Abstract
Background and Purpose
Hyperglycemia strongly predicts poor outcome in patients with aneurysmal subarachnoid hemorrhage (aSAH) but the effect of hyperglycemia management on outcome is unclear. We studied the impact of glycemic control on outcome of patients with aSAH.
Methods
A prospective intensive care unit database was used to identify 332 hyperglycemic aSAH patients admitted between January 2000 and December 2006. Patients treated with aggressive hyperglycemia management (AHM) protocol after 2003 (N=166) were compared with 166 patients treated using a standard hyperglycemia management (SHM) prior to 2003. Within the AHM group, outcome was compared between patients who achieved good (mean glucose burden < 1.1 mmol/L) and poor (mean glucose burden ≥1.1 mmol/L) glycemic control. Poor outcome was defined as modified Rankin scale ≥ 4 at 3–6 months. Multivariable logistic regression models correcting for temporal trend were used to quantify the effect of AHM on poor outcome.
Results
Poor outcome in AHM-treated patients was lower (28.31% vs. 40.36%) but was not statistically significant after correcting for temporal trend. However good glycemic control significantly reduced the incidence of poor outcome (OR 0.25, 95% CI [0.08, 0.80], p = 0.02) compared to patients with poor glycemic control within the AHM group. No difference in the rate of clinical vasospasm or the development of delayed ischemic neurological deficit was seen before and after AHM protocol implementation.
Conclusion
AHM results in good glucose control and significantly reduces the odds for poor outcome after aSAH in glucose-controlled patients. Further studies are needed to confirm these results.
Key Words: Subarachnoid hemorrhage, Intracranial aneurysm, Critical Care, Hyperglycemia, Outcome
The incidence of aneurysmal subarachnoid hemorrhage (aSAH) is 6–10/100,000 per year or about 30,000 people per year, accounting for up to 3% of all strokes and approximately 5% of stroke-related deaths.1 Women are preferentially affected with a mean age at presentation of 55 years.2 Approximately 51% die in the first 30 days. Of the survivors, 33% require life long care3 while only 50% achieve good outcome.4 Due to the substantial burden on health care resources, strategies to improve outcome is a desirable goal.
There is a growing body of experimental and clinical literature showing a significant association between persistent hyperglycemia and poor outcomes in different acute medical and surgical conditions.5–7 In aSAH, hyperglycemia has been linked to development of clinical vasospasm8 and poor outcome.9 Intensive insulin therapy has been shown to improve outcome in non-selected intubated patients in both medical and surgical intensive care units, with acceptable rate of complications related to hypoglycemia.10, 11 Hyperglycemia represents a very common problem in aSAH, occurring in 70–90% of the patients.8 Thus, treatment of persistent hyperglycemia has become an attractive management strategy to improve outcomes in aSAH patients.
Glucose management in patients with acute brain injury is complicated by the complex relationship of systemic and brain-specific factors governing the transport and utilization of glucose. Since human neurons are insulin-insensitive cells,12 the cellular uptake of glucose by neurons is not increased by insulin and is primarily regulated by supply.13 Lowering systemic glucose levels, for example with intensive insulin therapy, can potentially reduce extracellular glucose levels in the brain.14 Low levels of brain extracellular glucose have been associated with worse neurological outcome.15 Current recommendations advocate for a less restrictive target for systemic glycemic control in acutely brain-injured patients.16 This study investigated the effects of aggressive insulin therapy and resultant tight glycemic control on patient outcomes after aSAH.
Methods
Study Design
Using a prospectively collected institutional review board-approved database of patients admitted to the Massachusetts General Hospital for the management of SAH, we retrospectively identified all SAH patients that were hospitalized between January 1, 2000 and December 31, 2006 and met the following inclusion criteria: (1) age > 18; (2) survival through the first 72 hours of hospitalization; (3) documented aneurysm as the cause of SAH; (4) aneurysm repair by either endovascular coiling or surgical clipping within 72 hours of ictus; (5) hyperglycemia defined as blood sugar > 11.1 mmol/L (200 mg/dL) on admission or the first-24-hour mean blood sugar > 7.8 mmol/L. Two patient groups were subsequently identified: (1) patients admitted prior to 2003 and treated with standard hyperglycemia management (SHM), targeting the blood glucose levels to less than 11.1 mmol/L (200 mg/dL); and (2) patients treated after 2003 and treated with aggressive hyperglycemia management (AHM), targeting the blood glucose levels between 4.4 – 7.8 mmol/L (80–140 mg/dL). Prior to 2003, all patients were treated with SHM. In 2003, we developed a new institutional protocol (see Table 1) for aggressive hyperglycemia management. No other major changes in the management of aSAH patients occurred during the study period. Patients admitted during the year 2003 were excluded from the study in order to reduce the differential effects of process implementation and intensive care staff learning curve. Patients who received AHM were further categorized as achieving good glycemic control (mean glucose burden < 1.1 mmol/L [20 mg/dL], or poor glycemic control (mean glucose burden ≥ 1.1 mmol/L). Comparisons between the SHM and AHM groups were performed in order to establish any direct benefits of tight glycemic control on patient outcome. A secondary within-group analysis comparing patients who achieved good glycemic control with those who failed to achieve the target glucose level was also performed.
Table 1.
Protocol for Aggressive Hyperglycemia Management
Patient Management
|
Study population
A total of 332 patients met the inclusion criteria for the study out of 1139 patients admitted for management of SAH during the aforementioned time period. Of the 807 patients excluded, 261 had non-aneurysmal SAH, 174 were treated beyond 72 hours from symptom onset, 161 were admitted in 2003 during protocol development, 139 died in the ICU within 72 hours, 53 had normal blood sugar on admission and/or in first 24 hours, and 19 had incomplete records.
Standardized Clinical SAH Treatment Protocol
At our hospital, all SAH patients are treated according to a standardized clinical protocol. Patients are assigned a Hunt and Hess (HH) clinical score and Fisher grade based on their initial clinical presentation and head computed tomography (CT) imaging, respectively. Patients with known intracerebral aneurysm are treated with either surgical clipping or endovascular coiling within 24 hours of admission. The decision to clip or coil is based on collective decision among neurosurgery, neurointerventional and neurocritical care team. Patients with no visible aneurysm on conventional four-vessel cerebral angiography undergo repeat diagnostic cerebral angiography 7 days post presentation. All patients are monitored closely in the Neuro-Intensive Care Unit (NICU) until resolution of vasospasm or post hemorrhage day 10 (if they do not develop vasospasm). Patients receive continuous blood pressure monitoring with arterial catheter and continuous central venous pressure monitoring with a central venous catheter. Extraventricular drains (EVD) are placed prior to or during aneurysm repair in patients with clinical and radiographic evidence of hydrocephalus. Phenytoin is administered upon hospital admission and discontinued after the aneurysm has been secured or if the patient is awake and following commands. All patients are treated with a 21-day course of oral nimodipine. Hyperthermia is treated with acetaminophen and surface cooling to euthermia. Euvolemia with target central venous pressure of 8–10 mmHg is maintained. Patients are kept nothing per orem (NPO) and non-glucose containing intravenous fluids given in the first 24 hours. Enteral nutrition is started as soon as possible after the aneurysm is secured.
Patients receive daily transcranial Doppler (TCD) ultrasound screening for vasospasm. All patients with clinical, TCD, or angiographic vasospasm are treated with hemodynamic augmentation (systolic blood pressure >160 mmHg) and hypervolemia (central venous pressure >8 cm H2O) until the resolution of clinical and angiographic vasospasm. Those with medically refractory vasospasm are treated with balloon angioplasty or intra-arterial infusion of vasodilators (nicardipine and/or milrinone).
Data Collection
Electronic medical records of included patients were reviewed. The following demographic and clinical characteristics were recorded: age, sex, Hunt and Hess grade, Fisher group, aneurysm location and size, number of aneurysm, medical history of hypertension, diabetes, heart disease and stroke, presenting symptoms of sentinel headache, seizure, loss of consciousness and prehospital worsening, admission CT evidence of infarction, intraparenchymal bleed, hydrocephalus, cerebral edema, intraventricular hemorrhage and midline shift, and admission glucose level.
Important aspects of patient management were also documented and included: need for emergent extraventricular drainage (EVD), interval between symptom onset and aneurysm repair, method of aneurysm repair (clipping vs. coiling), blood transfusion, hemodynamic augmentation (HHH) therapy, perioperative steroid use and pattern of insulin use.
Outcome measures
Good and poor outcomes were defined as modified Rankin scale < 4 and ≥4, respectively and data were obtained at 3–6 months follow-up. In patients who did not have a follow-up record, the outcome was based on physical therapy notes recorded at the time of discharge. The occurrence of symptomatic vasospasm (defined as any TCD peak velocity >200cm/sec or radiologic evidence of vessel narrowing >25% from baseline or >50% from normal if no baseline study, associated with clinical deterioration in the absence of other causes such as worsening hydrocephalus, rebleeding, seizures, infection, other systemic illness)8 and delayed ischemic neurologic deficits (DINDs, defined as persistent neurologic abnormality and/or new CT or MRI evidence of ischemic infarction) were documented. Complications developing during hospitalizations were identified. Rebleed was defined as new or increased intracranial blood content from baseline; seizure was defined as clinical convulsive event or electroencephalogram (EEG) evidence of rhythmic epileptogenic activity; cardiac dysfunction was defined as new evidence of congestive heart failure (documented by abnormal wall motion contractility or hypokinesia and ejection fraction < 40% with clinical or radiographic evidence of pulmonary congestion) and/or development of acute myocardial infarction (documented by new electrocardiogram (ECG) evidence of myocardial injury associated with troponin elevation above 0.1 ng/mL and/or new segmental hypokinesia on echocardiography); respiratory failure was defined as hypoxemia requiring ventilatory support, or failure to extubate within 24 hours post-operatively; infection was defined as development of pneumonia (radiologic evidence of airspace disease associated with pathologic bacterial sputum growth and systemic inflammatory response) or CNS infection (CSF culture positive for pathologic bacteria/fungi); renal failure was defined as >50% elevation in baseline creatinine or azotemia requiring renal replacement therapy; venous thromboembolism was defined as sonologic or radiologic evidence deep venous thrombosis or development of pulmonary embolism. Hypoglycemia was defined as blood glucose < 3.88 mmol/L (70 mg/dL). Hospital and ICU length of stays were also documented.
Glucose burden
Admission blood glucose was recorded in all patients. Daily mean blood glucose was calculated by averaging the measured blood glucose for the day between day 1 and day 14. Overall mean blood glucose during ICU stay was calculated by averaging the daily mean blood glucose in the ICU. Daily mean glucose burden was defined as the daily mean blood glucose in excess of 7.8 mmol/L (140mg/dL). The reference glucose value of 7.8 mmol/dL was chosen based on our protocol and on prior studies.9 Total mean glucose burden was calculated by averaging the daily glucose burden. Poor glycemic control was defined as total mean glucose burden in excess of 1.1 mmol/L (20 mg/dL) above the target glucose level.
Statistical Analysis
All data were analyzed with SAS for Windows software version 9.1.3 (SAS Institute Inc., Cary, NC, USA). Categorical data was analyzed using Fisher’s exact test or Chi-square tests as appropriate. Continuous data with normal distribution were reported as mean ± standard deviation and analyzed with Student’s t-test, while those with non-normal distribution were reported as median (interquartile range) and analyzed with Wilcoxon 2-sample test. The primary outcome was poor clinical outcome. Secondary outcomes included clinical vasospasm, DINDs, medical complications, and length of stay in the hospital and NICU.
The crude effect of aggressive hyperglycemia management on dichotomized primary and secondary outcome was determined using a Chi-square analysis. Multivariable adjusted logistic regression models were then used to evaluate the relationship of aggressive hyperglycemia management on poor outcome. A separate exploratory analysis was performed to evaluate the influence of glucose control in each group on both primary and secondary outcome of interest. The effect of predictor variables on length of hospital and ICU stay were analyzed using linear regression models. For all regression models, adjustment was done by age, sex, Hunt and Hess clinical grade, Fisher group and method of aneurysm repair. A priori variables that were associated with poor outcome in prior studies such as interval from symptom onset to aneurysm repair, hypertension and admission glucose level, in addition to variables showing a p<0.20 on the respective univariate analyses (aneurysm size, seizure or loss of consciousness at symptom onset, diabetes and coronary artery disease, admission CT evidence of infarction, post-operative steroid use) were individually added in the regression model to detect changes in parameter estimate. A change of greater than 10% in the parameter estimate of the treatment group was considered criterion for including the variable of interest in the final model. To correct for the effect of temporal trend, an interrupted time series analysis using year of admission as a linear function was included in the regression model. The final regression model included treatment group, age, sex, method of aneurysm repair, Hunt and Hess grade, Fisher group, year of admission, post-operative steroid use, history of hypertension and admission CT evidence of infarction. Correction for multiple comparisons (AHM vs. SHM and poor vs. good glucose control within AHM or SHM group) were done using Bonferroni method and a p value <0.025 was considered significant.
Assuming a good outcome of 60%,8 the calculated sample size for the study to detect a 25% increase in good outcome with 80% power and 5% error rate was 152 per group, or total sample of 304 patients.
Results
The mean age of the cohort was 55.28± 13.47 years; 99 (29.8%) patients were men. A poor Hunt and Hess grade (>3) was documented in 129 (38.9%) of patients, while 279 (84%) were classified as Fisher group 3 on admission CT. Of the 332 patients included, 56 (16.9%) died, 218 (65.7%) had good outcome, and 105 (31.6%) developed clinical vasospasm.
The clinical and demographic characteristics of the AHM and SHM groups are presented in Table 2. A prior history of hypertension and admission CT signs of infarction were more common in AHM group, and post-operative steroid use was more common in the SHM group.
Table 2.
Patient characteristics by Treatment group
| Characteristics | SHM N=166 | AHM N=166 | p value |
|---|---|---|---|
| Male sex [N (%)] | 44 (26.51) | 55 (33.10) | 0.23** |
| Age in years [Mean ± SD] | 54.9 ± 13.88 | 55.6 ± 13.07 | 0.60** |
| Aneurysm Characteristics | |||
| Anterior Aneurysm Location [N (%)] | 103 (62.05) | 104 (62.65) | 1.00** |
| Number of Aneurysm [Median (25%,75%] | 1 (1,2) | 1 (1,2) | 0.29 |
| Aneurysm Size (mm) [Median (25%,75%] | 8 (5,10) | 7 (4,9) | 0.09** |
| Patient Presentation | |||
| Sentinel Headache [N (%)] | 19 (11.44) | 22 (13.25) | 0.74 |
| Seizure at onset [N (%)] | 11(6.63) | 21 (12.65) | 0.09** |
| Loss of consciousness [N (%)] | 87 (52.40) | 70 (42.16) | 0.08** |
| Prehospital worsening [N (%)] | 33 (19.88) | 43 (25.90) | 0.24 |
| History of Hypertension [N (%)] | 63 (40.12) | 87 (52.72) | 0.03** |
| History of Diabetes [N (%)] | 5 (3.18) | 17 (8.48) | 0.06** |
| History of stroke [N (%)] | 16 (10.30) | 13 (7.87) | 0.56 |
| History of CAD [N (%)] | 19 (12.17) | 10 (6.06) | 0.08** |
| Hunt and Hess Grade | |||
| Hunt and Hess 1 [N (%)] | 28 (16.87) | 26 (15.66) | 0.88 |
| Hunt and Hess 2 [N (%)] | 12 (7.23) | 32 (19.28) | 0.00 |
| Hunt and Hess 3 [N (%)] | 61 (36.75) | 44 (26.51) | 0.06 |
| Hunt and Hess 4 [N (%)] | 52 (31.33) | 47 (28.31) | 0.63 |
| Hunt and Hess 5 [N (%)] | 13 (7.83) | 17 (10.24) | 0.57 |
| Poor Hunt and Hess grade [N (%)] | 65 (39.10) | 64 (38.50) | 1.00** |
| Admission Lab/Imaging: | |||
| CT sign of edema [N (%)] | 46 (27.70) | 43 (25.90) | 0.80 |
| CT sign of infarction [N (%)] | 3 (1.80) | 12 (7.20) | 0.03** |
| CT sign of ICH [N (%)] | 37 (22.20) | 36 (21.60) | 0.89 |
| CT sign of IVH [N (%)] | 128 (77.10) | 134 (80.70) | 0.50 |
| CT sign of hydrocephalus [N (%)] | 133 (80.10) | 131 (78.90) | 0.89 |
| CT sign of MLS [N (%)] | 30 (18.07) | 25 (15.00) | 0.56 |
| Fisher Group: | |||
| Fisher Group 1 [N (%)] | 0 (0) | 1 (0.60) | 1.00 |
| Fisher Group 2 [N (%)] | 11 (6.63) | 13 (7.83) | 0.83 |
| Fisher Group 3 [N (%)] | 137 (82.53) | 142 (85.54) | 0.55 |
| Fisher Group 4 [N (%)] | 18 (10.84) | 10 (6.02) | 0.17 |
| Poor Fisher Group [N (%)] | 137 (82.53) | 142 (85.50) | 0.55** |
| Admission glucose [Mean ± SD] | 168.7±46.60 | 171.2±58.40 | 0.98** |
included in multivariate logistic regression analysis
Note: p values were computed using Fisher exact test, t-test or Wilcoxon 2-sample test where appropriate
Legend: SHM – Standard Hyperglycemia Management; AHM – Aggressive Hyperglycemia Management; CAD – Coronary Artery Disease; ICH – Intracerebral hemorrhage; IVH – intraventricular hemorrhage; MLS – midline shift
More patients in the SHM group (127, or 76.5%) did not receive insulin as compared to the AHM group (22, or 13.3%); however other aspects of patient treatment were similar between groups (See Table 3).
Table 3.
Patient management and glucose control by treatment assignment
| Characteristics | SHM N=166 | AHM N=166 | p value |
|---|---|---|---|
| Patient Management | |||
| Surgical clipping [N (%)] | 134 (80.70) | 121 (72.90) | 0.12** |
| Interval from symptom onset to aneurysm repair in days [Median (25%,75%] | 1 (0,1) | 1 (0,1) | 0.73** |
| Need for emergent EVD [N (%)] | 127 (76.50) | 137 (82.50) | 0.22 |
| Blood transfusion [N (%)] | 114 (68.67) | 109 (65.66) | 0.64 |
| HHH therapy [N (%)] | 116 (69.87) | 107 (64.44) | 0.35 |
| Post-op Steroid use [N (%)] | 141 (84.93) | 66 (39.75) | <0.01** |
| Insulin Treatment | |||
| SQ insulin only [N (%)] | 52 (31.33) | 30 (18.07) | 0.01 |
| IV insulin only [N (%)] | 9 (5.42) | 114 (68.67) | <0.01 |
| IV/SQ insulin [N (%)] | 61 (36.74) | 144 (86.74) | <0.01 |
| IV insulin first 72 hrs [N (%)] | 6 (3.60) | 103 (62.04) | <0.01 |
| IV insulin post 72 hrs [N (%)] | 3 (1.80) | 39 (23.40) | <0.01 |
| Glucose Control | |||
| Mean Glucose first 72 hrs in mmol/L [Mean ± SD] | 9.82 ± 1.56 | 8.84 ± 1.22 | <0.01 |
| Mean Glucose [Mean ± SD] | 8.98 ± 1.69 | 8.04 ± 0.93 | <0.01 |
| Mean Daily glucose burden [Mean ± SD] | 1.51 ± 1.51 | 0.79 ± 0.69 | <0.01 |
| Duration of hyperglycemia in days [Median (25%,75%] | 7 (5, 11) | 5 (3,8) | <0.01 |
| Hypoglycemia [N (%)] | 4 (2.40) | 21 (12.60) | <0.01 |
included in multivariate logistic regression analysis
Note: p values were computed using Fisher exact test, t-test or Wilcoxon 2-sample test where appropriate
Legend: SHM – Standard Hyperglycemia Management; AHM – Aggressive Hyperglycemia Management; EVD – extraventricular drainage; HHH – Hypervolemic, hypertensive, hemodilutional therapy; SQ – subcutaneous; IV – intravenous
Glucose Control
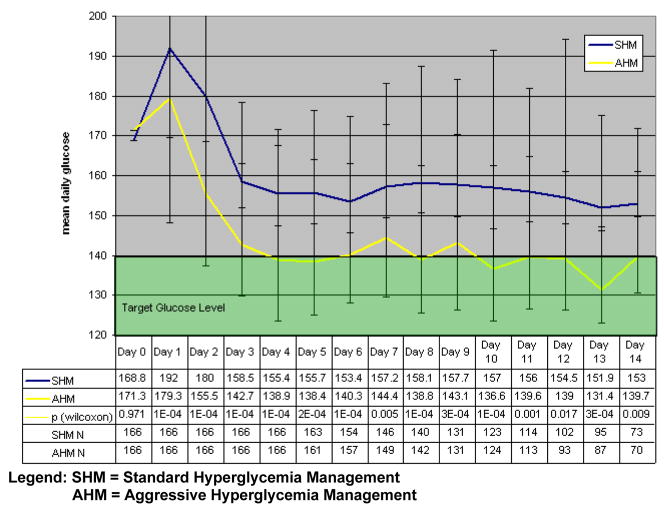
Admission glucose did not differ among the two groups. However, patients in the SHM group had significantly higher overall mean blood glucose (8.9 mmol/L [161.0 mg/dL] vs. 7.7 mmol/L [138.4 mg/dL], p<0.01), overall mean glucose burden (1.2 mmol/L [26.5 mg/dL] vs. 0.5 mmol/L [9.4 mg/dL], p<0.01), and longer hyperglycemia duration (7 days vs. 5 days, p<0.01), compared to the AHM group. In addition, daily mean glucose values were significantly higher in SHM group vs. AHM group from Day 1 to Day 14 (Figure 1). The proportion of patients achieving target glucose control was significantly higher in AHM compared to SHM group (80.1% vs. 52.4%, p<0.01).
Figure 1.
Mean Daily Glucose value by treatment group
Hypoglycemia occurred in 25 patients and all except one were receiving insulin during the hypoglycemic episode. As expected and consistent with other reports,10, 11 there were more hypoglycemic episodes in AHM compared to SHM group (12.7% vs. 2.4%, p<0.01). No immediate hypoglycemia-related complications were seen and none of the patients who developed hypoglycemia had clinical sequela.
Primary Outcome
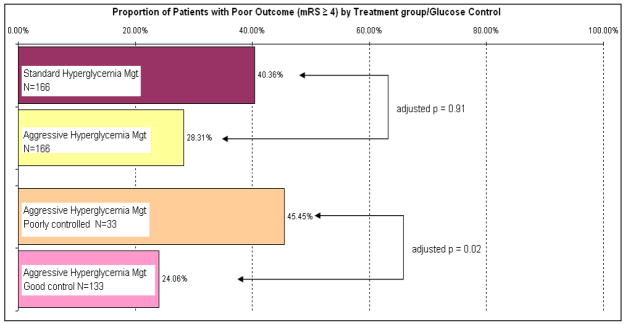
The proportion of poor outcome among patients treated with AHM after 2003 was significantly lower compared with patients treated with SHM before 2003 (28.3% vs. 40.4% p < 0.01), and remained statistically significant after adjusting for age, sex, Hunt and Hess Grade, Fisher group, method of aneurysm repair, history of hypertension, CT sign of infarction and post-operative steroid use. However, using interrupted time series analysis, the difference could not be explained entirely by AHM protocol implementation after correcting for temporal trend (adjusted OR 1.16, 95% CI [0.25, 5.29], p = 0.85).
Older age (adjusted OR 1.07, 95% CI [1.04, 1.10], p <0.01), poor Hunt and Hess grade (adjusted OR 5.73, 95% CI: [2.03, 16.19], p <0.01 for Hunt and Hess grade 3; OR 23.31, 95% CI [8.67, 62.68], p<0.01 for Hunt and Hess grade 4 and 5), and admission CT evidence of infarction (adjusted OR 5.27, 95% CI [1.20, 23.07], p=0.03) were found to be independently associated with poor outcome after multivariable adjustments (See Table 5).
Table 5.
Effect of hyperglycemia management on development of poor outcome in aSAH: Odds Ratio Estimates from Logistic Regression Model
| Predictor | Odds Ratio | 95% Wald Confidence Limits | p value |
|---|---|---|---|
| Treatment Effect (AHM vs SHM) | 1.16 | 0.25 – 5.29 | 0.85 |
| Year of Admission | 0.71 | 0.50 – 1.02 | 0.06 |
| Sex (Male vs Female) | 1.16 | 0.59 – 2.27 | 0.66 |
| Age in Years | 1.07 | 1.04 – 1.10 | <0.01 |
| Method of Aneurysm repair (Surgical vs Endovascular) | 0.56 | 0.27 – 1.14 | 0.11 |
| Hunt and Hess Grade 3 vs (1 and 2) | 5.73 | 2.03 – 16.19 | <0.01 |
| Hunt and Hess Grade (4 and 5) vs (1 and 2) | 23.31 | 8.67 – 62.68 | <0.01 |
| Poor Fisher Group (Group 3 and 4 vs Group 1 and 2) | 0.56 | 0.24 – 1.29 | 0.17 |
| Post-op Steroid use | 0.59 | 0.28 – 1.27 | 0.17 |
| Admission CT evidence of infarction | 5.27 | 1.20 – 23.07 | 0.03 |
| History of Hypertension | 1.21 | 0.66 – 2.21 | 0.54 |
Legend: SHM = Standard Hyperglycemia Management AHM = Aggressive Hyperglycemia Management
Admission glucose (10.4 mmol/L [186.9 mg/dL] vs. 9.0 mmol/L [161.2 mg/dL], p <0.01), mean glucose (9.0 mmol/L [162.6 mg/dL] vs. 8.3 mmol/L [148.5 mg/dL], p <0.01), duration of hyperglycemia (7.7 vs. 5.9 days, p < 0.01) and mean glucose burden (1.5 mmol/L [27.6 mg/dL] vs. 1.0 mmol/L [17.1 mg/dL], p < 0.01) were all significantly higher in patients with poor outcome. However, none of the above glucose parameters were independent predictors of poor outcome after multivariable adjustments.
Among patients treated with AHM, achievement of good glycemic control significantly reduced the chance of poor outcome (adjusted OR 0.25, 95% CI [0.08, 0.80], p = 0.02) compared to patients who had poor glycemic control (See Table 6).
Table 6.
Glycemic control and outcome within AHM group
| Variable | Good Control N=133 | Poor control N=33 | Adjusted OR (95% Confidence Interval) | p valuea |
|---|---|---|---|---|
| Primary Outcome | ||||
| Poor outcome defined as mRS ≥ 4 [N(%)] | 32 (24.06) | 15 (45.45) | 0.25 (0.08, 0.80) | 0.02 |
| Secondary Outcome | ||||
| Symptomatic vasospasm [N(%)] | 46 (34.59) | 15 (45.45) | 0.75 (0.33, 1.71) | 0.49 |
| DINDs [N(%)] | 72 (54.14) | 23 (69.70) | 0.73 (0.31, 1.72) | 0.48 |
| Complication rates | ||||
| Rebleed [N(%)] | 11 (8.27) | 2 (6.06) | 1.85 (0.34, 10.06) | 0.47 |
| Intracranial hypertension [N(%)] | 59 (44.36) | 15 (45.45) | 1.10 (0.47, 2.57) | 0.83 |
| Intracranial hemorrhage [N(%)] | 21 (15.79) | 6 (18.18) | 1.16 (0.40, 3.40) | 0.79 |
| DVT/PE [N(%)] | 14 (10.53) | 8 (24.24) | 0.49 (0.17, 1.43) | 0.19 |
| Cardiac dysfunction [N(%)] | 38 (28.57) | 14 (42.42) | 0.55 (0.23, 1.32) | 0.18 |
| Seizure [N(%)] | 5 (3.76) | 3 (9.09) | 0.35 (0.07, 1.81) | 0.21 |
| Infection [N(%)] | 62 (46.62) | 18 (54.55) | 1.00 (0.42, 2.40) | 0.99 |
| Respiratory Failure [N(%)] | 71 (53.38) | 24 (72.73) | 0.39 (0.12, 1.22) | 0.11 |
| Renal Failure [N(%)] | 6 (4.51) | 4 (12.12) | 0.50 (0.11, 2.35) | 0.38 |
| Length of Stays | ||||
| Hospital LOS [Median (25%,75%)] | 17 (13, 25) | 21 (12, 28) | −2.93 [2.28]b | 0.20c |
| ICU LOS [Median (25%,75%)] | 13 (10, 16) | 14 (9, 18) | 0.78 [1.14]b | 0.49c |
P values from logistic regression except Hosp LOS and ICU LOS
Parameter estimate (standard error)
P value from linear regression model
Legend: SHM = Standard Hyperglycemia Management AHM = Aggressive Hyperglycemia Deficit, OR = Odds Ratio, mRS = modified Rankin scale, DINDs = Delayed Ischemic Neurologic Deficits, DVT=Deep Venous Thrombosis, PE = Pulmonary Embolism, LOS = Length of Stay
More patients did not have follow-up record in SHM compared to AHM group (46 vs 17, respectively). However, the result of the analysis did not change when modified Rankin Score obtained at hospital discharge were used.
Secondary Outcomes
There was no difference in rate of clinical vasospasm, delayed ischemic neurological deficit and development of clinical complications before and after AHM protocol implementation (See Table 4). ICU and hospital length of stay was comparable between the two treatment groups. Surgical aneurysm repair and poor Hunt and Hess grade significantly increased ICU (2.5 ± 1.0 days [p=0.01] and 2.2 ± 0.5 days [p <0.01], respectively) and hospital length of stay (4.9 ± 1.9 days [p=0.01] and 3.2 ± 0.9 days [p <0.01].
Table 4.
Patient Outcome by Treatment group
| Variable | SHM N=166 | AHM N=166 | Adjusted OR (95% Confidence Interval) | p valuea |
|---|---|---|---|---|
| Primary Outcome | ||||
| Poor outcome defined as mRS ≥ 4 [N(%)] | 67 (40.36) | 47 (28.31) | 1.16 (0.25, 5.29) | 0.85 |
| Secondary Outcome | ||||
| Symptomatic vasospasm [N(%)] | 44 (26.51) | 61 (36.75) | 1.05 (0.28, 4.00) | 0.93 |
| DINDs [N(%)] | 93 (56.02) | 95 (57.23) | 1.32 (0.35, 5.03) | 0.68 |
| Complication rates | ||||
| Rebleed [N(%)] | 17 (10.24) | 13 (7.83%) | 0.54 (0.07, 4.32) | 0.56 |
| Intracranial hypertension [N(%)] | 70 (42.17) | 74 (44.58) | 1.27 (0.37, 4.34) | 0.70 |
| Intracranial hemorrhage [N(%)] | 25 (15.06) | 27 (16.27) | 1.02 (0.20, 5.15) | 0.99 |
| DVT/PE [N(%)] | 22 (13.25) | 22 (13.25) | 0.29 (0.05, 1.75) | 0.18 |
| Cardiac dysfunction [N(%)] | 46 (27.71) | 52 (31.33) | 1.40 (0.36, 5.38) | 0.63 |
| Seizure [N(%)] | 8 (4.82) | 8 (4.82) | 0.55 (0.03, 9.15) | 0.68 |
| Infection [N(%)] | 92 (55.42) | 80 (48.19) | 0.78 (0.22, 2.82) | 0.70 |
| Respiratory Failure [N(%)] | 100 (60.24) | 95 (57.23) | 1.19 (0.23, 6.11) | 0.83 |
| Renal Failure [N(%)] | 11 (6.63) | 10 (6.02) | 0.47 (0.04, 5.61) | 0.55 |
| Length of Stays | ||||
| Hospital LOS [Median (25%,75%)] | 20 (14, 29) | 17.5 (12, 25) | −4.17 [3.90]b | 0.28c |
| ICU LOS [Median (25%,75%)] | 13 (10, 18) | 13 (10, 17) | −3.10 [2.06]b | 0.13c |
P values from logistic regression
Parameter estimate (standard error)
P value from linear regression model
Legend: SHM = Standard Hyperglycemia Management; AHM = Aggressive Hyperglycemia Management; mRS – modified Rankin Scale; DINDs= Delayed Ischemic Neurologic Deficits; DVT=Deep Venous Thrombosis; PE = Pulmonary Embolism; ICU – intensive care unit; LOS = Length of Stay
Consistent with prior studies, Fisher group (adjusted OR 3.97, 95% CI [1.66, 9.48], p < 0.01) and poor Hunt and Hess grade (adjusted OR 2.18, 95% CI [1.14, 4.17], p = 0.02) strongly predicted development of symptomatic vasospasm while older age was associated with reduced risk for vasospasm (OR 0.97, 95% CI [0.95, 0.99], p = 0.01).
Discussion
In this study, we determined the impact of glucose control on outcome after aneurysmal subarachnoid hemorrhage in a cohort of patients using a prospectively collected database. We have shown that aggressive hyperglycemia management targeting a systemic blood glucose level of 4.4 – 7.8 mmol/L (80–140 mg/dL) is feasible and effective in achieving target glucose level with acceptable rates of hypoglycemia. Furthermore, we have demonstrated that among patients treated with aggressive hyperglycemia management, achievement of target glucose level was independently associated with good outcome (Figure 2).
Figure 2.
Patient Outcome by Treatment group and glucose control
Similar to a prior study 8, we did not find any association between admission hyperglycemia and poor outcome after multivariable adjustments suggesting that it is likely a marker of disease severity representing generalized catecholamine surge. However, failure to achieve the target glucose level in patients treated with aggressive hyperglycemia management was found to be significantly associated with poor outcome, highlighting the deleterious effect of persistent systemic glucose elevation.
Intensive insulin therapy is currently advocated for use in general critical care practice based on two landmark studies by van den Berghe. 10, 11 The first study was conducted in surgical critically ill patients showing significantly reduced ICU-related complications and mortality with intensive insulin use.11 The second study conducted in the medical intensive care unit reduced morbidity but did not have any impact on mortality.10 The results of these studies suggest that there may be differential effects of insulin therapy among various types of critically ill patients, including those with acute brain injuries. A recent report showed no difference in mortality, functional outcome and occurrence of vasospasm among aneurysmal subarachnoid patients treated with intensive insulin therapy compared to conventional glucose management.17 The study included 78 patients and only 69% of patients randomized to intensive insulin therapy achieved target glucose level. The small number of patients and the inability to achieve target glucose may have reduced the power of the study to detect significant difference. Oddo summarized the results of small clinical outcome studies comparing intensive insulin therapy to conventional glucose management and showed no significant effect on neurologic outcome and mortality.16
A major concern with intensive insulin therapy for neurocritically ill patients is the danger of brain tissue hypoglycemia. The human brain is an obligate glucose consumer. Because neuronal tissues are insulin-insensitive, cerebral glucose uptake and metabolism is likely supply-driven in humans.13 There are experimental18 and clinical14, 19 evidence that systemic lowering of glucose reduces brain tissue glucose concentration. Reduced cerebral tissue glucose in turn has been associated with elevated peri-ischemic cortical depolarization18 and with poor neurologic outcome.14 However, there is also convincing evidence that persistent hyperglycemia exacerbates secondary brain injury and independently predicts poor outcome.8, 9, 20–22 The challenge in critical care for SAH patients is in determining the optimal blood glucose target after acute brain injury. Because achievement of normoglycemia during insulin therapy may be associated with critical reduction in brain tissue glucose concentration, the lower limit of glucose target may need to be addressed in future studies.
Acute hyperglycemia in critically ill patients cause adverse effects from multiple mechanisms with positive feedback further upregulating the destructive processes.23 Although it has been postulated that insulin has direct beneficial effects for outcome after SAH 24, 25 our study is consistent with previous clinical studies in showing that blood glucose control rather than the use of insulin is the main reason for improvement in patient outcomes.26
Our study has a number of limitations, most of which are related to its retrospective nature. First, we were unable to quantify the amount of insulin given to each patient. Second, we were unable to quantify the daily caloric load of each patient in relation to the glucose levels. However, all patients were managed in a standardized fashion with appropriate nutritional support and uniform use of normal saline without dextrose. Hence, we do not believe significant differences in nutritional intake would be found in the two groups. Third, because of the extended period of the study, secular trend may have influenced the outcomes. This was addressed using an interrupted time series analysis with the inclusion of the year of admission as one of the predictor variables in the multivariable model.
Conclusion
Effective aggressive glucose management to maintain blood glucose below 140 mg/dL is associated with better neurologic outcome in patients with aneurysmal subarachnoid hemorrhage. Further studies are needed to validate our results and to explore the feasibility and safety of aggressive glucose management in a broader patient population in the neurointensive care unit.
Acknowledgments
We express gratitude to National Institute of Health for NCRR K30-RR022292-07 grant support to Dr. S. Chou.
We acknowledge Earl Francis Cook, S.D. for his invaluable help in statistical analysis.
We acknowledge Jeremy Shefner, M.D, Ph.D., for his critical appraisal of the manuscript.
Footnotes
Conflicts on Interest Disclosures – None
Funding Source – None
References
- 1.Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: Role of region, year, and rate of computed tomography: A meta-analysis. Stroke. 1996;27:625–629. doi: 10.1161/01.str.27.4.625. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP, Jr, Feinberg W, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Stroke. 1994;25:2315–2328. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- 3.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- 4.Rordorf G, Ogilvy CS, Gress DR, Crowell RM, Choi IS. Patients in poor neurological condition after subarachnoid hemorrhage: Early management and long-term outcome. Acta Neurochir (Wien) 1997;139:1143–1151. doi: 10.1007/BF01410974. [DOI] [PubMed] [Google Scholar]
- 5.Song EC, Chu K, Jeong SW, Jung KH, Kim SH, Kim M, Yoon BW. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke. 2003;34:2215–2220. doi: 10.1161/01.STR.0000088060.83709.2C. [DOI] [PubMed] [Google Scholar]
- 6.Cherian L, Goodman JC, Robertson CS. Hyperglycemia increases brain injury caused by secondary ischemia after cortical impact injury in rats. Crit Care Med. 1997;25:1378–1383. doi: 10.1097/00003246-199708000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 8.Badjatia N, Topcuoglu MA, Buonanno FS, Smith EE, Nogueira RG, Rordorf GA, Carter BS, Ogilvy CS, Singhal AB. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005;33:1603–1609. doi: 10.1097/01.ccm.0000168054.60538.2b. quiz 1623. [DOI] [PubMed] [Google Scholar]
- 9.Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, Temes R, Parra A, Ostapkovich ND, Mayer SA. Hyperglycemia after sah: Predictors, associated complications, and impact on outcome. Stroke. 2006;37:199–203. doi: 10.1161/01.STR.0000194960.73883.0f. [DOI] [PubMed] [Google Scholar]
- 10.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical icu. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 12.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 13.Seaquist ER, Damberg GS, Tkac I, Gruetter R. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes. 2001;50:2203–2209. doi: 10.2337/diabetes.50.10.2203. [DOI] [PubMed] [Google Scholar]
- 14.Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 15.Vespa PM, McArthur D, O’Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: A microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- 16.Oddo M, Schmidt JM, Mayer SA, Chiolero RL. Glucose control after severe brain injury. Curr Opin Clin Nutr Metab Care. 2008;11:134–139. doi: 10.1097/MCO.0b013e3282f37b43. [DOI] [PubMed] [Google Scholar]
- 17.Bilotta F, Spinelli A, Giovannini F, Doronzio A, Delfini R, Rosa G. The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: A randomized prospective pilot trial. J Neurosurg Anesthesiol. 2007;19:156–160. doi: 10.1097/ANA.0b013e3180338e69. [DOI] [PubMed] [Google Scholar]
- 18.Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- 19.Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care. 2008;12:R9. doi: 10.1186/cc6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of org 10172 in acute stroke treatment (toast) investigators. Neurology. 1999;52:280–284. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- 21.Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58:47–50. doi: 10.1097/01.ta.0000135158.42242.b1. [DOI] [PubMed] [Google Scholar]
- 22.Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335–342. doi: 10.1097/00006123-200002000-00015. discussion 342–333. [DOI] [PubMed] [Google Scholar]
- 23.Read JL, Cheng EY. Intensive insulin therapy for acute hyperglycemia. AACN Adv Crit Care. 2007;18:200–212. doi: 10.1097/01.AACN.0000269264.22041.1c. [DOI] [PubMed] [Google Scholar]
- 24.Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 25.Johan Groeneveld AB, Beishuizen A, Visser FC. Insulin: A wonder drug in the critically ill? Crit Care. 2002;6:102–105. doi: 10.1186/cc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]