Abstract
The concept of field cancerization was first introduced over six decades ago in the setting of oral cancer. Later, field cancerization involving histologic and molecular changes of neoplasms and adjacent tissue began to be characterized in smokers with or without lung cancer. Investigators also described a diffuse, non-neoplastic field of molecular injury throughout the respiratory tract that is attributable to cigarette smoking and susceptibility to smoking-induced lung disease. The potential molecular origins of field cancerization and the field of injury following cigarette smoke exposure in lung and airway epithelia are critical to understanding the impact of the field of injury on clinical diagnostics and therapeutics for smoking-induced lung disease.
Keywords: field of injury, field cancerization, lung cancer, tobacco smoke, molecular diagnosis and prognosis
“In all resected oral tumors it was found that the benign epithelium, beyond the confines of the malignant tumor, was abnormal.” — Danely P. Slaughter, 1953 (1)
Slaughter originated the concept of field cancerization in 1944 (2) and later observed its manifestation in epithelial hyperplasia and dysplasia in 783 patients with oral cancer (1). Field cancerization describes the site or sites of neoplasia and adjacent histologically normal-appearing tissue with molecular abnormalities in common with the neoplasm. The neoplasm and its adjacent tissue represent regional carcinogenesis, or a clonal patch, and may occur anywhere within the epithelial field (e.g., the airway and lung) exposed to a carcinogen. A distinct but related concept is “the field of injury,” which includes the molecular changes occurring throughout the tissue exposed to a carcinogen. The field of injury reflects host response to and damage from the carcinogen and may or may not be a precursor to premalignant lesions and frank malignancy. Field cancerization and the field of injury have both been implicated in many malignancies (including all epithelial cancers; reviewed in ref. 3) and potentially hold the keys to preventing and curing lung and other major epithelial cancers and to understanding in vivo epithelial carcinogenesis.
The concepts of field of injury and field cancerization are particularly relevant to the clinical settings of lung-cancer early detection and prevention. Eighty-five percent to 90% of lung cancer patients are current or former smokers, yet only 10%–20% of heavy smokers develop a primary lung malignancy (4). Lung-cancer—related mortality rates are high (80%–85% in five years) and result largely from the lack of effective lung-cancer chemoprevention or tools to diagnose the disease at an early stage. Furthermore, we are unable to identify which current and former smokers (of which there are 90 million in the United States) are at greatest risk for developing lung cancer and so would be most suitable for chemoprevention or early-detection approaches. Tissue injury changes occur in the respiratory tract of healthy smokers and can precede the development of smoking-induced lung cancer. This field of injury allows sampling of more accessible tissue (e.g., of the nasal passages or bronchial airway versus of the lung) that provides potential opportunities for risk assessment, early disease detection, therapeutic monitoring, and biologic insight into the mechanism of disease. The detection of dysplastic airway lesions on autofluorescent bronchoscopy, for example, is one method for identification of high-risk smokers who may benefit from ongoing trials with potential chemopreventive agents (5).
Although field cancerization and the field of injury have been studied most extensively in head and neck cancers and cigarette smokers, these effects also have been described in former uranium miners with radon exposure (6) and in esophageal, colorectal and other epithelial cancers (3, 7-9). It is conceivable that other inhaled carcinogenic agents such as unvented smoky coal would create a similar field of molecular injury (10-12).
In this article, we will discuss the potential biologic origins of molecular tissue injury and the relevance of the field of injury created by cigarette smoke to smoking-induced lung disease in humans. We also will discuss work from our and others’ laboratories in utilizing genomic and computational methodologies to leverage the concept of field tissue injury in developing clinical tools for risk assessment, diagnosis, treatment and prevention of smoking-induced lung disease.
Molecular Basis
In some cancers, the agent causing the diffuse field of epithelial cell injury is obvious. In the lung, it has been shown that all airway epithelial cells exposed to cigarette smoke develop genomic and epigenomic changes that are similar to some extent to molecular changes in the cancer in the distal lung (3, 13-24). The same is true for head and neck cancers. In skin cancer, exposure to sunlight is the common causal agent, and its mutagenic effects are evident in epithelial cells of skin distant from the site of melanoma (3). In colon, prostate and breast cancer the agent producing the field of injury is less clear, but in each case molecular changes similar to those that occur in the primary cancer have been demonstrated in normal-appearing cells of the same organ site (3, 25).
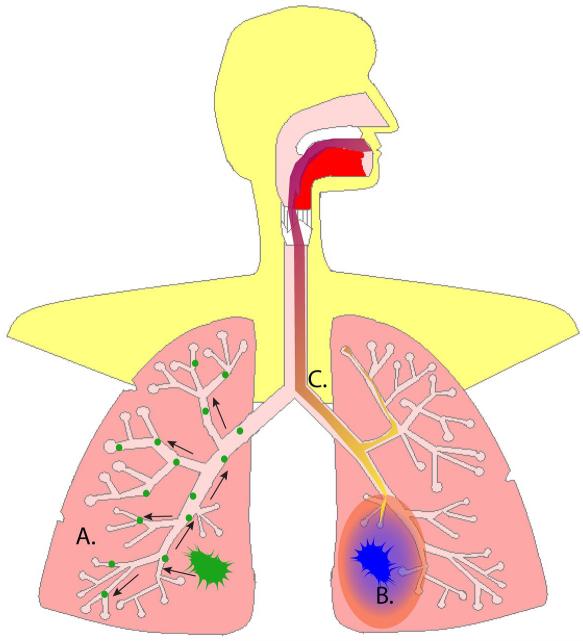
There are three major theories for the origin of the field of smoking-related injury, or how cells in the entire respiratory tract develop altered genomic, epigenomic, transcriptomic and proteomic modifications (Fig. 1). A single mutant epithelial cell clone could expand and extend into widespread areas of the organ’s epithelium, for example, the respiratory lining. Alternatively, tumors may arise from polyclonal tumor stem cells, as suggested by patients with multiple primary lung tumors each with different genetic alterations. This theory suggests that exposure to carcinogens in cigarette smoke damages the entire field and leads to stochastic genetic mutations at widely separated sites. This idea implies in turn that the field of injury actually reflects host response to and damage from the toxins in cigarette smoke. It has also been proposed that the cancer itself affects the surrounding tissue, contributing to genetic alterations and in turn malignancy, perhaps through tumor-associated macrophages. These theories likely are not entirely independent of each other and reflect phenomena that probably occur in combination. They demonstrate the multi-step process involved in carcinogenesis and provide some understanding of the molecular basis for the development of malignancy.
Fig. 1.
Hypotheses about the origins of the smoking-related field of epithelial injury in the lungs and airway. A) Based on the observation of p53 mutations throughout the airway epithelium of a smoker without overt lung cancer, Franklin et al. proposed that a malignant epithelial cell clone (green) propagates throughout the entire airway epithelium (14). B) Other studies have suggested that the field of epithelial injury reflects the host response (light blue- and orange-shaded areas) to the lung tumor itself (dark blue). This is supported by numerous observations of a gradient of changes radiating from the lung tumor throughout the adjacent noncancerous lung (17, 31), including tumor-associated macrophages (32). C) The field effect has been described not only in association with smoking-induced lung disease, but also in healthy and phenotypically normal smokers (16, 24, 34-36, 39). This suggests that the field effect represents the host’s response (the purple-to-yellow gradient in the airway) to toxins in cigarette smoke. It is likely that these mechanisms act together in varying degrees to create the field of injury observed in numerous studies.
Clonal Expansion
The idea that a single mutant epithelial cell clone expands and extends into widespread areas of the lung’s epithelium (Fig. 1A) is supported by the report of a smoker without overt lung cancer who had the same p53 mutation in numerous sites of the lower respiratory tract (14). Other observations potentially argue against clonal expansion as the sole mechanism of lung field injury. First, patients initially diagnosed with multiple primary lung tumors (albeit a rare occurrence) often have different genetic alterations in each tumor (26). Second, autopsy studies of paraffin-embedded tissues from patients with multiple synchronous primary lung cancers found that only 37.9% had the same clonality as measured by p53 and epidermal growth factor receptor (EGFR) somatic mutations (26). Other studies in patients with multiple primary lung tumors have shown that many tumors have discordant p53 or K-ras mutations (27, 28). Although the discordant p53 and K-ras mutations may occur after clonal expansion and may be allowed by an as yet unidentified mutation, they might also indicate that exposure to tobacco smoke carcinogens damages the entire respiratory epithelium and leads to different genetic mutations at different sites.
Tumor Effects on the Microenvironment
The idea that the cancer itself has an effect on the surrounding tissue, contributing to genetic alterations and in turn promoting carcinogenesis (Fig. 1B), is supported by several lines of evidence. Virchow first proposed in 1863 that cancer occurs at sites of chronic inflammation (7), and Dvorak later characterized tumors as “wounds that do not heal” (29). There is increasing molecular evidence that supports chronic inflammation as a critical oncogenic pathway and as the most-likely explanation for the diffuse nature of the field of injury (7). Therefore, inflammation likely is the inciting process in esophageal cancer (incited by chronic gastric reflux; ref. 9), many forms of colon cancer (inflammatory bowel disease; ref. 30), gastric cancer (infection with Helicobacter pylori; ref. 8), liver cancer (chronic hepatitis; ref. 7), prostate cancer (prostatitis; ref. 30), and cervical cancer (papilloma virus; ref. 8). The link between chronic inflammation and cancer has further been explored by Verma et al., who focused on inflammation induced by nuclear factor kappa B, or NF-κB, signaling as the “lynch-pin” of cancer and focal point of possible therapeutic strategies to treat and prevent inflammation-related cancer (9).
Tumors themselves can secrete pro-inflammatory cytokines that can lead to DNA damage (7). Inflammatory cells and cytokines act on epithelial cells, stromal cells, and the extracellular matrix to perpetuate inflammation (8) and may promote carcinogenesis through the production of reactive oxygen species causing additional DNA damage. In support of this concept, loss of heterozygosity (LOH) is more severe at locations closer to the primary lung tumor than at distal sites or in the contralateral airways (31). This phenomenon suggests that the field effect, while present throughout the lung epithelia, may have a gradient that emanates from the tumor. Like the LOH studies, EGFR mutations were present in a gradient with mutations occurring at higher frequencies at sites more proximal to the tumor(17).
In addition to a generalized inflammatory process incited by lung cancer, tumor-associated macrophages (TAMs) appear to promote inflammation-related carcinogenesis (8). In a mouse model of urethane-induced lung cancer, the lung tumor microenvironment modulated 46 genes, many of which were highly expressed in TAMs (32). Moreover, these genes were strongly predictive of lung cancer in macrophages isolated from bronchoalveolar lavage (32). TAMs, when appropriately activated, can kill tumor cells or can facilitate tumor growth, angiogenesis and local invasion (7).
Despite the growing evidence that chronic inflammation plays a key role in many cancers (9), not all subjects with predisposing inflammation develop cancer. There is evidence that heritable differences in genes protect against inducers of inflammation; in the case of smoking, these may include tobacco-metabolizing genes or genes that counteract the effects of the myriad of toxic products in tobacco smoke. Furthermore, there may be genetic differences in inflammatory pathway genes such as specific single-nucleotide polymorphisms, or SNPs, in interleukin genes associated with the occurrence of lung cancer (in smokers) possibly resulting from deregulated inflammatory responses to tobacco-induced lung damage (33).
Host Response to Toxins
Although the concept of a field of airway epithelial injury related to smoking was initially described in the setting of lung cancer (13), it has also been described in apparently healthy current and former smokers without known underlying lung cancer or chronic obstructive pulmonary disease (COPD; refs. 14-16, 34-39). This observation introduces the possibility that the field of epithelial injury reflects the host’s response to toxins contained in cigarette smoke independent of smoking-induced lung disease (Fig. 1C) and might reflect a subject’s risk for developing disease.
The concept of an epithelial field of injury induced by smoking alone is supported by studies of LOH (16) and from by our work showing alterations in gene expression (35) in the airway epithelium of apparently healthy smokers. Our group also found microRNA changes in the airway epithelium in response to smoking and that some of these microRNA modulate the impact of smoking on gene expression. Studies showing a relationship between bronchial and oral epithelial methylation (24) and gene expression (36) support the concept of a field of injury throughout the respiratory tract in response to cigarette smoke. Furthermore, our group found that lung tumor location had no effect on the accuracy of a lung-cancer—specific airway gene expression signature (40) in smokers suspected of (but not diagnosed with) lung cancer, further supporting the idea that this field reflects a generalized response to tobacco smoke rather than an effect of the tumor itself. Studies of patients with synchronous or metachronous lung tumors also suggest stochastic molecular changes at multiple sites throughout the respiratory tract (26-28, 41).
Biologic Implications
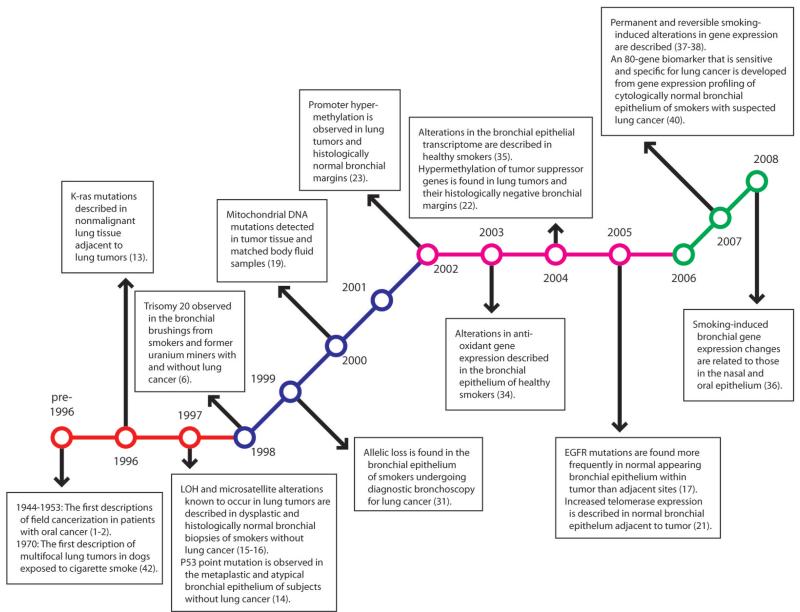
Numerous studies have begun to characterize the molecular changes that occur in the field of injury in healthy smokers and smokers with lung cancer. Auerbach et al. first described multicentric non-invasive lung tumors, observing them in dogs exposed to cigarette smoke (42). However, it was not until 1996 that studies described field cancerization and the field of injury with respect to smoking and lung cancer in humans. Since that time, rapid advances in biotechnology have driven our insights into the mechanism of the field of injury, characterization of specific changes in the respiratory tract exposed to cigarette smoking, and correlation of these changes to smoking-induced lung disease (Fig. 2).
Fig. 2.
Pivotal descriptions of the smoking-related field of injury in the lungs and airway. Although field cancerization was first described by Slaughter in 1944 for patients with oral cancer, the smoking-related field of injury in the human lungs and airway was not described until 1996. Initial studies (shown in red) were aimed at linking genetic changes in tumor tissue to noncancerous adjacent and peripheral lung. Later studies (shown in blue) described the epigenetic changes in both tumor tissue and bronchial epithelia. With the advent of microarray technology, large-scale gene expression studies further characterized the field effect previously observed and an initial push to move to sites distal from the tumor was made (shown in purple). The most recent studies (shown in green) have focused on applying this field of injury to a variety of clinical questions.
Genomics of the Field Effect
In 1996, Nelson and others reported the first descriptions of K-ras mutations in nonmalignant lung tissue adjacent to resected lung tumors (13), suggesting that field cancerization might also occur in the lung. P53 mutations, LOH and microsatellite alterations were subsequently described in the dysplastic and normal bronchial epithelium of smokers (14-16). Although these smokers did not have overt lung cancer, the changes paralleled what had been previously described in lung tumors. EGFR mutations were later described in the histologically normal bronchial epithelium of smokers without overt lung cancer (17).
In addition to somatic mutations and allelic loss, the field of injury within the respiratory tract has also been shown to include the DNA of mitochondria (mtDNA), an organelle that is particularly susceptible to oxidative damage (18). In a cross-sectional study of smokers and nonsmokers, mtDNA content of saliva was higher in smokers than nonsmokers independently of age, and there was no correlation with duration of smoking cessation (18). In another study, the same mutations occurred in small amounts of mtDNA obtained from bronchoalveolar lavage fluid and paired tumor samples of lung cancer patients (19).
Epigenetic Alterations in the Field of Smoking-Induced Injury
The epigenome serves a critical role in the differential expression of genes and in the specification of cellular functions. Deregulation of telomerase activity and aberrant methylation at CpG islands have been linked to field cancerization and the field of injury.
The enzyme telomerase functions to lengthen the telomere after cell division; therefore, deregulated telomerase activity contributes to carcinogenesis by causing chromosomal instability. In addition to deregulation in lung tumors themselves, telomerase is also deregulated in the nonmalignant adjacent bronchial epithelium of patients who later developed a recurrence or second primary lung malignancy (20). Telomerase activity has been found to be increased in preinvasive bronchial lesions (21).
Changes in gene methylation have also been found in both lung cancer and surrounding noncancerous lung tissue. Methylation-specific polymerase chain reaction (PCR) showed that the histologically negative margins of resected lung cancers have abnormal methylation of at least one gene and 85% concordance with the changes observed in matched tumor tissue (22). A later study found aberrant methylation of p16 in the nonmalignant bronchial epithelium of smokers (23), although most of these samples had dysplasia or metaplasia. More recent studies have identified changes in methylation in the oral epithelium of smokers (24, 43) and linked these changes to bronchial epithelium (24). Similar changes in methylation have been found in the sputum of smokers at a high lung-cancer risk and have the potential to become a tool for early lung-cancer detection (23, 44).
Alterations in Gene Expression
The development of high-throughput whole-genome expression arrays in the 1990s introduced a novel way to investigate large-scale alterations associated with smoking. Using this platform, our group and others found alterations in gene expression in the normal-appearing bronchial epithelium of healthy and phenotypically normal smokers (34, 35). The extent of gene expression changes seems to be similar in the large and small airway epithelium (34, 39), and we have recently found similar smoking-induced alterations in gene expression in the epithelia of the mainstem bronchus, mouth and nose (36). The degree of reversibility among smoking-altered genes in former smokers has been analyzed by whole-gene expression arrays in our laboratory and by Serial Analysis of Gene Expression (SAGE) libraries (37, 38). The presence of gene expression alterations despite smoking cessation suggests that smoking-induced damage may confer a survival advantage to the affected cells and that clonal growth of these cells may explain the persistence of gene expression changes.
Our ability to use gene expression in large-airway epithelium to determine the host response to tobacco smoke in healthy smokers led us to question whether smokers who developed lung cancer differ in host response from smokers who do not. Using bronchial brushings of normal-appearing large-airway epithelial cells, we have described an 80-gene—expression signature that can serve as both a sensitive and specific diagnostic biomarker for lung cancer in smokers (40). Our gene expression signature also accurately distinguished normal lung tissue in smokers from lung cancer tissue in previously published microarray datasets (40), suggesting that cancer-specific airway gene-expression differences are, at least in part, reflected in lung tumors.
Proteins and Proteomics
The available data on changes in protein expression that occur in smoking and smoking-induced lung diseases are limited. Some studies have validated changes in gene expression using immunohistochemistry (21, 40, 45, 46). Our group has begun to correlate gene expression changes in the airway epithelium of healthy smokers with proteomic changes measured by mass spectrometry (unpublished data). This work suggests that gene expression changes might be reflected in altered protein levels.
Only one study has examined proteomic profiles in fresh-frozen bronchial epithelial and lung tissue samples. Investigators using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) developed a proteomic profile based on a training subset of fresh-frozen samples of normal bronchial epithelial, premalignant and cancer tissues from 53 lung-cancer patients. This profile predicted tumor status of the entire blinded set of samples from the 53 patients and of a previously published dataset with a high degree of accuracy (74% overall; ref. 47).
Clinical Applications
Our improved understanding of the origins of and changes within the field of injury can be applied clinically and may fundamentally change the risk assessment, screening, diagnosis, and treatment of patients with or at risk for lung disease.
Early Diagnostic Biomarkers
The epithelial field of injury created by cigarette smoking can be used as an early diagnostic biomarker for lung cancer. We previously profiled gene expression in histologically normal large-airway epithelial cell brushings obtained from current and former smokers undergoing flexible bronchoscopy for suspected lung cancer (40). We identified an 80-gene biomarker that distinguishes smokers with and without lung cancer. The biomarker had an accuracy of 83% (80% sensitive, 84% specific) in an independent test set, and was subsequently validated on an independent prospective series (40). Our biomarker had ∼90% sensitivity for stage-1 cancer across all subjects (40). Combined with cytopathology of lower-airway cells, the biomarker yielded 95% sensitivity and a 95% predictive value. We subsequently demonstrated that the biomarker gave indications of the presence or absence of cancer independently of other clinical risk factors (48). The biomarker’s overall accuracy improved when used in combination with clinical features, providing a paradigm for a clinicogenomic approach to comprehensive lung-cancer diagnosis and treatment (48).
Screening and Chemoprevention
Even in early-stage disease, the incidence of recurrence and second primary lung cancers remain high following resection (49). An important implication of the field cancerization hypothesis is that part of the field of injury may remain after complete removal of the tumor and wide areas of negative margins, potentially increasing the likelihood of recurrence or a second primary tumor. Changes in promoter hypermethylation of tumor suppressor genes have been found in the noncancerous margins of resected non-small cell lung carcinoma (22) and resected squamous-cell carcinoma of the head and neck (50), suggesting that the field of injury might persist in vivo after surgical resection of the primary tumor. The field of injury might reflect prognosis in addition to the risk of a new cancer. A cytokine gene expression profile derived from lung tumor tissue and normal-appearing adjacent tissue has been linked to prognosis of stage I lung adenocarcinoma (51).
Molecular alterations in the field of injury of otherwise healthy smokers may also reflect susceptibility to future lung cancer development. In a large screening program, changes in methylation of key tumor suppressor genes previously described as methylated in lung tumors were also methylated in the sputum of cancer-free heavy smokers (52). Similar changes were found in BAL and biopsy specimens from these patients (52). One of the smokers with p53 mutations in sputum went on to develop lung cancer (52), and a subsequent study of this high-risk cohort found increased lung-cancer risk among smokers with promoter hypermethylation of these tumor suppressor genes (44).
Trials conducted thus far to prevent lung cancer have not been effective (5). However, the ability to identify the subset of smokers at high risk for developing lung cancer or recurrence of lung cancer based on changes in the field of injury might allow not only for more intensive surveillance but also for early targeted chemoprevention based on deregulated molecular pathways in the field of injury. Analysis of c-ErbB1/EGFR and c-ErbB1/HER-2 expression in bronchial dysplasia has already shown an association between EGFR expression and severity of bronchial dysplasia (53). If the proliferative state is mediated by EGFR, this provides a potential pathway for pharmacologic intervention. Also, chronic inflammation caused by cigarette smoking promotes tissue repair, production of reactive oxygen species causing further DNA damage, and cell proliferation, which may increase lung cancer risk in certain smokers (54). Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin can decrease colon cancer development among subjects with cyclooxygenase-2 (COX-2) overexpression in colorectal tumor specimens (55). Although cancer chemoprevention studies of COX-2—inhibiting NSAIDs in the lung have not been completed, there appears to be an interaction between a COX-2 polymorphism and NSAID use, suggesting that NSAID use may decrease the risk of lung cancer in a targeted at-risk population (56). Other medications with anti-inflammatory properties, such as lovastatin, have also shown potential as chemopreventive agents (57). Our group recently identified increased PI3K activity in the cytologically normal proximal airway of smokers with lung cancer and high-risk smokers with dysplastic airway lesions, providing a target and intermediate endpoint for evaluating the efficacy of chemopreventive strategies (58).
Extending the Field of Injury to Less Invasive Sites
Tissues in the field of injury used to determine lung cancer risk should be in an easily accessible site and obtained via a non-invasive collection method. Although several studies have screened for lung cancer in samples of bronchial epithelial cells obtained during fiberoptic bronchoscopy, this procedure cannot be used as a large-scale screening tool for smokers because of the invasiveness of the procedure.
Given that the smoking-induced field of injury to the airway epithelial lining extends to cytologically normal bronchial epithelial cells, it is logical to question whether upper airway epithelial cells of the mouth or nose might also reflect these changes. Hypermethylation of p16 (43), p53 overexpression (59), and p53 mutations (60) have been described in the normal oral mucosa of heavy smokers. Gene expression studies using oral epithelium are challenging because of the degree of RNA degradation that occurs in the mouth due to RNAses, but methods for collecting oral epithelial cells for gene expression analysis have been described (61). As an alternative, nasal epithelium may provide a non-invasive source of airway epithelial cells reflecting the field of smoking-induced molecular injury given their exposure to both exhaled and sidestream smoke. Alterations in methylation and gene expression in both the oral and nasal epithelium have been linked to similar changes in the bronchial epithelium (24, 36, 62, 63).
The Future of the Field
Studies are currently underway to further characterize the molecular changes that occur in the airway epithelium in response to smoking and in smoking-induced lung disease. New classes of molecules, such as microRNAs, may be altered in this field of injury and serve to modulate the downstream gene expression changes. The role of alternative splice events in the field of injury remains undefined, and detailed proteomic studies of tissues in the field remain on the horizon. Several questions regarding the origins of the field effect and how this might change in various disease states remain unanswered. It is not clear if the field remains affected or if it reverts to a more normal molecular profile in patients with lung cancer that is resected or treated with chemotherapy or radiation therapy. In addition, the accuracy of our airway gene expression biomarker for lung cancer has not been tested in nonsmokers with lung cancer, and doing so might better characterize whether this gene expression signature is a host response to cigarette smoking or represents the host response to the lung tumor itself. The field of injury induced by second-hand smoke and other environmental pollutants also remains uncharacterized.
Our group and others have begun to integrate clinical markers of lung-cancer risk with alterations in gene expression (48) or DNA repair capacity (64) in constructing comprehensive models for lung-cancer diagnosis. A similar approach can improve lung cancer screening. Molecular alterations in the field of injury that persist after smoking cessation (37, 38, 65) may help explain persistent lung-cancer risk in former smokers, and risk-related enzymes that metabolize carcinogens in cigarette smoke (65-67) suggest possible targets for lung cancer chemoprevention.
Just as we have begun to apply our knowledge of the field effect to lung cancer, this knowledge might also be applied to other smoking-induced lung diseases such as COPD (68). An improved understanding of the molecular changes that occur in heterogeneous and phenotypically complex diseases such as lung cancer and COPD might lead to improved treatments for these diseases. Work to characterize the relationship between alterations in nasal and buccal gene expression and changes in the bronchial epithelium may help extend the field of epithelial injury to the more accessible sites of the nasal passages and oral cavity and thus allow minimally invasive screening and early targeted intervention for lung cancer and possibly COPD.
Detailing the molecular changes that occur in the airway epithelium in smoking and lung cancer using genome-wide approaches has the potential to provide biologic insight into the mechanism of smoking-induced lung diseases by sampling more accessible sites than the diseased lung tissue. This would provide not only a less invasive opportunity to diagnose smoking-induced lung disease, but also provides a potential mechanism for screening high-risk populations. The ability to characterize the pathways affected in smoking and smoking-induced lung diseases might allow rational targeted therapy designed to normalize the affected molecules or pathways (69).
Acknowledgements
We thank Marc E. Lenburg and Christina Anderlind for their critical review of this manuscript. This work was supported by NIH/NCI R01CA124640 (AS), and NIH/NIEHS U01ES016035 (AS).
References
- (1).Slaughter DP, Southwick HW, Smejkai W. “Field cancerization” in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer. 1953;6:693–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- (2).Slaughter DP. The multiplicity of origin of malignant tumors: collective review. Internat Abstr Surg. 1944;79:89–98. [Google Scholar]
- (3).Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Shields PG. Molecular epidemiology of lung cancer. Ann Oncol. 1999;10(Suppl 5):S7–11. doi: 10.1093/annonc/10.suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- (5).Gray J, Mao JT, Szabo E, et al. Lung cancer chemoprevention: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3):56S–68S. doi: 10.1378/chest.07-1348. [DOI] [PubMed] [Google Scholar]
- (6).Neft RE, Crowell RE, Gilliland FD, et al. Frequency of trisomy 20 in nonmalignant bronchial epithelium from lung cancer patients and cancer-free former uranium miners and smokers. Cancer Epidemiol Biomarkers Prev. 1998;7:1051–4. [PubMed] [Google Scholar]
- (7).Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- (8).Moss SF, Blaser MJ. Mechanisms of disease inflammation and the origins of cancer. Nature Clinical Practice Oncology. 2005;2(2):90–7. doi: 10.1038/ncponc0081. [DOI] [PubMed] [Google Scholar]
- (9).Li Qiutang, Withoff S, Verma IM. Inflammation-associated cancer: NF-kB is the lynchpin. TRENDS in Immunology. 2005;26(6):318–25. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- (10).DeMarini DM, Landi S, Tian D, et al. Lung tumor KRAS and TP53 mutations in nonsmokers reflect exposure to PAH-rich coal combustion emissions. Cancer Res. 2001;61(81):6679–81. [PubMed] [Google Scholar]
- (11).Keohavong P, Lan Q, Gao WM, et al. K-ras mutations in lung carcinomas from nonsmoking women exposued to unvented coal smoke in China. Lung Cancer. 2003;41(1):21–7. doi: 10.1016/s0169-5002(03)00125-9. [DOI] [PubMed] [Google Scholar]
- (12).Keohavong P, Lan Q, Gao WM, et al. Detection of p53 K-ras mutations in sputum of individuals exposed to smoky coal emissions in Xuan Wei County, China. Carcinogenesis. 2005;26(2):303–8. doi: 10.1093/carcin/bgh328. [DOI] [PubMed] [Google Scholar]
- (13).Nelson MA, Wymer J, Clements N. Detection of K-ras gene mutations in non-neoplastic lung tissue and lung cancers. Cancer Lett. 1996;105:115–21. doi: 10.1016/0304-3835(96)04202-4. [DOI] [PubMed] [Google Scholar]
- (14).Franklin WA, Gazdar AF, Haney Jerry, et al. Widely dispersed p53 mutation in respiratory epithelium. A novel mechanism for field carcinogenesis. J Clin Invest. 1997 Oct;100(8):2133–7. doi: 10.1172/JCI119748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wistuba, Lam S, Behrens C, et al. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst. 1997 Sep 17;89:1366–73. doi: 10.1093/jnci/89.18.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mao L, Lee JS, Kurie JM, et al. Clonal genetic alterations in the lungs of current and former smokers. J Natl Cancer Inst. 1997;89(12):857–62. doi: 10.1093/jnci/89.12.857. [DOI] [PubMed] [Google Scholar]
- (17).Tang X, Shigematsu H, Bekele BN, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65(17):7568–72. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- (18).Masayesva BG, Mambo E, Taylor RJ, et al. Mitochondrial DNA content increase in response to cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2006;15(1):19–24. doi: 10.1158/1055-9965.EPI-05-0210. [DOI] [PubMed] [Google Scholar]
- (19).Fliss MS, Usadel H, Caballero OL, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–9. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- (20).Yashima K, Litzky LA, Kaiser L, et al. Telomerase expression in respiratory epithelium during the multistage pathogenesis of lung carcinomas. Cancer Res. 1997;57:2373–7. [PubMed] [Google Scholar]
- (21).Miyazu YM, Miyazawa T, Hiyama K, et al. Telomerase Expression in Noncancerous Bronchial Epithelia Is a Possible Marker of Early Development of Lung Cancer. Cancer Res. 2005 Nov 1;65:9623–7. doi: 10.1158/0008-5472.CAN-05-0976. [DOI] [PubMed] [Google Scholar]
- (22).Guo M, House MG, Hooker C, et al. Promoter Hypermethylation of Resected Bronchial Margins: A Field Defect of Changes? Clin Cancer Res. 2004 Aug 1;10:5131–6. doi: 10.1158/1078-0432.CCR-03-0763. [DOI] [PubMed] [Google Scholar]
- (23).Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promotor methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62:2370–7. [PubMed] [Google Scholar]
- (24).Bhutani M, Pathak AK, Fan YH, et al. Oral epithelium as a surrogate tissue for assessing smoking-induced molecular alterations in the lungs. Cancer Prev Res. 2008;1(1):39–44. doi: 10.1158/1940-6207.CAPR-08-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kopelovich L, Henson DE, Gazdar AF, et al. Surrogate anatomic/functional sites for evaluating cancer risk: an extension of the field effect. Clin Cancer Res. 1999;5:3899–905. [PubMed] [Google Scholar]
- (26).Chang YL, Wu CT, Lin SC, et al. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin Cancer Res. 2007;13(1):52–8. doi: 10.1158/1078-0432.CCR-06-1743. [DOI] [PubMed] [Google Scholar]
- (27).Wang X, Christiani DC, Mark EJ, et al. Carcinogen exposure, p53 alteration, and K-ras mutation in synchronous multiple primary lung carcinoma. Cancer. 1998;85(8):1734–9. [PubMed] [Google Scholar]
- (28).Mitsudomi T, Yatabe Y, Koshikawa T, et al. Mutations of the p53 tumor suppressor gene as a clonal marker for multiple primary lung cancers. Journal of Thoracic and Cardiovascular Surgery. 1997;114:354–60. doi: 10.1016/S0022-5223(97)70180-6. [DOI] [PubMed] [Google Scholar]
- (29).Dvorak HF. Tumors: Wounds that do not heal: Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- (30).Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(24):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- (31).Powell CA, Klares S, O’Connor G, Brody JS. Loss of Heterozygosity in Epithelial Cells Obtained by Bronchial Brushing: Clinical Utility in Lung Cancer. Clin Cancer Res. 1999;5:2025–34. [PubMed] [Google Scholar]
- (32).Stearman RS, Dwyer-Nield L, Grady MC, Malkinson AM, Geraci MW. A macrophage gene expression signature defines a field effect in the lung tumor microenvironment. Cancer Res. 2008;68(1):34–43. doi: 10.1158/0008-5472.CAN-07-0988. [DOI] [PubMed] [Google Scholar]
- (33).Engels EA, Wu X, Gu J, et al. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67(13):6520–7. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- (34).Hackett NR, Heguy A, Havey BG, et al. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol. 2003;29:331–43. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- (35).Spira A, Beane J, Shah V, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004 Jun 21;101(27):10143–8. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sridhar S, Schembri F, Zeskind J, et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics. 2008;9:259. doi: 10.1186/1471-2164-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Beane J, Sebastiani P, Liu G, et al. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8(9) doi: 10.1186/gb-2007-8-9-r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chari R, Lonergan KM, Ng RT, et al. Effect of active smoking on the human bronchial epithelium transcriptome. BMC Genomics. 2007;8:297. doi: 10.1186/1471-2164-8-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Harvey BF, Heguy A, Leopold LP, et al. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med. 2006;85:39–53. doi: 10.1007/s00109-006-0103-z. [DOI] [PubMed] [Google Scholar]
- (40).Spira A, Beane JE, Shah V, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13(3):361–6. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- (41).Neugut AI, Sherr D, Robinson E, Murray T, Nieves J. Differences in histology between first and second primary lung cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:109–12. [PubMed] [Google Scholar]
- (42).Auerbach O, Hammond EC, Kirman D, Garfinkel L. Effect of cigarette smoking on dogs. II. Pulmonary neoplasms. Arch Environ Health. 1970;21(6):754–68. doi: 10.1080/00039896.1970.10667329. [DOI] [PubMed] [Google Scholar]
- (43).vonZeidler SV, Miracca EC, Nagai MA, Birman EG. Hypermethylation of the p16 gene in normal oral mucosa of smokers. Int J Mol Med. 2004;14(5):807–11. doi: 10.3892/ijmm.14.5.807. [DOI] [PubMed] [Google Scholar]
- (44).Belinsky SA, Liechty KC, Gentry FD, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66(6):3338–44. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- (45).Merrick DT, Haney J, Petrunich S, et al. Overexpression of vascular endothelial growth factor and its receptors in bronchial dysplsia demonstrated by quantitative RT-PCR analysis. Lung Cancer. 2005;48:31–45. doi: 10.1016/j.lungcan.2004.07.049. [DOI] [PubMed] [Google Scholar]
- (46).Martinet N, Bonnard L, Regnaul V, et al. In vivo transglutaminase type 1 expression in normal lung, previnvasive bronchial lesions, and lung cancer. Am J Respir Cell Mol Biol. 2003;28:428–35. doi: 10.1165/rcmb.2002-0114OC. [DOI] [PubMed] [Google Scholar]
- (47).Rahman SMJ, Shyr Y, Yildiz PB, et al. Proteomic Patterns of Preinvasive Bronchial Lesions. Am J Respir Crit Care Med. 2005 Sep 22;172:1556–62. doi: 10.1164/rccm.200502-274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Beane J, Sebastiani P, Whitfield TH, et al. A prediction model for lung cancer diagnosis that integrates genomic and clinical features. Cancer Prev Res. 2008;1(1):56–64. doi: 10.1158/1940-6207.CAPR-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. Journal of Thoracic Cardiovascular Surgery. 1995;109:120–9. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- (50).Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathologic staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–35. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- (51).Seike M, Yanaihara N, Bowman EDZK, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst. 2007;99:1257–69. doi: 10.1093/jnci/djm083. [DOI] [PubMed] [Google Scholar]
- (52).Baryshnikova E, Destro A, Infante MV, et al. Molecular alterations in spontaneous sputum of cancer-free heavy smokers: results from a large screening program. Clin Cancer Res. 2008;14(6):1913–9. doi: 10.1158/1078-0432.CCR-07-1741. [DOI] [PubMed] [Google Scholar]
- (53).Merrick DT, Kittelson J, Winterhalder R, et al. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: Evaluation of potential targets for chemoprevention of lung cancer. Clin Cancer Res. 2006;12(7):2281–8. doi: 10.1158/1078-0432.CCR-05-2291. [DOI] [PubMed] [Google Scholar]
- (54).Siegfried JM. Biology and chemoprevention of lung cancer. Chest. 1998;113(1):40S–5S. doi: 10.1378/chest.113.1_supplement.40s. [DOI] [PubMed] [Google Scholar]
- (55).Chan AT, Ogino S, Fuchs CS. Aspirin and risk of colon cancer in relation to expression of COX-2. N Engl J Med. 2007;356(21):2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- (56).Vogel U, Christensen J, Wallin H, et al. Polymorphisms in genes involved in the inflammatory response and interaction with NSAID use or smoking in relation to lung cancer risk in a prospective study. Mutat Res. 2008;639:89–100. doi: 10.1016/j.mrfmmm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- (57).Maksimova E, Yie TA, Rom WN. In vitro mechanisms of lovastatin on lung cancer cell lines as a potential chemopreventive agent. Lung. 2008;186:45–54. doi: 10.1007/s00408-007-9053-7. [DOI] [PubMed] [Google Scholar]
- (58).Gustafson AM, Lam S, McWilliams A, et al. Deregulation of phosphatidylinositol 3-kinase pathway in the airway epithelium of smokers is associated with lung cancer. Am J Respir Crit Care Med. 2008:A541. Abstracts Issue. [Google Scholar]
- (59).Avan N, Avan I, Alatli C, et al. P53 overexpression in normal oral mucosa of heavy smokers. J Exp Clin Cancer Res. 2000;19(4):525–9. [PubMed] [Google Scholar]
- (60).Colucci S, el-Gehani R, Flint S, Mothersill C. p53 mutations and protein expression in primary cultures of normal oral mucosa in smokers and non-smokers. Oral Oncol. 1997;33(4):240–6. doi: 10.1016/s0964-1955(97)00027-4. [DOI] [PubMed] [Google Scholar]
- (61).Spira A, Beane J, Schembri F, et al. Noninvasive method for obtaining RNA from buccal mucosa epithelial cells for gene expression profiling. Biotechniques. 2004;36(3):484–7. doi: 10.2144/04363RN03. [DOI] [PubMed] [Google Scholar]
- (62).Jain RJ, Varma S, Hurteau GJ, Spivack S. Buccal-lung comparison of quantitative expression of carcinogen and oxidant metabolism genes in human subjects. Chest. 2004;125:107–8. doi: 10.1378/chest.125.5_suppl.107s-a. [DOI] [PubMed] [Google Scholar]
- (63).Spivack SD, Hurteau GJ, Jain R, et al. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosa cells. Cancer Res. 2004;64:6805–13. doi: 10.1158/0008-5472.CAN-04-1771. [DOI] [PubMed] [Google Scholar]
- (64).Spitz MR, Etzel CJ, Dong Q, et al. An expanded risk prediction model for lung cancer. Cancer Prev Res. 2008;1(4):250–4. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Zhang L, Lee JJ, Tang H, et al. Impact of smoking cessation on global gene expression in the bronchial epithelium of chronic smokers. Cancer Prev Res. 2008;1(2):112–8. doi: 10.1158/1940-6207.CAPR-07-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Gumus ZH, Du B, Kacker A, et al. Effects of tobacco smoke on gene expression and cellular pathways in a cellular model of oral leukoplakia. Cancer Prev Res. 2008;1(2):100–11. doi: 10.1158/1940-6207.CAPR-08-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Penning TM, Lerman C. Genomics of smoking exposure and cessation: Lessons for cancer prevention and treatment. Cancer Prev Res. 2008;1:80–3. doi: 10.1158/1940-6207.CAPR-08-0047. [DOI] [PubMed] [Google Scholar]
- (68).Pierrou S, Broberg P, O’Donnell RA, et al. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(6):577–86. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- (69).Herbst RS, Heymach JV, Lippman SM. Molecular Origins of Cancer: Lung Cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]