peguillet I, Khosrotehrani K, Lantz O, Carosella ED. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005;65:1755–1760. doi: 10.1158/0008-5472.CAN-04-2783. [DOI] [PubMed] [Google Scholar]
- 2.Avital I, Moreira AL, Klimstra DS, Leversha M, Papadopoulos EB, Brennan M, Downey RJ. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–2909. doi: 10.1634/stemcells.2007-0409. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 4.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr, Feng L, Sampsel JW. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 7.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 9.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 10.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth D, Haley JD, Bruskin AM, Potten CS. Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int J Cancer. 2000;86:53–59. doi: 10.1002/(sici)1097-0215(20000401)86:1<53::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K, Goldman J, Alison MR, Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty A, Lazova R, Davies S, Backvall H, Ponten F, Brash D, Pawelek J. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–186. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 15.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells: perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 16.Cogle CR, Theise ND, Fu D, Ucar D, Lee S, Guthrie SM, Lonergan J, Rybka W, Krause DS, Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells. 2007;25:1881–1887. doi: 10.1634/stemcells.2007-0163. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 20.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan LM, Richards LA, Mihm MC., Jr Increased mast cell density in invasive melanoma. J Cutan Pathol. 1998;25:11–15. doi: 10.1111/j.1600-0560.1998.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 23.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743–747. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- 25.Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, Marcusson EG, Karras JG, Na S, Sedgwick JD, Hertzog PJ, Jenkins BJ. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 27.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 28.Goessel G, Quante M, Hahn WC, Harada H, Heeg S, Suliman Y, Doebele M, von Werder A, Fulda C, Nakagawa H, Rustgi AK, Blum HE, Opitz OG. Creating oral squamous cancer cells: a cellular model of oral-esophageal carcinogenesis. Proc Natl Acad Sci USA. 2005;102:15599–15604. doi: 10.1073/pnas.0409730102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 32.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 33.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 36.Hold GL, El-Omar ME. Genetic aspects of inflammation and cancer. Biochem J. 2008;410:225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- 37.Houghton J, Morozov A, Smirnova I, Wang TC. Stem cells and cancer. Semin Cancer Biol. 2007;17:191–203. doi: 10.1016/j.semcancer.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 39.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 41.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Judd LM, Bredin K, Kalantzis A, Jenkins BJ, Ernst M, Giraud AS. STAT3 activation regulates growth, inflammation, and vascularization in a mouse model of gastric tumorigenesis. Gastroenterology. 2006;131:1073–1085. doi: 10.1053/j.gastro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 46.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 47.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 49.Kayahara T, Sawada M, Takaishi S, Fukui H, Seno H, Fukuzawa H, Suzuki K, Hiai H, Kageyama R, Okano H, Chiba T. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 50.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 51.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 52.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 53.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 54.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 55.Loeffler M, Birke A, Winton D, Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J Theor Biol. 1993;160:471–491. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- 56.Macarthur M, Hold GL, El-Omar EM. Inflammation and cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- 57.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 59.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 60.Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 61.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 62.McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol. 2001;11:25–27. doi: 10.1016/s0960-9822(00)00038-5. [DOI] [PubMed] [Google Scholar]
- 63.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA, Wright NA. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 64.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 66.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murata H, Tsuji S, Tsujii M, Nakamura T, Fu HY, Eguchi H, Asahi K, Okano H, Kawano S, Hayashi N. Helicobacter pylori infection induces candidate stem cell marker Musashi-1 in the human gastric epithelium. Dig Dis Sci. 2008;53:363–369. doi: 10.1007/s10620-007-9858-5. [DOI] [PubMed] [Google Scholar]
- 68.Nomura S, Esumi H, Job C, Tan SS. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–135. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 69.Nomura S, Kaminishi M, Sugiyama K, Oohara T, Esumi H. Clonal analysis of isolated intestinal metaplastic glands of stomach using X linked polymorphism. Gut. 1998;42:663–668. doi: 10.1136/gut.42.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, Maruszynski M, Aukerman SL, Wiktor-Jedrzejczak W. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer. 1996;65:112–129. doi: 10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 71.Nygren JM, Liuba K, Breitbach M, Stott S, Thoren L, Roell W, Geisen C, Sasse P, Kirik D, Bjorklund A, Nerlov C, Fleischmann BK, Jovinge S, Jacobsen SE. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 72.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671–1681. doi: 10.1038/emboj.2008.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 74.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 75.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 76.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol. 2006;291:F902–F912. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 77.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 78.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 79.Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 80.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 81.Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 83.Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA. 2006;103:6321–6325. doi: 10.1073/pnas.0508593103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5(Suppl 1):5–11. doi: 10.1007/s10120-002-0203-6. [DOI] [PubMed] [Google Scholar]
- 85.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 87.Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 90.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 95.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang XF. Immunology of stem cells and cancer stem cells. Cell Mol Immunol. 2007;4:161–171. [PubMed] [Google Scholar]
- 97.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]