Abstract
Erythropoietin (EPO), recognized for its central role in erythropoiesis, also mediates neuroprotection when the recombinant form (r-Hu-EPO) is directly injected into ischemic rodent brain. We observed abundant expression of the EPO receptor at brain capillaries, which could provide a route for circulating EPO to enter the brain. In confirmation of this hypothesis, systemic administration of r-Hu-EPO before or up to 6 h after focal brain ischemia reduced injury by ≈50–75%. R-Hu-EPO also ameliorates the extent of concussive brain injury, the immune damage in experimental autoimmune encephalomyelitis, and the toxicity of kainate. Given r-Hu-EPO's excellent safety profile, clinical trials evaluating systemically administered r-Hu-EPO as a general neuroprotective treatment are warranted.
Erythropoietin (EPO) and its receptor (EPO-R) function as primary mediators of the normal physiologic response to hypoxia. EPO, a glycoprotein that increases red cell mass to improve tissue oxygenation, is produced by the kidney in response to hypoxia. Recombinant human EPO (r-Hu-EPO) is effective and widely used for the treatment of anemia associated with renal failure, HIV infection, cancer, and surgery. However, like other members of the cytokine superfamily to which EPO and its receptor belong, both are expressed by other tissues, including the nervous system. Similar to its regulation in the periphery, EPO within the central nervous system is inducible by hypoxia (1–4). An in vivo neuroprotective function for EPO has been demonstrated by the observation that direct intracerebraventricular injection of r-Hu-EPO in advance of hypoxic/ischemic stress offers significant protection of neuronal tissue (5–7). A critical neuroprotective role for endogenous EPO in the central nervous system has been confirmed by the administration of soluble EPO-R, which neutralizes EPO, consequently exacerbating ischemic stress and increasing tissue injury (7).
Hypoxia may not be the only relevant stimulus for brain EPO production, however, as metabolic disturbances, including hypoglycemia and strong neuronal depolarization, generate mitochondrial reactive oxygen species that may increase brain EPO expression through hypoxia inducible factor 1 (8). EPO may thus protect nervous tissue under any condition characterized by a relative deficiency of ATP in the face of increased metabolic demands. EPO has been shown to exhibit classic neurotrophic effects in vivo and in vitro (2, 9–11). The mechanism of action of EPO in erythropoiesis, neuroprotection, and neurotrophic effects ultimately may involve activation of the bcl-x family of antiapoptotic genes, promoting survival rather than apoptosis (12–14).
Despite the demonstrated benefit of intrathecally administered r-Hu-EPO in preventing ischemic neuronal damage, direct delivery of r-Hu-EPO into the brain is not a practical approach in most clinical contexts. Systemic delivery of r-Hu-EPO has not been evaluated because of the perception that the brain EPO system is parallel and distinct from the control of peripheral hemoglobin levels. However, this concept is based on the untested assumption that the blood–brain barrier (BBB) effectively excludes large glycosylated molecules such as EPO (1, 6, 7, 15, 16). Although in the classic view the BBB is considered to be impermeable to large molecules, recent study clearly establishes that some large molecules can be specifically transported into the brain across the capillary endothelium (17–19) to affect brain function. Specific vectorial movement of macromolecules into the brain parenchyma begins by binding to receptors present on the luminal surfaces of the endothelial cells. This initiates endocytosis, followed by translocation across the BBB (reviewed in ref. 20). Using immunohistochemistry, we observed that the EPO-R is abundantly expressed at brain capillaries. Thus, we hypothesized that systemically administered r-Hu-EPO would be transported across the BBB, and if so, generally would defend against brain injury. In the present report we describe the ability of systemically administered r-Hu-EPO to function as a neuroprotective agent in animal models of focal brain ischemia, concussive brain injury, experimental autoimmune encephalomyelitis (EAE), and kainate-induced seizures.
Materials and Methods
Reagents.
The r-Hu-EPO used is a human 165-aa glycoprotein manufactured by using recombinant DNA technology, which contains the identical amino acid sequence of isolated natural EPO and possesses the same biologic activity (21, 22). R-Hu-EPO is approximately 80% homologous to rodent EPO, and it has been shown to be biologically active in rodents for erythropoietic as well as neurotrophic functions. Although an immune response against human antigens can be elicited in rodents, it requires several weeks to obtain even a weak response (data not shown) and thus is not important in the context of these short-term studies. All experiments were performed by using r-Hu-EPO (Epoetin alfa, Procrit, Ortho Biotech, Raritan, NJ), which is formulated as a sterile, colorless liquid in an isotonic sodium chloride/sodium citrate or a sodium chloride/sodium PBS with added 1.25% human albumin.
EPO Assay.
R-Hu-EPO concentrations in mouse serum obtained by serial phlebotomy via the orbital sinus were determined by using a commercially available enzyme-linked immunosorbent assay (Immunobiological Laboratories, Hamburg, Germany) following the manufacturer's protocol. The lower limit of detection was ≈2 milliunits/ml.
Biotinylation of R-Hu-EPO.
R-Hu-EPO was isolated from the albumin present in the clinical material by using cibacron blue columns (Aff, Gel Blue, Bio-Rad) followed by molecular weight-selective spin columns (Centricon, Millipore). Isolated r-Hu-EPO then was biotinylated by using a commercially available kit (Boehringer Mannheim). Briefly, 0.2 mg of a long arm biotin (Vector Laboratories) was dissolved in 100 μl of DMSO. This solution was added to the concentrated r-Hu-EPO solution and vortexed immediately. The mixture then was incubated at room temperature for 4 h protected from light with aluminum foil, while gently stirring. The unbound biotin was removed from this solution by using a Centricon-10 column. Confirmation of successful biotinylation and separation of r-Hu-EPO was confirmed by visualizing an ≈34-kDa product after electrophoresis in a 6% agarose gel by using a streptavidin-biotin peroxidase kit (Vectostain, Vector Laboratories).
Immunochemistry.
Human hippocampal specimens with adjacent white matter and temporal cortex were freshly isolated as 0.5-cm blocks cut from surgical specimens obtained during margin resection for temporal lobe tumors or vascular malformations. These were immediately postfixed by 5% acriline in 0.1 M phosphate buffer, pH 7.4 for 3 h. Sections for histological analyses were cut with a vibrating microtome (Vibratome, Ted Paella, Redding, CA) at 40 μm thick. We previously have shown that these sections are anatomically normal (23). Immunohistochemical staining was performed as described (24) by using free floating sections and the indirect antibody peroxidase-antiperoxidase method with a 1:500 dilution EPO-R antiserum (Santa Cruz Biotechnology). Tissue controls by antibody omission and antibody specificity controls by use of the appropriate blocking peptide (Santa Cruz Biotechnology) also were carried out to confirm that staining was specific for EPO-R. Endogenous peroxidase activity was quenched by pretreatment of tissue sections with hydrogen peroxide (3% in methanol for 30 min). These antibodies have been previously validated for study of human tissue (2, 25). Cytochemical localization of biotinylated r-Hu-EPO in sections of perfused mouse brain was visualized by methodology similar to the immunocytochemistry, except for elimination of primary and secondary antibodies.
Animal Experimentation.
Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with Italian and international laws and policies. Specific protocols were approved by the Animal Use and Care Committee of the Kenneth S. Warren Laboratories. All statistics were computed by using the analysis program jmp (SAS Institute, Cary, NC).
Middle Cerebral Artery (MCA) Occlusion.
Sprague–Dawley male rats weighing ≈250 g were anesthetized with pentobarbital [60 mg/kg body weight (BW)]. Body core temperature was thermostatically maintained at 37°C by using a water blanket and a rectal thermistor (Harvard Apparatus) for the duration of the anesthesia. The carotid arteries were visualized, and the right carotid was occluded by two sutures and cut. A burr hole adjacent and rostral to the right orbit allowed visualization of the MCA, which was cauterized distal to the rhinal artery. Animals then were positioned on a stereotaxic frame. To produce a penumbra surrounding this fixed MCA lesion, the contralateral carotid artery was occluded for 1 h by using traction provided by a fine forceps. Saline or r-Hu-EPO (250–5,000 units/kg BW) was administered at time points determined from the onset of the reversible carotid occlusion. To evaluate the extent of injury, the animals were killed after 24 h, the brains were removed, and serial 1-mm thick sections through the entire brain were cut by using a brain matrix device (Harvard Apparatus). Each section subsequently was incubated in a solution of 2% triphenyltetrazolium chloride (wt/vol) in 154 mM NaCl for 30 min at 37°C and stored in 4% paraformaldehyde until analysis. Quantification of the extent of injury was determined by using a computerized image analysis system (MCID, Imaging Research, St. Catharine's, ON, Canada). To accomplish this, a digital image of each section was obtained and the area of injury delineated by outlining the region in which the tetrazolium salt was not reduced, i.e., nonviable tissue. For cases in which the necrosis was so severe that tissue was actually lost and therefore the borders could not be directly assessed, an outline of the contralateral side was used to estimate the volume of injured brain. Total volume of infarct was calculated by reconstruction of the serial 1-mm thick sections. An indirect neuroprotective role for r-Hu-EPO via its effects on the circulating red cell mass was ruled out by the time frame of this experiment, which was shorter than the minimum required to produce a measurable erythropoietic effect (longer than 1 week).
Blunt Trauma.
To produce trauma to the temporal and frontal cortices reproducibly, a pneumatic piston was precisely driven by using miniature precision valves (Clippard, Cincinnati, OH) powered by nitrogen. Displacement and velocity of the piston was determined by a digital motion detector (EPD Technologies, Elmsford, NY). Female BALB/c mice were anesthetized with pentobarbital, and their heads were placed securely in a stereotaxic frame. A scalp incision was made to locate the begma. A 3-mm diameter stainless steel piston then was positioned to deliver the blow 2 mm caudal and 2 mm ventral to the bregma. Once the piston was activated, the velocity and time of impact was noted, as well as the amount of damage to the skull. The scalp incision was closed by using sutures. R-Hu-EPO was administered before, at the time of, or 3 or 6 h after impact and continued daily for a total of 5 days. Ten days after impact, the animals were anesthetized with pentobarbital and their brains were fixed by perfusion of 4% paraformaldehyde. The brains then were embedded in paraffin and 20-μm sections were cut through the region of injury and stained with hematoxylin/eosin. Quantitative analysis of volume of injury was determined by using the MCID system as described above. Qualitative analysis of degree of inflammatory infiltrate was performed by a blinded observer scoring each slide by using a scale of 0–5, 0 corresponding to no visible inflammation and 5 to the densest infiltrate.
EAE.
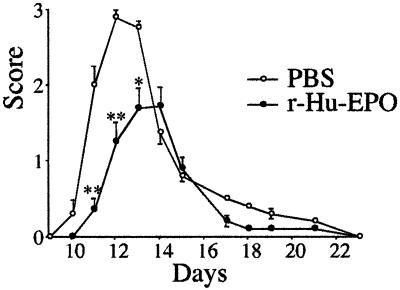
Female Lewis rats, 6–8 weeks of age, were purchased from Charles River (Calco, Italy). Animals were immunized by injecting 50 μg of guinea pig myelin basic protein (Sigma) in water, emulsified in equal volumes of complete Freund's adjuvant (CFA; Sigma) into both hind footpads along with an additional 7 mg/ml of heat-killed Mycobacterium tuberculosis (H37Ra; Difco) administered under light ether anesthesia. The final volume was 100 μl. Control mice received CFA alone. Rats were observed in a blinded fashion daily for signs of EAE and scored as follows: 0, no symptoms; 1, flaccid tail; 2, ataxia; and 3, complete hind limb paralysis with urinary incontinence. Statistical significance of experimental results was assessed by using a two-tailed Student's t test.
Kainate-Induced Seizures.
Female BALB/c mice received r-Hu-EPO (5,000 units/kg i.p.) or saline at different time intervals with respect to administering kainate i.p. (Sigma) at various concentrations. Latency and seizure severity were assessed as described (26) by using a numerical scale of 0 to 5: 0 = no evident seizure activity; 1 = mild, nonsustained activity (e.g., wet dog shakes, immobility); 2 = mild limbic activity (e.g., forelimb clonus, tooth chattering); 3 = brief bursts of sustained seizure activity; 4 = status epilepticus with rearing and loss of balance; and 5 = status epilepticus with inability to stand. End points were time of onset of status epilepticus and time of death. Because seizure activity developed only within 20–30 min in the BALB/c mouse model, these experiments was limited to a maximum of 60 min, at which time surviving animals were killed.
Results
EPO and EPO-R Are Expressed at Capillaries of the Brain-Periphery Interface.
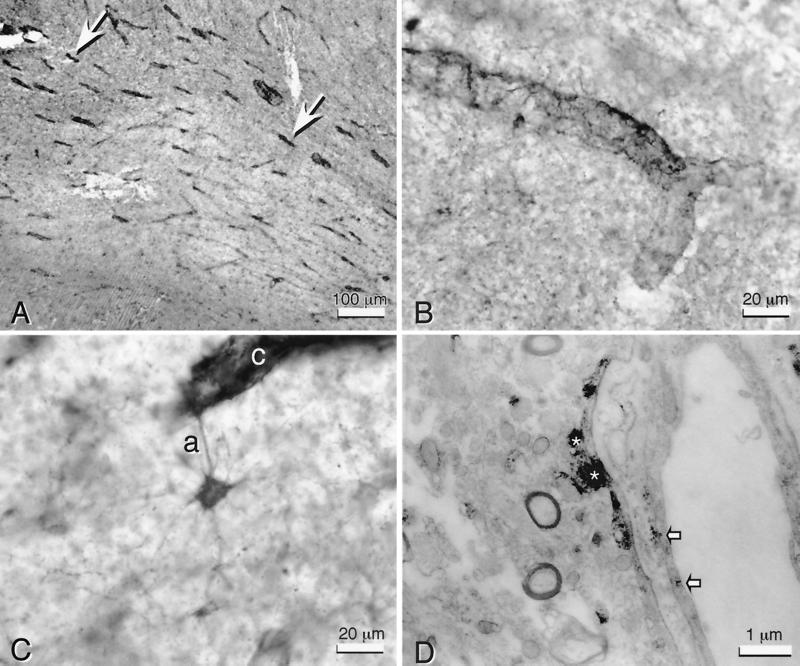
We first determined the expression of EPO and EPO-R in the normal brain by using specific polyclonal antibodies applied to sections of human, rat, and mouse tissue (unpublished work). Similar to many brain regions examined, the frontal cortex and hippocampus exhibited intense immunoreactivity for EPO-R in many medium to large neurons, but in a pattern restricted to the somata and proximal dendrites, and capillaries, particularly within white matter (Fig. 1A, arrows). In contrast, larger vessels and most astroglia were generally unreactive for anti-EPO-R. Under higher magnification, capillaries appeared enveloped by numerous EPO-R immunopositive processes (Fig. 1B) derived from nearby stellate astrocytes (Fig. 1C). Transmission electron microscopy confirmed that the predominance of anti-EPO-R immunoreactivity was located within the astrocytic endfeet surrounding the capillaries (Fig. 1D, *). In addition, substantial EPO-R immunoreactivity was observed within or on the surface of capillary endothelial cells (Fig. 1D, arrows).
Figure 1.
EPO-R is found within and around human brain capillaries. (A) Anti-EPO-R staining in white matter (human hippocampal fimbria) is primarily localized to capillaries (arrows). (B) High-power view of capillaries illustrates the distinctly fibrous quality of EPO-R immunoreactivity around the capillary wall. (C) This immunoreactivity at the capillary (c) often could be identified as an astrocytic process (a). The entire cytoplasmic volume of such stellate astrocytes contain EPO-R immunoreactivity. (D) Transmission electron microscopy confirms that the predominant EPO-R immunoreactivity is within astrocytic foot processes (*), but it is also present within endothelial cells (arrows).
Biotinylated r-Hu-EPO Crosses the BBB.
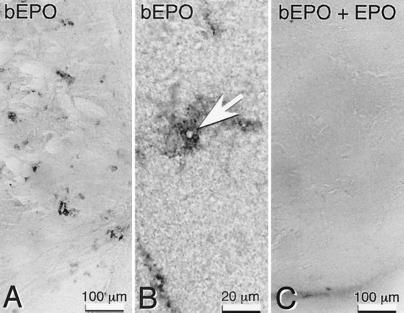
These observations derived from immunocytochemical staining clearly suggest an anatomical basis for direct transport of EPO within the systemic circulation into the central nervous system in the absence of any neural insult. To test this hypothesis, biotinylated r-Hu-EPO was injected i.p. into mice and subsequently visualized in brain sections by using streptavidin-peroxidase methodology. Two time intervals of 5 and 17 h were selected for evaluation, based on the pharmacokinetics of a single 5,000 units/kg BW r-Hu-EPO dose administered i.p. R-Hu-EPO administered in this fashion reaches a peak serum concentration at approximately 4 h (≈25,000 milliunits/ml; 1 milliunit ≈10 ng) and subsequently decays slowly to baseline levels over the next 20–30 h. We selected a 5-h time point for analysis to allow for an adequate exposure of the capillary endothelium to peak concentrations of r-Hu-EPO and compared this to 17 h later, when the serum levels had decreased to <0.1% of the peak.
Five hours after i.p. administration of biotinylated r-HuEPO (5,000 units/kg BW), peroxidase reaction product was observed surrounding capillaries (Fig. 2A) extending into the brain parenchyma a distance 3–4 times that of the thickness of the capillary wall (Fig. 2B). Simultaneous administration of unlabeled r-Hu-EPO (100-fold excess) with biotinylated r-Hu-EPO resulted in a markedly reduced or absent reaction product around the capillaries (Fig. 2C). Brain sections prepared 17 h after biotinylated r-Hu-EPO administration also lacked pericapillary reaction product. Instead, the label was localized to scattered neurons (data not shown). These anatomical studies provided evidence supportive of an active translocation of peripheral EPO across the BBB and further suggested that neurons might be one target for EPO. To test this idea further, we administered r-Hu-EPO systemically in a rodent focal stroke model, for which intracerebraventricular r-Hu-EPO administration is known to reduce the infarct volume in the penumbra (6).
Figure 2.
Systemically administered biotinylated r-Hu-EPO labels capillaries within the mouse brain. (A) Localization of biotinylated r-Hu-EPO (b-EPO) is around capillaries 5 h after i.p. injection into mice but is not observed (C) if given with 100 times excess of unlabeled r-Hu-EPO (bEPO + EPO). Tissue sections are from the striatum. (B) Biotinylated r-Hu-EPO surrounds the lumen of capillaries (arrow) 5 h after administration.
R-Hu-EPO Administered Systemically Is Neuroprotective in Focal Ischemic Stroke.
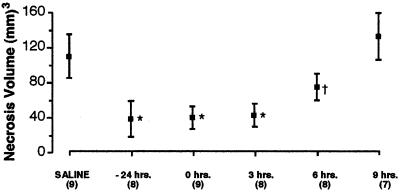
Ischemia that recapitulates damage caused by human stroke can be induced in adult male rats by permanently occluding both the right MCA (27) and carotid artery, followed by a reversible 1-h occlusion of the left carotid artery (28). Using this model, a large penumbral region of ischemia was obtained in the right frontal cortex and r-Hu-EPO (5,000 units/kg BW) or vehicle was injected i.p. 24 h before, simultaneously, or 3, 6, or 9 h after MCA occlusion. The volume of brain infarcted 24 h after ischemia, determined by computerized volumetric analysis of triphenyltetrazolium reduction (i.e., to distinguish living from dead tissue) within serial sections, was markedly reduced in animals receiving r-Hu-EPO administered systemically 24 h before or up to 3 h after occlusion (Fig. 3). By 6 h after occlusion this protective effect was partially lost and r-Hu-EPO provided no apparent protection when given 9 h after occlusion. The minimum effective dose for r-Hu-EPO administered at the same time as vascular occlusion was found to be ≈450 units/kg BW (data not shown). Thus in this model of stroke, a window up to 6 h after the onset of ischemia appears open for intervention by using r-Hu-EPO, which can be administered systemically over a dose ranging from 450 units/kg BW to 5,000 units/kg BW.
Figure 3.
Systemic administration of r-Hu-EPO reduces infarct volume after cerebral artery occlusion. Animals given r-Hu-EPO (5,000 units/kg BW i.p.) before, during, or 3 h after carotid artery occlusion showed significant (*, P < 0.01) and equivalent reduction of necrosis volume compared with controls. In contrast, animals receiving r-Hu-EPO 6 h after the onset of reversible ischemia exhibited a significant, but substantially smaller, decrease in injury volume compared with animals receiving r-Hu-EPO sooner (†, P < 0.05). High-dose r-Hu-EPO given 9 h after the onset of occlusion was ineffective in reducing the cortical volume of injury. Numbers in parentheses indicate number of animals studied under each condition.
R-Hu-EPO Administration Reduces Injury by Blunt Trauma.
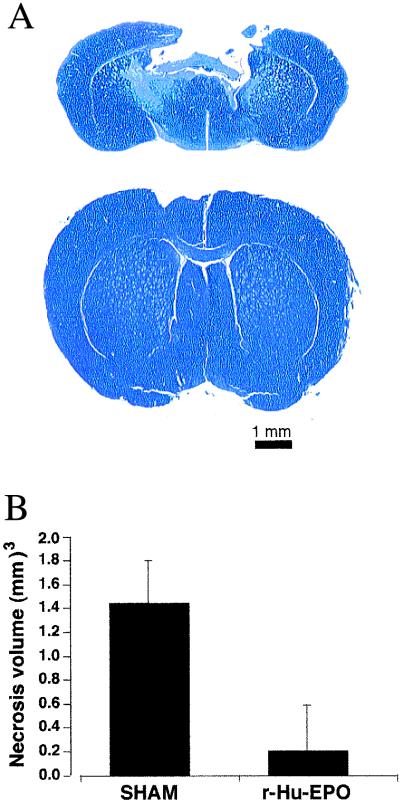
A mechanical insult delivered to the brain elicits elements of ischemic, excitotoxic, and inflammatory injury and, if severe enough, produces a cavitary lesion after 7–10 days (29). To determine whether systemically administered r-Hu-EPO is also protective of such injury, we used a mouse model in which the frontal cortex was subjected to a blow delivered to the intact calvaria by a calibrated pneumatic piston. Under pentobarbital anesthesia, animals received a blow of moderate severity (4 m/s; 2-mm displacement) and received an i.p. injection of r-Hu-EPO (5,000 units/kg BW i.p.) 24 h before or 0, 3, or 6 h after blow delivery. Animals continued to receive r-Hu-EPO once daily for 4 additional days (five doses total). Ten days after injury, each animal was killed, and their brains were perfused, fixed, serially sectioned and stained with hemotoxylin and eosin or cresyl violet. Animals not receiving r-Hu-EPO exhibited extensive cavitary injury 10 days after blow delivery, in marked contrast to animals receiving r-Hu-EPO 24 h before, as Fig. 4A illustrates for brain sections obtained from representative animals. Quantitative analysis of injury volume for animals given r-Hu-EPO 24 h in advance of injury (Fig. 4B) illustrates that r-Hu-EPO pretreatment significantly reduced this concussive injury. As observed in the model of focal ischemia, qualitative examination of animals receiving r-Hu-EPO at 0, 3, or 6 h in relationship to trauma revealed a similar protection as pretreatment with r-Hu-EPO (data not shown).
Figure 4.
Systemic administration of r-Hu-EPO attenuates injury after blunt trauma. (A) Mice receiving a nonpenetrating blow to the frontal cortex exhibited extensive cavitary necrosis when examined 10 days after injury if treated with saline (Upper) in contrast to the minimal injury observed if they had received r-Hu-EPO (Lower). Cresyl violet stain of representative brain sections through site of injury. (B) Results of a representative experiment for r-Hu-EPO (5,000 units/kg BW) given 24 h before delivery of the impact. n = 6 animals each group; P < 0.05. The experiment was repeated four times with similar results.
Histologic examination of serial brain sections showed that for animals receiving saline alone the region immediately surrounding the necrotic core was densely populated with mononuclear inflammatory cells. In contrast, regions surrounding the necrotic core in r-Hu-EPO-treated animals were characterized by a markedly reduced inflammatory infiltrate (not shown). Thus, as in the experimental model for stroke, the results of these experiments demonstrate the ability of systemically administered r-Hu-EPO to protect brain tissue from blunt trauma.
R-Hu-EPO Reduces the Clinical Severity of EAE.
Observations obtained from study of the blunt trauma model were striking in the absence of a prominent mononuclear cell infiltrate at the site of injury. These findings suggested that r-Hu-EPO also might reduce nervous system inflammation caused by other pathological processes. To test this hypothesis, we administered r-Hu-EPO in a rat model of EAE. In these experiments, EAE is induced in female Lewis rats by using guinea pig myelin basic protein and complete Freund's adjuvant. Immunized rats develop clinical symptoms by day 10, which peak with an increasing degree of paralysis by day 12. Daily administration of r-Hu-EPO (5,000 units/kg BW) or saline was initiated on day 3 after receiving myelin basic protein/complete Freund's adjuvant and continued until day 18. As shown in Fig. 5, r-Hu-EPO administration both significantly delayed the onset and reduced the severity of symptoms compared with saline-administered controls. Animals were followed for a total of 3 weeks after discontinuing r-Hu-EPO administration, during which time no “rebound” of symptoms occurred, as is typically observed after discontinuing treatment with glucocorticoids or IFN-β (30).
Figure 5.
Systemic administration of r-Hu-EPO ameliorates EAE. Lewis rats receiving r-Hu-EPO (5,000 units/kg BW) beginning at day 3 after immunization with myelin basic protein demonstrate both a delay in onset and a marked reduction of clinical symptoms (n = 18 animals in each group, three separate experiments; **, P < 0.01; *, P < 0.05).
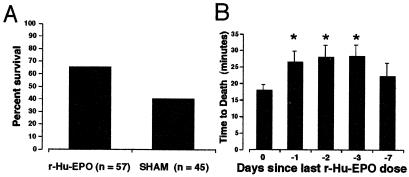
R-Hu-EPO Ameliorates the Latency and Severity of Seizures Induced by Kainate.
Excitotoxicity is a prominent component common to many forms of brain injury (reviewed in refs. 31–33) and could be a target for EPO action. To test for this possibility, we determined whether r-Hu-EPO reduced the toxicity of the glutamate analogue kainic acid, as assessed by latency and severity of seizures. To accomplish this, first the relationship between doses of systemically administered kainate and its toxicity was established by determining the latency and severity of seizures. In mice, death caused by status epilepticus occurred with a latency of ≈ 18 min when kainate was administered alone at the ED50 dose of ≈20 mg/kg. Mice were treated with r-Hu-EPO (5,000 units/kg BW) or saline 24 h before receiving kainate. Animals receiving both r-Hu-EPO and 20 mg/kg of kainate exhibited a significant delay of onset of status epilepticus with a markedly reduced motor involvement. The reduction in status epilepticus severity was reflected by the fact that there was a significant reduction in mortality of ≈45% compared with controls (Fig. 6A) as well as an increase in mean survival time (25.8 min for r-Hu-EPO treatment compared with 18.2 min for control (P < 0.0002; n = 68 animals each group). Additionally, for each dose of kainate studied below the ED50 of 20 mg/kg that did not produce status epilepticus, the behavioral seizure activity was significantly less severe than that observed for the sham-treated controls (data not shown).
Figure 6.
Systemic administration of r-Hu-EPO delays and lessens kainate-induced seizures. (A) Mortality subsequent to a convulsant dosage of kainate (20 mg/kg BW i.p.) is significantly reduced (P < 0.01) by a 24-h pretreatment of mice with r-Hu-EPO (5,000 units/kg BW i.p.). (B) A single dose of r-Hu-EPO (5,000 units/kg BW) administered on day 0, 1, 2, 3, or 7 before testing continues to provide protection from kainate (n = 18, 36, 17, 8, or 8 respectively; *, P < 0.05 compared with control).
In contrast to r-Hu-EPO administered 24 h before kainate, no protection from seizures was afforded by administering r-Hu-EPO 30 min before kainate (Fig. 6B) or after the development of any grade of motor seizure (data not shown). Further, a single exposure to r-Hu-EPO (5,000 units/kg BW) provided protection from kainate for at least 3 days (Fig. 6B). Thus, r-Hu-EPO is clearly not acting in the manner of conventional antiepileptic agents, which can terminate ongoing seizure activity but require a continued presence for efficacy. Presumably, EPO is inducing the expression of an array of genes that continue protection even in the absence of the cytokine.
Discussion
It is now clear that EPO possesses biological activities in addition to the erythropoietic effects that originally provided its name. Diverse cell types have been demonstrated to produce EPO and many cells besides erythroid progenitors express the EPO-R, including in the brain. The discovery that astrocytes produce EPO in response to hypoxia and that the EPO could protect nearby neuronal cells from ischemic injury in vivo (7) added further support for the pleiotropic nature of this cytokine. However, these findings also have historically suggested that the brain and peripheral EPO systems are separate. This concept is further reinforced by the known impermeability of the BBB to most plasma proteins. In the present paper, we have provided evidence that cross talk is possible between the peripheral and central EPO systems. Perhaps the most striking effect of these interacting systems is the ability of peripherally injected r-Hu-EPO to protect brain tissue from a variety of insults including ischemia/hypoxia, as well as trauma, immune-mediated inflammation, and excessive neuronal excitation.
A full understanding of how EPO mediates its effects across the BBB has not yet been elucidated, but we report here a plausible manner in which this is accomplished. First, our observation that the EPO-R is present on the capillaries of the brain, including the endothelial cells, but excluded from larger vessels, is consistent with a location at the anatomical locus of the BBB. Second, although previous studies report that small amounts of large proteins such as albumin can cross the BBB (34, 35), the mechanism is not receptor-mediated. At 5 h after systemic injection of biotinylated r-Hu-EPO, we observed biotin label only surrounding the BBB capillaries and not around larger vessels, as would be expected for a nonspecific transport mechanism. Third, movement of the biotin label was eliminated by coinjection of excess amounts of unlabeled r-Hu-EPO, consistent with a specific and saturable transport mechanism. In sum, these observations are consistent with a specific receptor-mediated translocation of EPO into the brain.
The inflammatory response elicited by trauma results in a permeable BBB and thus could “deliver” in a nonspecific way any plasma substance into the site of injury. Although this mechanism certainly could contribute to the site-specific delivery of r-Hu-EPO after an injury, it cannot explain the protective effects of 24-h pretreatment in the MCA occlusion and kainate models, for which the serum r-Hu-EPO levels were low at the time of injury. Further, a minimum effective dose of 450 units/kg BW argues against a nonspecific leakage into the brain, which presumably could take place at much lower dosages as only small amounts of r-Hu-EPO are needed intrathecally (7). Systemic delivery of r-Hu-EPO has the advantage in that it is universally available to the capillary endothelium, and thus potentially present everywhere in the brain, in contrast to intrathecal injection, which is highly localized and not practical in clinical settings.
The quantity of r-Hu-EPO we administered in the MCA occlusion studies is much higher than that needed for erythropoiesis and substantially higher than most conventional clinical dosages. Nonetheless, large doses equivalent to those administered in this study have been tested in preclinical and phase I clinical trials without adverse effects (36) and many cancer patients now receive r-Hu-EPO as a weekly injection of 40,000–60,000 units.‖ We reasoned that a bolus of r-Hu-EPO should be presented to the brain capillaries as quickly as possible to be available for transport across the BBB to provide protection. Using these conditions, we found that systemic administration of r-Hu-EPO in the MCA model extended neuroprotective effects for up to 6 h after the initiating injury. This window of protection may be explained by the ability of exogenous r-Hu-EPO to induce protective genes in potentially viable cells within the ischemic penumbra before they enter into programmed cell death. In acute in vivo situations, the endogenous production of EPO by astrocytes is likely not sufficient or fast enough to provide significant protection to adjacent neural tissue.
The manner in which r-Hu-EPO provides this impressive neuroprotection is currently unclear. R-Hu-EPO could rescue cells from death through modulation of apoptosis, a role defined for EPO action in erythropoiesis and later extended to neuronal-like cells in vitro (9, 10, 37), modulation of necrosis, or immune-mediated injury. All three forms of neuronal demise are thought to play a role in a wide variety of brain injury syndromes (reviewed in refs. 31–33). The experiments reported here were not designed to distinguish between these possibilities. Further work is obviously needed to evaluate these complex interactions in vivo.
Inflammatory processes play a key role in many forms of brain injury. The reduction in inflammatory infiltrate we observed in the blunt trauma experiments suggests that EPO may play an immunomodulatory role not previously described, but typical of many other cytokines. In the EAE model, both the inflammatory and immune systems are activated, whereas cell death is not thought to be a significant component of the clinical syndrome. The action of r-Hu-EPO in this model is to delay and blunt clinical manifestations in a manner consistent with known anti-inflammatory agents such as glucocorticoids. Whether one or both of these systems is responsible for the neuroprotective effects of EPO remains to be determined. However, our observation that mononuclear cell infiltration is reduced in r-Hu-EPO-treated animals suffering traumatic injury taken with the results of the EAE experiments suggest that both the inflammatory and immune responses are affected. If true, EPO may then exemplify a new therapeutic class.
In addition to an antiapoptotic role for EPO in the brain, one other function is suggested by the kainate experiments: r-Hu-EPO also can modulate neuronal excitability. However, EPO does not appear to have acute activity, clearly implying that its effects are secondary to activation of gene expression. The relevance of this type of neuronal activity to the now amply documented beneficial effects of r-Hu-EPO on quality of life (38) and possible improvement of cognitive function in patients receiving r-Hu-EPO (39) is intriguing.
Over the last decade, r-Hu-EPO has proven to be a safe therapeutic agent with minimal adverse effects. The results of studies presented here constitute a basis for examining the effectiveness of this agent for the treatment of several human maladies that currently are therapeutically underserved. Hopefully, r-Hu-EPO will prove as effective in humans as it appears to be in animals.
Acknowledgments
This research was supported in part by Ortho Biotech Inc. and The Kenneth S. Warren Laboratories. D.A. is a fellow of the Alfredo Leonardi Fund and G. L. Pfeiffer Foundation.
Abbreviations
- EPO
erythropoietin
- r-Hu-EPO
recombinant human EPO
- EPO-R
EPO receptor
- BBB
blood–brain barrier
- EAE
experimental autoimmune encephalomyelitis
- MCA
middle cerebral artery
- BW
body weight
Note Added in Proof.
In further support of the transport of r-Hu-EPO across the BBB, we have measured EPO in the cerebral spinal fluid of male rats obtained from the cisterna magna. The administration of r-Hu-EPO at 5,000 units/kg BW i.p. is associated with an ≈100 milliunits/ml increase in EPO in the spinal fluid within 30 min.
Footnotes
Gabrilove, J. L., Einhorn, L. H., Livingston, R. B., Winer, E. & Cleeland, C. S. (1999) Proc. Am. Soc. Clin. Oncol. 18, 2216 (abstr.).
References
- 1.Juul S E, Stallings S A, Christensen R D. Pediatr Res. 1999;46:543–547. doi: 10.1203/00006450-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Juul S E, Anderson D K, Li Y, Christensen R D. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Marti H H, Wenger R H, Rivas L A, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 4.Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. J Biol Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- 5.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 6.Bernaudin M, Marti H H, Roussel S, Divoux D, Nouvelot A, MacKenzie E T, Petit E. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Sakanaka M, Wen T C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel N S, Maltepe E, Goldwasser E, Mathieu C E, Simon M C, Schumacker P T. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabira T, Konishi Y, Gallyas F., Jr Int J Dev Neurosci. 1995;13:241–252. doi: 10.1016/0736-5748(94)00020-4. [DOI] [PubMed] [Google Scholar]
- 10.Konishi Y, Chui D H, Hirose H, Kunishita T, Tabira T. Brain Res. 1993;609:29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- 11.Campana W M, Misasi R, O'Brien J S. Int J Mol Med. 1998;1:235–241. doi: 10.3892/ijmm.1.1.235. [DOI] [PubMed] [Google Scholar]
- 12.Silva M, Grillot D, Benito A, Richard C, Nunez G, Fernandez-Luna J L. Blood. 1996;88:1576–1582. [PubMed] [Google Scholar]
- 13.Silva M, Benito A, Sanz C, Prosper F, Ekhterae D, Nunez G, Fernandez-Luna J L. J Biol Chem. 1999;274:22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- 14.Gregory T, Yu C, Ma A, Orkin S H, Blobel G A, Weiss M J. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- 15.Buemi M, Allegra A, Corica F, Floccari F, D'Avella D, Aloisi C, Calapai G, Iacopino G, Frisina N. Nephrol Dial Transplant. 2000;15:422–423. doi: 10.1093/ndt/15.3.422. [DOI] [PubMed] [Google Scholar]
- 16.Marti H H, Gassmann M, Wenger R H, Kvietikova I, Morganti-Kossmann M C, Kossmann T, Trentz O, Bauer C. Kidney Int. 1997;51:416–418. doi: 10.1038/ki.1997.55. [DOI] [PubMed] [Google Scholar]
- 17.Pardridge W M, Eisenberg J, Yang J. Metabolism. 1987;36:892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 18.Golden P L, Maccagnan T J, Pardridge W M. J Clin Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy K R, Pardridge W M, Rosenfeld R G. Metabolism. 1988;37:136–140. doi: 10.1016/s0026-0495(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 20.Pardridge W M. J Cereb Blood Flow. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Egrie J C, Strickland T W, Lane J, Aoki K, Cohen A M, Smalling R, Trail G, Lin F K, Browne J K, Hines D K. Immunobiology. 1986;172:213–224. doi: 10.1016/S0171-2985(86)80101-2. [DOI] [PubMed] [Google Scholar]
- 22.Egrie J C, Browne J, Lai P, Lin F K. Prog Clin Biol Res. 1985;191:339–350. [PubMed] [Google Scholar]
- 23.de Lanerolle N C, Kim J H, Brines M L. Clin Neurosci. 1994;2:64–81. [Google Scholar]
- 24.de Lanerolle N C, Kim J H, Robbins R J, Spencer D D. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 25.Juul S E, Yachnis A T, Christensen R D. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 26.Brines M L, Dare A O, de Lanerolle N C. Neurosci Lett. 1995;191:145–148. doi: 10.1016/0304-3940(95)11577-j. [DOI] [PubMed] [Google Scholar]
- 27.Coyle P. Stroke. 1982;13:855–859. doi: 10.1161/01.str.13.6.855. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerman G A, Meistrell M, 3rd, Bloom O, Cockroft K M, Bianchi M, Risucci D, Broome J, Farmer P, Cerami A, Vlassara H, et al. Proc Natl Acad Sci USA. 1995;92:3744–3748. doi: 10.1073/pnas.92.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon C E, Lighthall J W, Anderson T E. J Neurotrauma. 1988;5:91–104. doi: 10.1089/neu.1988.5.91. [DOI] [PubMed] [Google Scholar]
- 30.van der Meide P H, de Labie M C, Ruuls S R, Groenestein R J, Botman C A, Olsson T, Dijkstra C D. J Neuroimmunol. 1998;84:14–23. doi: 10.1016/s0165-5728(97)00207-5. [DOI] [PubMed] [Google Scholar]
- 31.Martin L J, Al-Abdulla N A, Brambrink A M, Kirsch J R, Sieber F E, Portera-Cailliau C. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 32.Juurlink B H, Paterson P G. J Spinal Cord Med. 1998;21:309–334. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- 33.Dirnagl U, Iadecola C, Moskowitz M A. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 34.Broadwell R D, Balin B J, Salcman M. Proc Natl Acad Sci USA. 1988;85:632–636. doi: 10.1073/pnas.85.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poduslo J F, Curran G L, Berg C T. Proc Natl Acad Sci USA. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung W K, Goon B L, Guilfoyle M C, Wacholtz M C. Clin Pharmacol Ther. 1998;64:412–423. doi: 10.1016/S0009-9236(98)90072-8. [DOI] [PubMed] [Google Scholar]
- 37.Ling Z D, Potter E D, Lipton J W, Carvey P M. Exp Neurol. 1998;149:411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- 38.Demetri G D, Kris M, Wade J, Degos L, Cella D. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 39.Pickett J L, Theberge D C, Brown W S, Schweitzer S U, Nissenson A R. Am J Kidney Dis. 1999;33:1122–1130. doi: 10.1016/S0272-6386(99)70150-2. [DOI] [PubMed] [Google Scholar]