Abstract
Altered redox signaling and regulation in cancer cells represent a chemical vulnerability that can be targeted by selective chemotherapeutic intervention. Here, we demonstrate that 3,7-diaminophenothiazinium-based redoxcyclers (PRC) induce selective cancer cell apoptosis by NAD(P)H:quinone oxidoreductase (NQO1)-dependent bioreductive generation of cellular oxidative stress. Using PRC lead compounds including toluidine blue against human metastatic G361 melanoma cells, apoptosis occurred with phosphatidylserine-externalization, loss of mitochondrial transmembrane potential, cytochrome C release, caspase-3 activation, and massive ROS production. Consistent with reductive activation and subsequent redoxcycling as the mechanism of PRC cytotoxicity, co-incubation with catalase achieved cell protection, whereas reductive antioxidants enhanced PRC-cytotoxicity. Unexpectedly, human A375 melanoma cells were resistant to PRC-induced apoptosis, and PRC-sensitive G361 cells were protected by preincubation with the NQO1-inhibitor dicoumarol. Indeed, NQO1 specific enzymatic activity was nine fold higher in G361 than in A375 cells. The critical role of NQO1 in PRC-bioactivation and cytotoxicity was confirmed, when NQO1-transfected breast cancer cells (MCF7-DT15) stably overexpressing active NQO1 displayed strongly enhanced PRC-sensitivity as compared to vector-control transfected cells with base line NQO1 activity. Based on the known overexpression of NQO1 in various tumors these findings suggest the feasibility of developing PRC lead compounds into tumor-selective bioreductive chemotherapeutics.
Keywords: Apoptosis, Bioreductive Activation, Cancer, Melanoma, Methylene Blue, NQO1, Phenothiazinium Redox Cycler, Redox Chemotherapy, ROS, Toluidine Blue
Introduction
The involvement of reactive oxygen species (ROS) in cancer initiation and progression is now firmly established. ROS-induced mutagenesis is an early causative factor in carcinogenesis, and ROS-mediated mitogenic signaling and redox modulation of apoptotic and survival pathways contribute to malignant transformation and progression [1–4]. ROS production from mitochondrial and other sources is increased in cancer cells leading to alterations of proliferative and apoptotic control by constitutive activation of multiple redox sensitive targets, including components of signaling cascades (e.g. Akt/protein kinase B and MAP kinases) as well as transcription factors (e.g. nuclear factor κB and activator protein 1) [5–7]. For example, constitutive activation of nuclear factor κB signaling in human melanoma cells occurs as a result of autocrine generation of ROS contributing to proliferative signaling and the notorious resistance of melanoma cells to induction of apoptosis by chemotherapeutic agents [8, 9]. In addition to substantiating the emerging role of the cellular redox environment in the general regulation of proliferation, differentiation, and survival [10], recent research has revealed the differential redox control of proliferation and viability in nontransformed versus malignant cells [1, 2, 6, 11]. For example, in nontransformed NIH 3T3 cells, constitutive ROS production occurs at low levels, whereas in CT26 colon and Hepa 1–6 liver tumor cells, high concentrations of ROS, close to the threshold of cytotoxicity, are produced by mitochondria. Importantly, increased in vitro proliferation of NIH 3T3 cells results from exposure to small molecule SOD mimetics that elevate H2O2 levels through superoxide dismutation, whereas extensive tumor cell death was observed as a consequence of the same redox intervention [1]. Thus, constitutively elevated levels of cellular oxidative stress and dependence on ROS-signaling may represent a redox vulnerability of malignancy that can be targeted by chemotherapeutic intervention using redox modulators, and both anti- and prooxidant agents have been shown to exert anti-cancer activity. According to this hypothesis, pro-oxidant pharmacological agents that substantially increase cellular ROS would induce deviations from redox homeostasis that do not reduce viability of untransformed cells, but cannot be tolerated by malignant cells that are already under high constitutive oxidative stress [1, 12]. Indeed, prooxidant redox agents including metal-based drugs such as dithiocarbamate chelates [13] and therapeutics in advanced clinical development such as texaphyrins [14] can achieve cancer cell-selective cytotoxicity [15, 16].
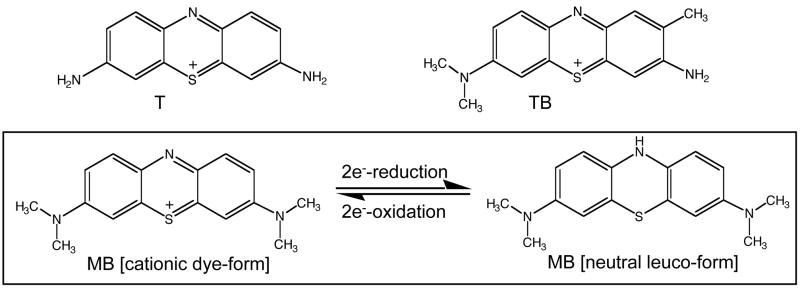
In an attempt to identify promising molecular agents that target the emerging redox Achilles heel of malignancy by selective induction of oxidative stress in cancer cells, we have examined the anti-cancer activity of 3,7-diaminophenothiazinium-derivatives, an established class of synthetic redox dyes. Phenothiazinium redox dyes have diverse applications as biological redox indicators, vital stains and diagnostic dyes [17], modulators of mitochondrial respiration [18], infusional antidotes against cyanide poisoning and methemoglobinemia [19], anti-infective agents [20, 21], and photosensitizers [22–24], but their therapeutic potential and mechanism of action as anti-cancer redox chemotherapeutics are largely unexplored [23, 25]. Compounds containing the 3,7-diaminophenothiazinium redox pharmacophore including thionine (T, 3,7-diamino-phenothiazinium acetate), methylene blue (MB, 3,7-bis(dimethylamino)-phenothiazinium chloride), and toluidine blue O (TB, 2-methyl-3-amino-7-dimethylamino-phenothiazinium chloride) are two-electron redox systems with standard reduction potentials [e.g. E′o (MB) = + 0.01 V] compatible with nonenzymatic and enzyme-dependent cycling between the oxidized dye-form and the colorless reduced leuco-form under cellular redox conditions as summarized in Fig. 1 [23, 26–28]. Spontaneous autoxidation of the leuco-form of these phenothiazinium redox cyclers (PRC) under physiological conditions can regenerate the dye-form [29]. Importantly, electron transfer from the PRC leuco-form to molecular oxygen may induce the nonenzymatic formation of ROS including H2O2, and spontaneous ROS formation by PRC redoxcycling may be driven by biological reducing agents including glutathione and NAD(P)H [26, 30, 31].
Figure 1. Phenothiazinium redox cyclers.
Compounds containing the 3,7-diaminophenothiazinium redox pharmacophore including thionine (T), toluidine blue (TB), and methylene blue (MB) are two-electron redox systems comprising an oxidized dye-form and a colorless reduced leuco-form.
Here, we demonstrate for the first time that (I) PRC compounds including MB and TB selectively induce cancer cell apoptosis, and that (II) NAD(P)H:quinone oxidoreductase (NQO1)-dependent bioreductive generation of intracellular oxidative stress is an important mechanistic determinant of cancer cell-sensitivity to PRC compounds.
Material and Methods
Chemicals
Most chemicals were from Sigma Chemical Co, St. Louis, MO. The cell permeable pancaspase inhibitor Z-VAD-(OMe)-fmk and the cell permeable caspase 8 inhibitor (Ac-AAVALLPAVLLALLAP-IETD-CHO) were from Calbiochem-Novabiochem, San Diego, CA. N-Benzoyl-leucomethylene blue was from ABCR GmbH, Karlsruhe, Germany.
General cell culture
G-361 Human melanoma cells from ATCC (Manassas, VA, USA) were cultured in McCoy’s 5a medium containing 10% bovine calf serum (BCS). Human A375 and LOX metastatic melanoma cells (ATCC) were cultured in RPMI medium containing 10% BCS and 2 mM L-glutamine. Human MIA PaCa-2 pancreatic carcinoma cells (ATCC) and two dermal neonatal foreskin fibroblast lines (Hs27 cells from ATCC and CF-3 cells, a gift from Dr. Robert Dell’Orco, Noble Center for Biomedical Research, Oklahoma City, USA) were cultured in DMEM containing 10% BCS. Human MDA-MB231 breast carcinoma cells were obtained from Dr. D. Zhang, University of Arizona, and cultured in MEM supplemented with 10% fetal bovine serum, 25 mM HEPES, 26 mM sodium bicarbonate, 5000 units/mL penicillin G, 5000 ug/mL streptomycin, and 6 ng/mL bovine insulin (Sigma, St. Louis, MO). Cells were maintained at 37 °C in 5% CO2, 95% air in a humidified incubator (referred to as ‘normoxia’). Cytotoxicity of test compounds was also assessed under hypoxic conditions (18 h at 1% O2, 5% CO2) using an Invivo Hypoxia Workstation 400 with a Ruskin hypoxic gas mixer (Biotrace, Cincinnati, OH).
Apoptosis analysis and assessment of cytotoxicity
Viability and induction of cell death (early and late apoptosis/necrosis) were examined by annexin-V-FITC/propidium iodide (PI) dual staining of cells followed by flow cytometric analysis as published previously [32, 33]. Cells (150,000) were seeded on 35 mm dishes and received drug treatment 24 hours later. To exclude photodynamic effects associated with phenothiazinium dyes cell manipulations were performed under dimmed light conditions or with complete light exclusion. Cells were harvested at various time points after treatment and cell staining was performed using an apoptosis detection kit according to the manufacturer’s specifications (APO-AF, Sigma, St. Louis, MO). The same methodology was used to establish the dose response relationship of PRC-induced cell death, and LD50 values (drug concentration that induces cell death in 50% of treated cells within 24 h exposure ± SD, n = 3) were established after 24h continuous exposure.
Mitochondrial Transmembrane Potential
Mitochondrial transmembrane potential (Δψm) was assessed using the potentiometric dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) following a published procedure [32]. At high Δψm, JC-1 forms red-fluorescent ‘J-aggregates’ distinct from the green-fluorescent monomer observed at low Δψm. J-aggregate formation increases linearly with Δψm over the range of 30–180 mV. In brief, cells were trypsinized, washed in PBS, resuspended in 300 μL PBS containing 5 μg/mL JC-1 for 15 min at 37°C and 5% CO2 in the dark, then washed twice in PBS and resuspended in 300 μL PBS. Bivariate analysis was performed by flow cytometry with excitation at 488 nm, and mitochondrial function was assessed as JC-1 green (depolarized mitochondria, detector FL-1) or red (polarized mitochondria, detector FL-2) fluorescence.
Caspase-3 activation assay
PRC-induced caspase-3 activation was examined in G361 melanoma cells using a cleaved/activated caspase-3 (asp 175) antibody (Alexa Fluor 488 conjugate, Cell Signaling, Inc.) followed by flow cytometric analysis according to the manufacturer’s procedure. Briefly, G361 cells (800,000) were exposed to PRC compounds and harvested by trypsinization. Cells were resuspended in PBS and fixed in 1% formaldehyde. Cells were then permabilized using 90% methanol and resuspended in incubation buffer (PBS, 0.5% BSA). After rinsing by centrifugation, cells were resuspended in incubation buffer (90 μl) and cleaved caspase-3 antibody (10 μl) was added. After incubation (40 min) followed by rinsing and centrifugation in incubation buffer, cells were resuspended in PBS and analyzed by flow cytometry.
Detection of cytochrome C release
Cells were harvested by trypsinization and resuspended in ice-cold isolation buffer (20 mM HEPES, pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 10 mM phenylmethanesulphonyl fluoride, 10 μM leupeptin, 250 mM sucrose) as described recently [34]. After homogenization using a Dounce homogenizer, the cell lysate was centrifuged at 12,000 g for 20 min at 4°C. Protein content of cytosolic fractions was determined using the BCA assay (Pierce) and samples containing 20 μg protein were analyzed by 15% reducing SDS-PAGE. After electrophoresis and Western transfer to nitrocellulose, the membrane was reversibly stained using Ponceau S (0.1% in 1% acetic acid) in order to confirm equal protein loading and transfer. Cytosolic cytochrome c was detected using a cytochrome c rabbit mAB (136F3, Cell Signaling, 1:1000) followed by enhanced chemiluminescence visualization (Amersham) using an HRP-conjugated goat anti-rabbit secondary antibody (Jackson laboratories, 1:10000).
Detection of intracellular oxidative stress by flow cytometric analysis
Drug-induced generation of intracellular oxidative stress was analyzed by flow cytometry using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) as a sensitive non-fluorescent precursor dye according to a published standard procedure [35]. In brief, PRC-treated and untreated control cells on dishes were washed with PBS and incubated for 60 min in the dark (37°C, 5% CO2) with culture medium containing DCFH-DA (5 μg/mL final concentration). Cells were then washed with PBS, harvested by trypsinization, resuspended in 300 μl PBS and immediately analyzed by flow cytometry.
Measurement of NQO1-specific activity
Determination of NQO1 specific activity was performed according to a published standard procedure [36]. In brief, cells (2 × 106) were harvested by trysinization and resuspended in ice-cold TE (20 mM Tris-HCl with 2 mM EDTA, pH 7.4). Cells were disrupted in three cycles of freeze/thawing using liquid nitrogen and a 37°C waterbath, followed by centrifugation (12,000 g, 5 min). Protein concentration in the supernatant was determined using the BCA assay (Pierce). For determination of NQO1 specific activity the reaction mixture (1 mL final volume) contained: 25 mM Tris-HCl (pH 7.4), 180 μM NADPH, BSA (0.2 mg/mL), Tween 20 [0.01 % (v/v)], and cell lysate (5 μl). The reaction was started by the addition of 2 μL 2,6-dichlorophenolindophenol (DCPIP, 20 mM stock in DMSO). Reduction of DCPIP was measured at room temperature for 1 min at 600 nm (ε = 21 × 103 M−1cm−1) with or without 20 μM dicoumarol. The dicoumarol-inhibitable part of DCPIP reduction was used to calculate NQO1 activity expressed as nmol DCPIP/mg protein/min. A minimum of triplicate cultures were assayed.
MCF-7 human breast cancer NQO1 transfectants
MCF-7 cells with increased NQO1 activity have been generated previously by stable transfection of an expression vector containing the rat NQO1 cDNA under the control of the β-actin promotor and encoding for neomycin resistance (neor) [37]. Control transfectants (MCF-7-neo2) and the NQO1 overexpressing clone MCF-7 DT15 with up to 30-fold greater NQO1 specific activity were obtained as a kind gift from Dr. M. Briehl, University of Arizona. Cells were maintained in DMEM containing 10% fetal bovine serum (FBS) and G418 (0.3 mg/mL).
Statistical Analysis
The results are presented as means ± S.D. of at least three independent experiments.
Results
Selective induction of G361 melanoma cell apoptosis by PRC compounds
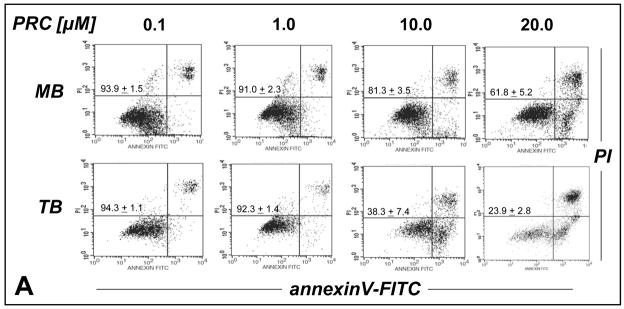
First, the potential of various phenothiazinium-type redox cyclers [thionine (T), methylene blue (MB), and toluidine blue O (TB)] for the chemotherapeutic induction of melanoma cell apoptosis was evaluated as summarized in Fig. 2. Using flow cytometric analysis of annexinV-FITC/propidium iodide-stained cells, dose-response relationship (Fig. 2A) and time course (Fig. 2B) of PRC-induction of melanoma cell apoptosis were established. All PRC compounds induced melanoma cell death in the low micromolar range. Among the PRC compounds tested TB was identified as the most potent apoptogenic agent that induced pronounced melanoma cell death within 18 h continuous exposure (Fig. 2B). The following apoptogenic potencies based on comparative LD50 determination (drug concentration that induces cell death in 50% of treated cells within 24 h exposure ± SD) were established: TB (7.8 + 1.1 μM) > MB (23.4 ± 7.3 μM) > T (26.3 + 5.9)] (data not shown). In contrast, no PRC-induction of cell death was observed when normal human Hs27 skin fibroblasts (or human CF3 fibroblasts, data not shown), an established and readily available model of untransformed proliferating human cells, were exposed to concentrations of MB (20 μM) or TB (10 μM) that were strongly apoptogenic in melanoma cells (Fig. 2C). These findings suggest that the apoptogenicity of MB and TB observed in cancer cells was not due to general cytotoxicity of these agents.
Figure 2. Preferential induction of apoptosis by PRC compounds in malignant G361 melanoma cells.
(A) Dose-response relationship (24 h continuous exposure) of induction of G361 melanoma cell apoptosis by the PRC compounds MB and TB (0.1–20 μM, each) were established by flow cytometric analysis of annexinV-FITC/propidium iodide-stained cells. (B) Time course of TB-induction (10 μM) of G361 melanoma cell apoptosis. (C) No induction of cell death was observed when normal human skin fibroblasts (Hs27) were exposed to MB (20 μM) or TB (10 μM, 24 h continuous exposure). Early apoptotic and late apoptotic/necrotic cells are located in the lower right (AV+, PI−) and upper right quadrant (AV+, PI+), respectively. One representative experiment of three similar repeats is shown. The numbers indicate viable cells (AV−, PI−, lower left quadrant) in percent of total gated cells (mean ± SD, n=3).
PRC-induced intracellular oxidative stress, loss of mitochondrial transmembrane potential, cytochrome C release, and caspase 3 activation
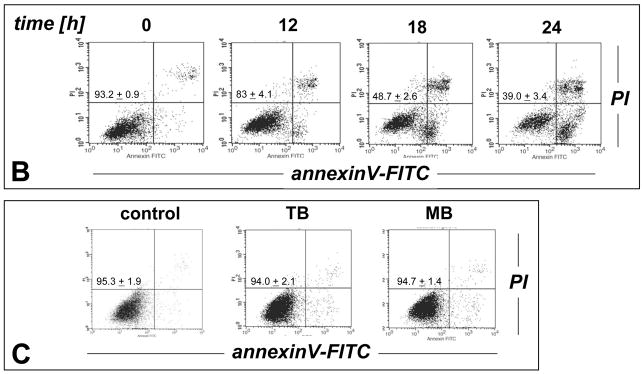
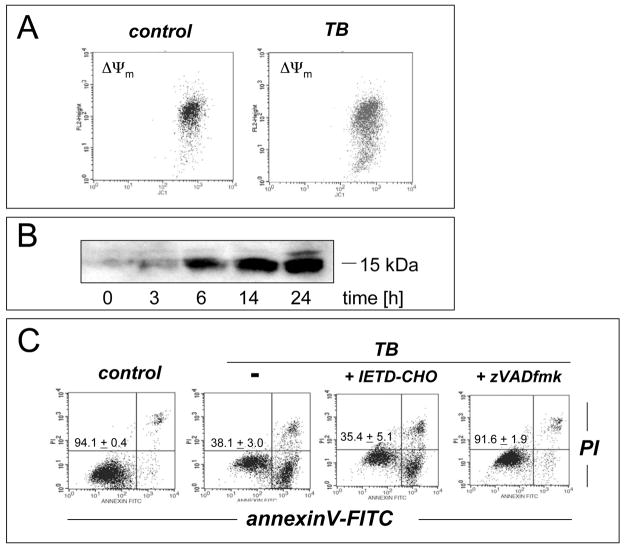
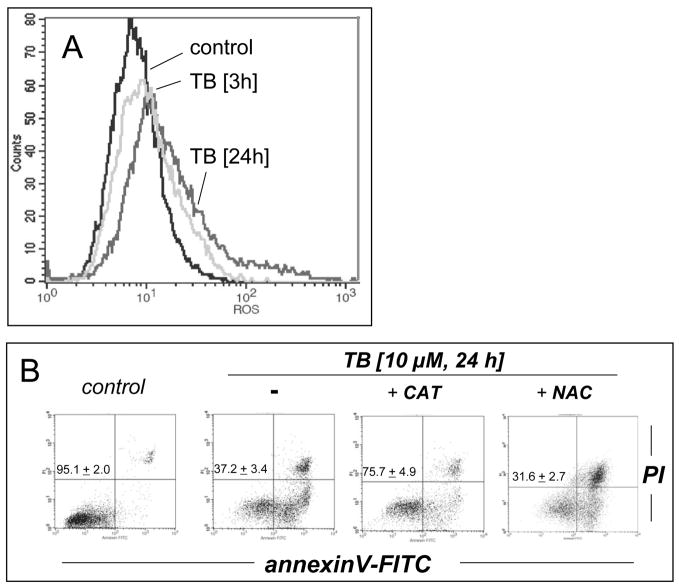
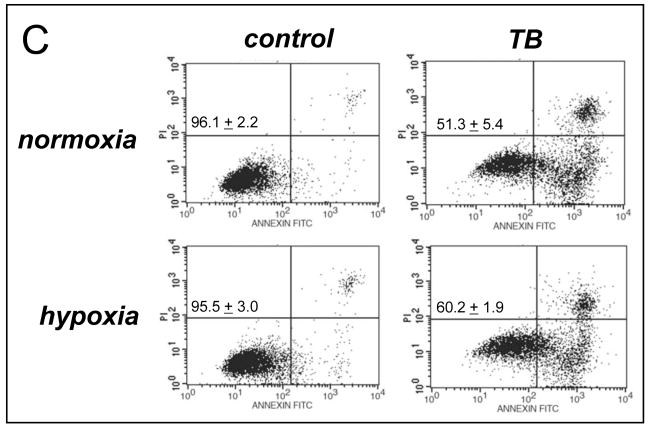
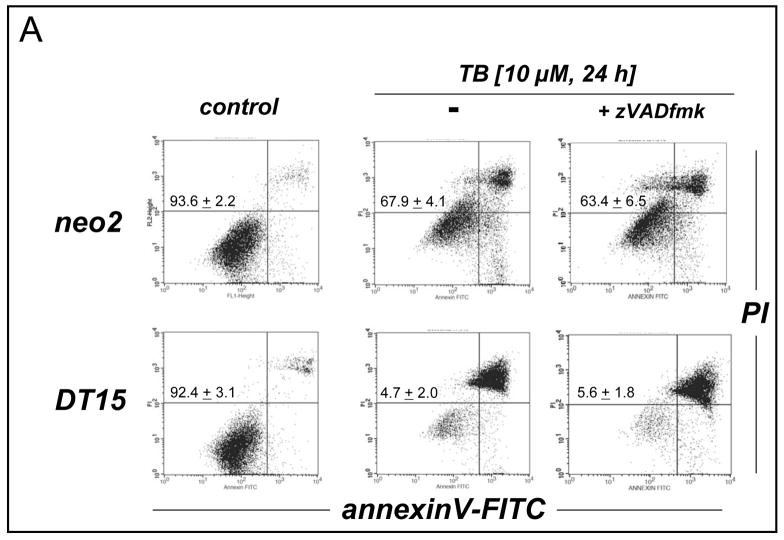
Next, the molecular mechanism of induction of melanoma cell death by TB, the most potent of the three PRC compounds examined above, was further elucidated. Consistent with TB-induction of apoptotic pathways of cell death, already suggested by annexinV-FITC staining indicative of apoptosis-specific phophatidylserine externalization as shown in Fig. 2A and B, it was demonstrated that TB-induced melanoma cell death (I) depends on caspase activation as evidenced by cell protection by pretreatment with the pan-caspase inhibitor Z-VAD(OMe)-fmk (Fig. 3C) [38], (II) does not depend on caspase 8-activation as evidenced by the lack of protective effects of pretreatment with the cell-permeable caspase 8 inhibitor peptide Ac-AAVALLPAVLLALLAP-IETD-CHO (Fig. 3C) [38], and (III) occurs dose dependently with activation of caspase-3 as evidenced by flow cytometric detection of cleaved procaspase-3 (Fig. 3D). Moreover, TB-induced cell death occurred with pronounced loss of mitochondrial transmembrane potential (Δψm) (Fig. 3A) and early mitochondrial release of cytochrome C (Fig. 3B), hallmarks of the intrinsic, mitochondria-controlled pathway of apoptosis known to be activated by cellular oxidative stress and various chemotherapeutics [39]. Indeed, TB-induced apoptosis was accompanied by massive ROS production as assessed by 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) staining followed by flow cytometric analysis (Fig. 4A). Increased cellular oxidative stress could be detected after 3h PRC-exposure of G361 melanoma cells and was even more pronounced after 24h treatment as obvious from an approximately fourfold increase in green fluorescence intensity over untreated control cells exposed to DCFH-DA only. The exact molecular nature of the oxidizing species involved in PRC-induced cytotoxicity was not examined at this point, and various ROS and other reactive species including PRC metabolites could participate in the intracellular oxidation of the nonfluorescent precursor dye [40]. However, H2O2 is a likely candidate mediator of PRC-induced intracellular oxidative stress, a hypothesis consistent with earlier reports that document H2O2 formation by PRC redox cycling [26]. PRC-induced formation of H2O2 in G361 melanoma cells was also suggested by the observation that significant protection against TB-induction of cell death was achieved, when G361 cells were co-treated with high doses of catalase (Fig. 4B). A similar degree of protection was achieved, when cells were co-treated with the cell-permable SOD- and catalase-mimetic EUK 134 (data not shown). In contrast, co-treatment using SOD (from bovine erythrocytes) or the small molecule SOD mimetic MnTBAP did not protect against PRC-induced apoptosis (data not shown), and flow cytometric analysis of dihydroethidium-stained PRC treated cells gave no indication for an involvement of superoxide radical anions as mediators of PRC-induced oxidative stress (data not shown). Pre-treatment (24 h, Fig. 4B) or co-treatment with various concentrations of Nα-acetyl-L-cysteine (NAC, 0.1 up to 10 mM) and other reducing antioxidants (L-ascorbate, data not shown) was equally ineffective and even enhanced PRC-cytotoxicity, consistent with a cyclic mechanism of ROS formation by PRC compounds that is based on spontaneous electron transfer from the reduced leucoform to molecular oxygen followed by subsequent reduction of the oxidized PRC compound by reducing factors including glutathione and NAD(P)H [26, 30]. Similar results with regard to caspase activation, mitochondrial cytochrome C release, and induction of intracellular oxidative stress were obtained, when G361 melanoma cells were exposed to 25 μM MB (data not shown). Moreover, no induction of cellular oxidative stress and apoptosis were observed, when G361 melanoma cells were exposed to N-benzoyl-leucomethylene blue, a redox inactive leuco-derivative of MB [41]. Taken together, these data provide evidence for a mechanism of PRC-induced melanoma cell apoptosis that depends on the initial generation of cellular oxidative stress by formation of ROS that triggers the mitochondrial pathway of apoptosis with cytochrome C release and activation of caspase-3. Next, we examined the possibility that the ROS dependence of PRC-induction of cancer cell apoptosis may limit the efficacy of theses agents under conditions of tumor hypoxia, an important hallmark of many cancers. To this end, potency of induction of G361 melanoma cell apoptosis by TB (10 μM, 18 hr) was examined in a regular cell culture incubator (‘normoxia’) and in a hypoxic chamber (1% oxygen, ‘hypoxia’) as summarized in Fig. 4C. TB-cytotoxicity was only slightly attenuated under hypoxic conditions with an approximately 9% increase in cell viability over cells treated under normoxic conditions. These data indicate that induction of melanoma cell apoptosis by PRC compounds occurs even under conditions of reduced oxygen availability, suggesting feasibility of using theses agents for cancer cell elimination even under conditions of tumor hypoxia.
Figure 3. TB-induction of mitochondrial apoptosis in human melanoma cells.
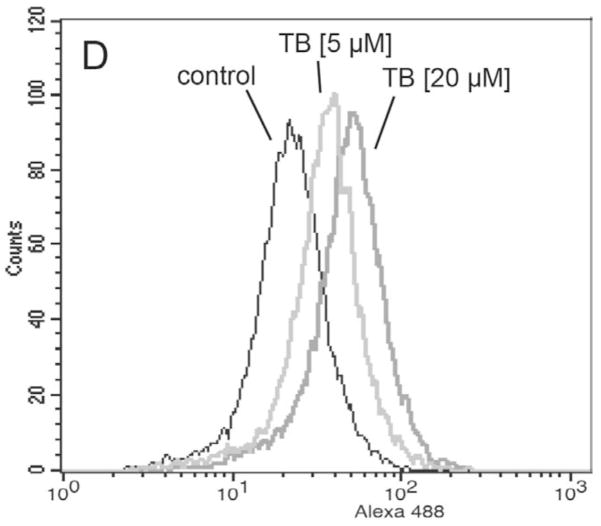
(A) G361 melanoma cells were exposed to TB (10 μM) as described above and loss of mitochondrial transmembrane potential Δψm was monitored after 18 hours using JC-1 flow cytometric analysis. (B) Mitochondrial release of cytochrome C in TB-treated melanoma cells was monitored over time by Western blot analysis of cytosolic protein fractions. Samples containing 20 μg protein were analyzed by 15% reducing SDS-PAGE. After Western transfer to nitrocellulose, equal protein loading and transfer were confirmed by Ponceau S staining and the membrane was probed for cytosolic cytochrome C. (C) Caspase-dependence of TB-induced melanoma cell apoptosis was examined by annexinV-PI flow cytometric analysis after pretreatment with cell-permeable pan-caspase [zVAD-fmk (42 μM)] and caspase 8 [IETD-CHO (42 μM)] inhibitors added 1 h before exposure to TB (10 μM, 24h). The numbers indicate viable cells (AV−, PI−, lower left quadrant) in percent of total gated cells (mean ± SD, n=3). (D) TB-induced (5 and 20 μM, 24 h) caspase-3 activation was examined by flow cytometric detection using an Alexa Fluor 488-conjugated monoclonal antibody against cleaved procaspase-3. One representative experiment of three similar repeats is shown.
Figure 4. TB-induction of intracellular oxidative stress in human melanoma cells.
(A) Generation of intracellular oxidative stress during TB-induced apoptosis (10 μM, 3 and 24 h) was assessed by 2′,7′-dichloro-dihydrofluorescein diacetate staining of human G361 melanoma cells followed by flow cytometric analysis. One representative experiment of three similar repeats is shown. (B) Protection against TB-induction of cell death (10 μM, 24 h) was detected by flow cytometric analysis of annexinV-FITC/propidium iodide-stained cells, when cells were co-treated with catalase (400 u per mL). In contrast, pre-treatment (24 h) with NAC (10 mM) followed by PBS wash and TB exposure (10 μM, 24 h) increased cytotoxicity. (C) Potency of induction of G361 cell apoptosis by TB (10 μM, 18 hr) was examined in a regular cell culture incubator (termed ‘normoxia’) and in a hypoxic chamber (1% oxygen, termed ‘hypoxia’) as detailed in Materials and Methods. The numbers indicate viable cells (AV−, PI−, lower left quadrant) in percent of total gated cells (mean ± SD, n=3).
Differential PRC-sensitivity of human melanoma cell lines and dicoumarol protection of PRC-sensitive G361 melanoma cells
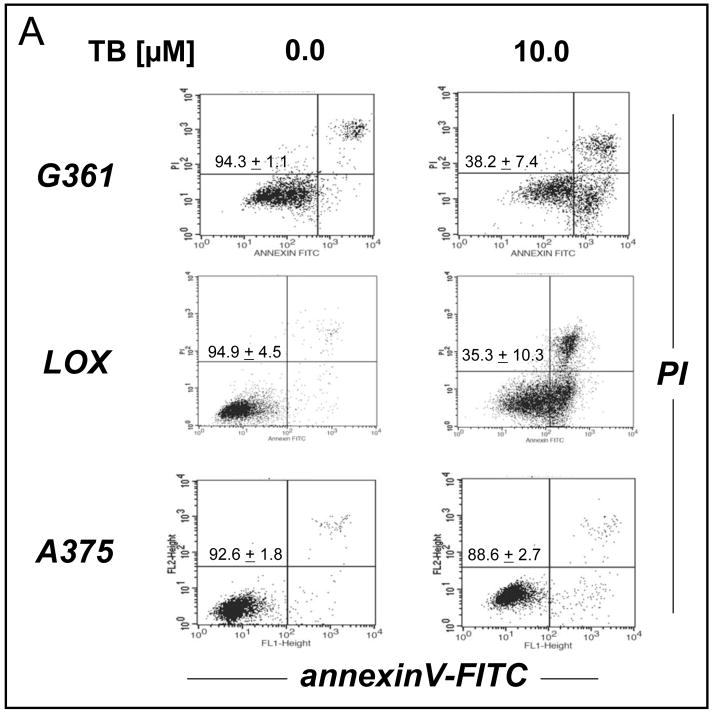
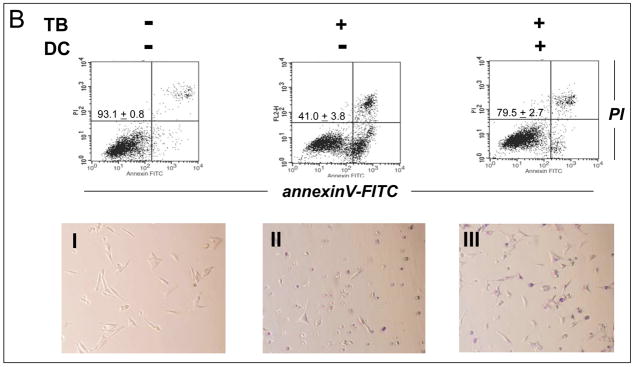
Based on the results obtained with G361 human melanoma cells, PRC-induction of cell death was examined in other human metastatic melanoma cell lines. As shown in Fig. 5A, LOX melanoma cells displayed high sensitivity to TB-induction of apoptosis that paralleled the earlier observations obtained with G361 melanoma cells. Unexpectedly, A375 human metastatic melanoma cells were highly resistant to TB-induced apoptosis, and significant apoptosis occurred only upon exposure to high doses above 40 μM (data not shown). Similar results were also obtained with MB (data not shown). Next, a range of small molecule redox and metabolic modulators was examined for potential protection of PRC-sensitive G361 cells against TB-induced apoptosis. Among all tested modulators including the mitochondrial electron transport chain (complex I) inhibitor rotenone, the flavoenzyme inhibitor diphenylene iodonium, the mitochondrial ATPase inhibitor oligomycin, and the NAD(P)H:quinone oxidoreductase (NQO1) inhibitor dicoumarol [42], only dicoumarol exhibited strong protective efficacy against MB- and TB-induction of G361 melanoma cell apoptosis as summarized in Fig. 5B (data obtained with MB not shown). TB-treatment reduced viability of G361 melanoma cells down to 40% within 24 hours continuous exposure, whereas viability of TB-treated cells was largely preserved by cotreatment with 30 μM dicoumarol as obvious from flow cytometric analysis of annexinV-FITC/PI-stained cells. Analysis by light microscopy (Fig. 5B, lower panels) further confirmed that dicoumarol co-treatment largely prevented the pronounced morphological changes observed in TB-treated G361 melanoma cells indicative of induction of apoptosis, including cell rounding, membrane blebbing, cytoplasmic condensation, and cell detachment (Fig. 5B, panels I-III). These findings demonstrate that PRC-induced apoptosis targets selected cancer cell lines and does not occur as a consequence of general cytotoxicity, a result already suggested by the complete PRC-resistance of human fibroblast cell lines presented in Fig. 2C. Moreover, dicoumarol protection of PRC-sensitive G361 melanoma cells indicates that NQO1 enzymatic activity may play a causative role in PRC-induction of G361 melanoma cell apoptosis.
Figure 5. Differential TB-sensitivity of cultured human melanoma cell lines and dicoumarol protection of TB-sensitive G361 melanoma cells.
(A) The dose response relationship of TB-induction of cell death was examined in G361, LOX, and A375 human metastatic melanoma cell lines using flow cytometry as indicated above. (B) Protection of PRC-sensitive G361 cells against TB-induced apoptosis (10 μM, 24 h) by dicoumarol (DC, 30 μM, added 1h before TB) was examined using flow cytometry (upper panels) and light microscopy (panels I-III) as described above. One representative experiment of three similar repeats is shown. The numbers in the upper panels indicate viable cells (AV−, PI−, lower left quadrant) in percent of total gated cells (mean ± SD, n=3).
NQO1-modulation of PRC-induced cancer cell death
Since protection of G361 melanoma cells against PRC-induced apoptosis by the specific NQO1-inhibitor dicoumarol strongly suggested NQO1 expression as a molecular determinant of cell-sensitivity to PRC-induced cell death, NQO1 specific enzymatic activity was determined in PRC sensitive (G361 and LOX) and PRC-resistant melanoma cell lines (A375) using the DCPIP reduction assay [36]. Indeed, NQO1 specific enzymatic activity was approximately nine fold higher in G361 than in A375 cells, and PRC-sensitive LOX melanoma cells displayed high NQO1 specific activity as summarized in Table 1. Moreover, the inverse relationship between NQO1 specific activity and TB-sensitivity (LD50) was also observed in various non-melanoma human cancer cell lines. Human MIA PaCa-2 pancreatic carcinoma cells, known to express high levels of active NQO1 [43], displayed high PRC-sensitivity, whereas human MDA-MB231 breast carcinoma cells known to constitutively express an inactive NQO1 mutant (NQO1*2/*2) were PRC-resistant [44]. In contrast, PRC-resistant normal human Hs27 and CF3 dermal fibroblasts (data not shown) displayed low basal NQO1 activity as summarized in Table 1.
Table 1. NQO1 specific enzymatic activity of PRC-sensitive and PRC-resistant malignant human cell lines.
NQO1 specific enzymatic activity of cytosolic protein preparations from diverse cancer and non-malignant cell lines was determined using the DCPIP reduction assay. TB-sensitivity was determined as potency of TB-induced cell death, expressed as the drug concentration that induces cell death in 50% of treated cells within 24 h exposure [LD50 ± SD, n=3] as specified in Materials and Methods.
| NQO1 specific activity [nmol DCPIP/mg protein/min] | LD50 [TB, μM] | |
|---|---|---|
| G361 | 2403 ± 215 | 7.8 ± 1.1 |
| LOX | 1387 ± 114 | 4.0 ± 0.7 |
| A375 | 283 ± 37 | > 40 |
| MDA-MB231 | N.D. [NQO1*2/*2] | > 40 |
| MIA-PaCa-2 | 1342 ± 187 | 11.4 ± 2.3 |
| MCF7-neo2 | 371 ± 42 | 24.6 ± 3.0 |
| MCF7-DT15 | 8558 ± 316 | 2.1 ± 0.4 |
| Hs27 | 203 ± 21 | > 40 |
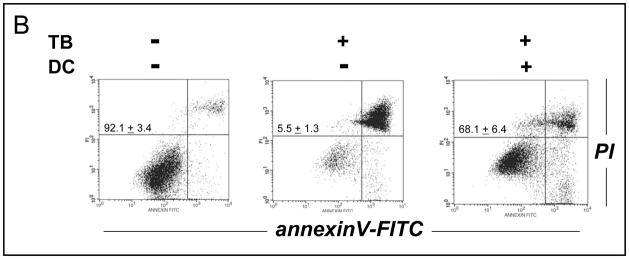
The critical role of NQO1 in PRC-bioactivation and apoptogenicity was further examined using NQO1-transfected human breast cancer cells (MCF7-DT15) stably overexpressing active NQO1 as summarized in Table 1 and Fig. 6 [37]. MCF7-DT15 cells display an approximately 20 to 25 fold increase in specific NQO1 enzymatic activity compared to vector-control transfected (MCF7-neo2) cells with moderate NQO1 activity, and these cells have been used earlier to demonstrate the NQO1-dependent cytotoxicity of the bioreductive indoloquinone anti-cancer drug EO9 [37]. Remarkably, MCF7-DT15 cells displayed an approximately 12 fold increased TB-sensitivity (based on LD50 values) as compared to MCF7-neo2 cells (Fig. 6A and Table 1). Unexpectedly, annexinV-FITC/PI flow cytometric analysis of TB-induced cell death in MCF7-DT15 cells revealed the necrotic nature of PRC-induced cytotoxicity in these cells: Over a time course of 24 h after addition of TB, cells stained increasingly positive for annexinV-FITC and PI (upper right quadrant), but no significant cell staining indicative of early apoptosis (annexinV-FITC positive/PI negative cells, lower right quadrant) was observed at any time after initiation of drug exposure. The necrotic nature of PRC-induced MCF7 cell death most likely originates from the known lack of caspase 3 expression and impaired apoptotic execution in these cells that have a nonfunctional caspase 3 gene due to a 47-base pair deletion within exon 3 [45]. Indeed, in stark contrast to our earlier observations with G361 melanoma cells (Fig. 3C), pan-caspase inhibition using zVADfmk did not protect MCF7-neo2 or MCF-DT15 against TB-induced cell death, consistent with a caspase-independent mechanism of cell elimination (Fig. 6A). Finally, pronounced cell protection against TB-induced MCF7-DT15 cell death was achieved by dicoumarol co-treatment as summarized in Fig. 6B. Almost 70% viability was maintained in TB-treated MCF7-DT15 cells that received co-treatment with dicoumarol, whereas almost complete necrotic elimination of TB-treated cells was observed in the absence of dicoumarol, a protective effect by this compound observed earlier with PRC-sensitive G361 melanoma cells (Fig. 5B). Taken together, these data strongly suggest the causative role of NQO1 overexpression and enzymatic activity in PRC-chemosensitization of MCF7-DT15 cells.
Figure 6. Increased TB-Sensitivity of MCF-7 NQO1-Transfectants.
(A) NQO1-transfected human breast cancer cells (MCF7-DT15) stably overexpressing active NQO1 and vector-control transfected (MCF7-neo2) cells were exposed to TB (10 μM, 24 h) and cell death was analyzed by flow cytometric analysis as described above. Caspase dependence of TB-induced cell death was examined using zVAD-fmk as indicated above (Fig. 3C) (B) TB-induced cell death of MCF7-DT15 was modulated by preincubation with dicoumarol (DC) as detailed in Fig. 5B. The numbers indicate viable cells (AV−, PI−, lower left quadrant) in percent of total gated cells (mean ± SD, n=3). One representative experiment of three similar repeats is shown.
Discussion
The emerging causative role of redox alterations in cancer cell proliferative control, survival, invasion, and metastasis suggests feasibility of using pro- and antioxidant redox modulators for molecularly targeted chemotherapeutic intervention. Here we demonstrate that dyes that contain a redox active 3,7-diaminophenothiazinium pharmacophore (PRC compounds) selectively target melanoma and other cancer cells with induction of apoptosis and that expression of NAD(P)H:quinone oxidoreductase (NQO1) enzymatic activity is an important determinant of PRC-cytotoxicity, most likely due to NQO1-dependent PRC-mediated bioreductive generation of cellular oxidative stress.
Due to their redox versatility, extensive research has examined the clinical use of various PRC compounds as antimicrobial, antimethemoglobinemic, and photodynamic agents [17]. Importantly, MB is currently used systemically for the treatment of urinary bladder infections and as an intravenous emergency medication for cyanide poisoning and the reversal of drug-induced methemoglobinemia [19] suggesting the feasibility of administering these agents as systemic chemotherapeutic agents. Earlier reports have described cytotoxic effects of selected PRC compounds, which occur independent of their action as light-activated photodynamic agents [23, 25]. Interestingly, some PRC compounds have high affinity for human tumor tissue in vivo, an effect that has been used earlier for experimental melanoma radiotherapy using single injections of 131I-labeled MB [46]. Moreover, MB is currently in clinical use as a diagnostic tool for sentinel lymph node biopsy in breast cancer surgery and esophageal chromoendoscopy [48], and TB is currently employed for the detection of oral epithelial dysplasia and squamous cell carcinoma [47].
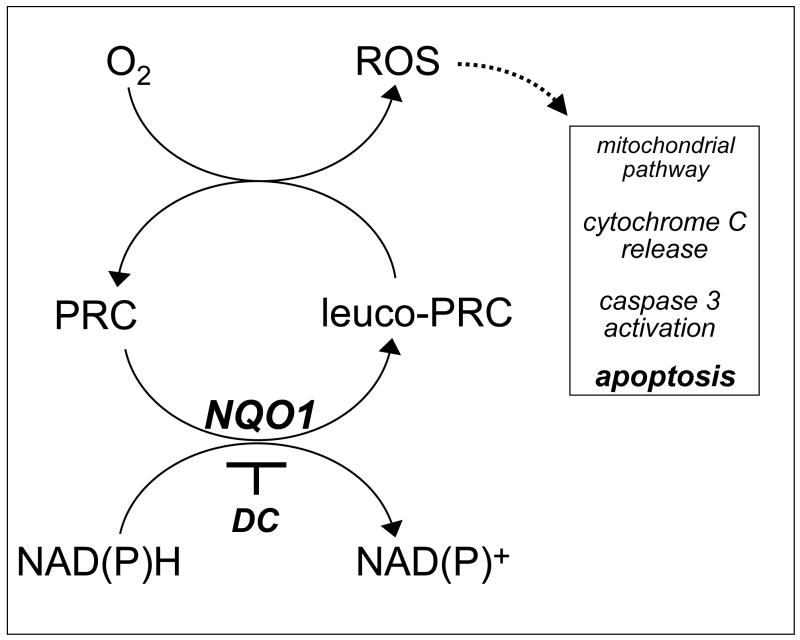
Our experimental evidence supports a novel role of PRC compounds as cancer cell-selective redox chemotherapeutics and elucidates the molecular mechanism of their apoptogenic activity. Using the PRC prototype agents T, MB, and TB in the low micromolar range, apoptotic elimination of human metastatic G361 melanoma cells was achieved. In contrast, no PRC-cytotoxicity was observed in untransformed human skin Hs27 and CF3 fibroblasts at concentrations that were strongly apoptogenic against G361 melanoma cells (Fig. 2C). Consistent with PRC-induction of the mitochondrial (intrinsic) pathway of apoptosis triggered by oxidative stress, MB- and TB-induced melanoma cell death occurred with early phosphatidylserine-externalization, loss of mitochondrial transmembrane potential, cytochrome C release, and caspase 3 activation, and was accompanied by induction of pronounced intracellular oxidative stress (Figs. 3 and 4) [39]. Similar results were obtained when other cultured cancer cell lines including LOX melanoma and MIA PaCa-2 pancreas carcinoma cells were exposed to PRC compounds (Fig. 5A and Table 1). However, other human cancer cell lines including A375 melanoma and MDA-MB231 breast carcinoma were highly resistant to PRC-induced apoptosis. Importantly, PRC-sensitive G361 cells were protected from PRC-cytotoxicity by pre-incubation with the specific NQO1-inhibitor dicoumarol suggesting a role of NQO1 cellular specific activity in the bioactivation of PRC cytotoxicity (Fig. 5B). Indeed, an inverse relationship between cellular specific NQO1 activity and LD50 values for TB-induced cancer cell apoptosis was observed (Table 1). The critical role of NQO1 in PRC-bioactivation and apoptogenicity was confirmed, when NQO1-transfected human breast cancer cells (MCF7-DT15) stably overexpressing active NQO1 displayed strongly enhanced PRC-sensitivity as compared to vector-control transfected (MCF7-neo2) cells with base line NQO1 activity (Fig. 6A). Dicoumarol co-treatment provided significant protection of NQO1 overexpressing cells against PRC-cytotoxicity (Fig. 6B). A reaction scheme compatible with our results is shown in Fig. 7, where enzymatic reduction by NQO1 is an important mechanistic determinant of bioreductive PRC-activation and apoptogenicity against cancer cells. In addition, PRC compounds including MB and TB are known to undergo spontaneous redoxcycling, where non-enzymatic PRC-reduction by NAD(P)H is followed by ROS formation, particularly H2O2, via autoxidation of the reduced leuco form of the dye by molecular oxygen [26]. This nonenzymatic mechanism of PRC activation together with alternative enzymatic reductive pathways may also contribute to PRC-induced cancer cell elimination independent of NQO1 activity, consistent with significant PRC-cytotoxicity observed even in the presence of dicoumarol (Figs. 5B and 6B).
Figure 7. PRC compounds as potential redox chemotherapeutics for the targeted induction of cancer cell apoptosis.
PRC compounds may selectively target cancer cells with high NQO1 enzymatic activity by oxidative-stress dependent induction of the mitochondrial pathway of apoptosis. In this model, NQO1-dependent bioreductive activation is followed by spontaneous electron transfer between reduced PRC leuco-form and molecular oxygen leading to intracellular ROS formation with regeneration of the oxidized PRC dye-form. Consistent with this model, NQO1-inhibition by dicoumarol (DC), antioxidant intervention by catalase, and caspase inhibition by zVADfmk suppress PRC-induced apoptosis in human G361 melanoma cells. See discussion for detailed explanation.
NQO1 (EC 1.6.99.2, also called DT-diaphorase) is a flavoprotein that catalyzes the obligatory two-electron reduction of quinones and quinone imines using NAD(P)H [42], and PRC compounds are known NQO1 substrates that are reductively converted into the leucoform [27, 29, 49, 50]. Importantly, constitutive NQO1 overexpression leading to high specific enzymatic activity is associated with various human malignancies including non-small cell lung cancer, pancreas carcinoma, and breast and colon adenocarcinoma, and specific enzymatic activity can differ by up to 20 fold between malignant and unaffected surrounding tissue [42, 51]. Recent research suggests that NQO1-overexpression in tumors may serve to accommodate the needs of rapidly metabolizing cells to regenerate NAD+ from NADH, a metabolic adaptation of cancer cells that ensures high glycolytic flux independent of NAD+ regeneration by mitochondrial respiration that is often compromised in cancer cells [43]. Based on the known overexpression and increased specific enzymatic activity of NQO1 in various human tumors, NQO1-activated bioreductive alkylating agents including mitomycin C and other aziridinylquinones (EO9, diaziquone, RH1) have been developed as molecularly targeted anti-cancer chemotherapeutic prodrugs [42]. In analogy, PRC compounds may therefore represent a novel class of bioreductive experimental anti-cancer agents that eliminate cancer cells by NQO1-driven redox cycling with induction of apoptosis without imposing the burden of genotoxic alkylating stress observed with conventional bioreductive chemotherapeutic agents [52]. Interestingly, enhanced cytotoxicity of mitomycin C can be achieved in human tumor cells treated with small molecule inducers of NQO1 including sulforaphane and 1,2-dithiole-3-thione [53], and it remains to be seen if NQO1-induction by these classic chemopreventive inducers of phase II detoxification enzymes including NQO1 further sensitizes cancer cells to PRC-induction of apoptosis.
Our studies on PRC-induction of cancer cell-selective apoptosis suggest that simple PRC compounds such as MB and TB may serve as lead compounds for future development of more advanced experimental therapeutics. Further preliminary studies to be published elsewhere indicate that the octanol/PBS partition coefficient (log P oct/PBS) of a selected PRC compound correlates positively with its cytotoxic potency, suggesting that compound lipophilicity is an important structural determinant that may explain the differential apoptogenicity of T, MB, TB, and other more lipophilic PRC compounds. However, the detailed structure-activity relationship (SAR) of PRC-induction of cancer cell apoptosis remains to be elucidated, and future preclinical PRC-drug development will aim at refining compound lipophilicity, redox potential, and NQO1-dependent activation in order to optimize cancer-cell selectivity and apoptogenic potency.
In summary, our experiments suggest that PRC compounds may constitute a unique class of anti-cancer redox chemotherapeutics that promises to achieve selectivity based not only on the known vulnerability of cancer cells to prooxidant intervention but also on tumor-specific NQO1-expression as an important molecular determinant of bioreductive activation and apoptogenicity. Feasibility of using PRC compounds as redox chemotherapeutics for anti-cancer intervention in vivo is currently tested in murine xenograft models of human pancreatic carcinoma and melanoma.
Acknowledgments
We are grateful for receiving NQO1-transfected MCF7 cells as a gift from Dr. Margaret Briehl, AZCC, University of Arizona. Technical support from Barb Carolus and Debbie Sakiestewa, AZCC Flow Cytometry Core Facility, is gratefully acknowledged. This research was supported in part by grants from NIH (SPORE in GI Cancer CA95060; ES06694) and Arizona Biomedical Research Commission (ABRC 0721). Preliminary data from this research were part of an oral presentation at the 13th Annual Meeting of the Society for Free Radical Biology and Medicine, November 18, 2006, in Denver, CO.
Abbreviations
- AV
annexinV
- DC
dicoumarol
- DCPIP
2,6-dichlorophenolindophenol
- GSH
glutathione
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide
- MB
methylene blue
- NAC
Nα-acetyl-L-cysteine
- NQO1, NAD(P)H
quinone oxidoreductase
- PI
propidium iodide
- PRC
phenothiazinium redox cycler
- ROS
reactive oxygen species
- SDS-PAGE
Sodium Dodecylsulfate Polyacrylamide Gel Electrophoresis
- T
thionine
- TB
toluidine blue O
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. [PubMed] [Google Scholar]
- 2.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–43. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 3.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 4.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–70. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 5.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Rosenberger SF, Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;20:2063–73. doi: 10.1093/carcin/20.11.2063. [DOI] [PubMed] [Google Scholar]
- 7.Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28:1338–48. doi: 10.1016/s0891-5849(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 8.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, et al. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–24. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 9.Brar SS, Kennedy TP, Whorton AR, Sturrock AB, Huecksteadt TP, Ghio AJ, et al. Reactive oxygen species from NAD(P)H:quinone oxidoreductase constitutively activate NF-kappaB in malignant melanoma cells. Am J Physiol Cell Physiol. 2001;280:C659–76. doi: 10.1152/ajpcell.2001.280.3.C659. [DOI] [PubMed] [Google Scholar]
- 10.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 11.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, et al. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]; 11 Meyskens FL, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–154. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 12.Hileman EO, Liu J, Albitar M, Keating MJ, Huang P. Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity. Cancer Chemother Pharmacol. 2004;53:209–19. doi: 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- 13.Cen D, Brayton D, Shahandeh B, Meyskens FL, Jr, Farmer PJ. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47:6914–20. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 14.Miller RA, Woodburn KW, Fan Q, Lee I, Miles D, Duran G, et al. Motexafin gadolinium: a redox active drug that enhances the efficacy of bleomycin and doxorubicin. Clin Cancer Res. 2001;7:3215–21. [PubMed] [Google Scholar]
- 15.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–40. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Fry FH, Holme AL, Giles NM, Giles GI, Collins C, Holt K, et al. Multifunctional redox catalysts as selective enhancers of oxidative stress. Org Biomol Chem. 2005;3:2579–87. doi: 10.1039/b502197a. [DOI] [PubMed] [Google Scholar]
- 17.Moura JC, Cordeiro N. 3,7-bis(dialkylamino)phenothiazin-5-ium derivatives: biomedical applications and biological activity. Curr Drug Targets. 2003;4:133–41. doi: 10.2174/1389450033346902. [DOI] [PubMed] [Google Scholar]
- 18.Visarius TM, Stucki JW, Lauterburg BH. Stimulation of respiration by methylene blue in rat liver mitochondria. FEBS Lett. 1997;412:157–60. doi: 10.1016/s0014-5793(97)00767-9. [DOI] [PubMed] [Google Scholar]
- 19.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–56. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 20.Guttmann P, Ehrlich P. Ueber die Wirkung des Methylenblau bei Malaria. Berliner Klin Wochenschrift. 1891;28:953–6. [Google Scholar]
- 21.Atamna H, Krugliak M, Shalmiev G, Deharo E, Pescarmona G, Ginsburg H. Mode of antimalarial effect of methylene blue and some of its analogues on Plasmodium falciparum in culture and their inhibition of P. vinckei petteri and P. yoelii nigeriensis in vivo. Biochem Pharmacol. 1996;51:693–700. doi: 10.1016/s0006-2952(95)02258-9. [DOI] [PubMed] [Google Scholar]
- 22.Wondrak GT, Jacobson MK, Jacobson EL. Identification of quenchers of photoexcited states as novel agents for skin photoprotection. J Pharmacol Exp Ther. 2005;312:482–91. doi: 10.1124/jpet.104.075101. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright M, Phoenix DA, Rice L, Burrow SM, Waring J. Increased cytotoxicity and phototoxicity in the methylene blue series via chromophore methylation. J Photochem Photobiol B. 1997;40:233–9. doi: 10.1016/s1011-1344(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay JF, Dussault S, Viau G, Gad F, Boushira M, Bissonnette R. Photodynamic therapy with toluidine blue in Jurkat cells: cytotoxicity, subcellular localization and apoptosis induction. Photochem Photobiol Sci. 2002;1:852–6. doi: 10.1039/b204385h. [DOI] [PubMed] [Google Scholar]
- 25.Kirszberg C, Rumjanek VM, Capella MA. Methylene blue is more toxic to erythroleukemic cells than to normal peripheral blood mononuclear cells: a possible use in chemotherapy. Cancer Chemother Pharmacol. 2005;56:659–65. doi: 10.1007/s00280-005-1014-3. [DOI] [PubMed] [Google Scholar]
- 26.Biaglow JE, Koch CJ, Tuttle SW, Manevich Y, Ayene IS, Bernhard EJ, et al. The measurement of bioreductive capacity of tumor cells using methylene blue. Int J Radiat Oncol Biol Phys. 1998;42:769–73. doi: 10.1016/s0360-3016(98)00313-7. [DOI] [PubMed] [Google Scholar]
- 27.Rice L, Phoenix DA, Wainwright M, Waring JJ. Effect of increasing methylation on the ability of methylene blue to cause diaphorase-catalyzsed oxidation of NADH. Biochem Soc Trans. 1998;26:S319. doi: 10.1042/bst026s319. [DOI] [PubMed] [Google Scholar]
- 28.Wainwright M, Amaral L. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop Med Int Health. 2005;10:501–11. doi: 10.1111/j.1365-3156.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 29.Audi SH, Olson LE, Bongard RD, Roerig DL, Schulte ML, Dawson CA. Toluidine blue O and methylene blue as endothelial redox probes in the intact lung. Am J Physiol Heart Circ Physiol. 2000;278:H137–50. doi: 10.1152/ajpheart.2000.278.1.H137. [DOI] [PubMed] [Google Scholar]
- 30.Kelner MJ, Alexander NM. Methylene blue directly oxidizes glutathione without the intermediate formation of hydrogen peroxide. J Biol Chem. 1985;260:15168–71. [PubMed] [Google Scholar]
- 31.Andranovich T, Kelner MJ. In vitro oxidation of uric acid in serum by methylene blue. Clin Chem. 1986;32:177–9. [PubMed] [Google Scholar]
- 32.Wondrak GT, Jacobson MK, Jacobson EL. Antimelanoma activity of apoptogenic carbonyl scavengers. J Pharmacol Exp Ther. 2006;316:805–14. doi: 10.1124/jpet.105.094953. [DOI] [PubMed] [Google Scholar]
- 33.Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. 3-hydroxypyridine chromophores are endogenous sensitizers of photooxidative stress in human skin cells. J Biol Chem. 2004;279:30009–20. doi: 10.1074/jbc.M404379200. [DOI] [PubMed] [Google Scholar]
- 34.Choi HJ, Yee SB, Park SE, Im E, Jung JH, Chung HY, et al. Petrotetrayndiol A induces cell cycle arrest and apoptosis in SK-MEL-2 human melanoma cells through cytochrome c-mediated activation of caspases. Cancer Lett. 2006;232:214–25. doi: 10.1016/j.canlet.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Pani G, Colavitti R, Bedogni B, Anzevino R, Borrello S, Galeotti T. Determination of intracellular reactive oxygen species as function of cell density. Methods Enzymol. 2002;352:91–100. doi: 10.1016/s0076-6879(02)52010-3. [DOI] [PubMed] [Google Scholar]
- 36.De Haan LH, Boerboom AM, Rietjens IM, van Capelle D, De Ruijter AJ, Jaiswal AK, et al. A physiological threshold for protection against menadione toxicity by human NAD(P)H:quinone oxidoreductase (NQO1) in Chinese hamster ovary (CHO) cells. Biochem Pharmacol. 2002;64:1597–603. doi: 10.1016/s0006-2952(02)01383-7. [DOI] [PubMed] [Google Scholar]
- 37.Siemankowski LM, Morreale J, Butts BD, Briehl MM. Increased tumor necrosis factor-alpha sensitivity of MCF-7 cells transfected with NAD(P)H:quinone reductase. Cancer Res. 2000;60:3638–44. [PubMed] [Google Scholar]
- 38.Dobos J, Timar J, Bocsi J, Burian Z, Nagy K, Barna G, et al. In vitro and in vivo antitumor effect of 2-methoxyestradiol on human melanoma. Int J Cancer. 2004;112:771–6. doi: 10.1002/ijc.20473. [DOI] [PubMed] [Google Scholar]
- 39.Debatin KM, Poncet D, Kroemer G. Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene. 2002;21:8786–803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 40.Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27:146–59. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 41.Auerbach BJ, Kiely JS, Cornicelli JA. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem. 1992;201:375–80. doi: 10.1016/0003-2697(92)90354-a. [DOI] [PubMed] [Google Scholar]
- 42.Danson S, Ward TH, Butler J, Ranson M. DT-diaphorase: a target for new anticancer drugs. Cancer Treat Rev. 2004;30:437–49. doi: 10.1016/j.ctrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, Zhang YP, et al. Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003;63:5513–20. [PubMed] [Google Scholar]
- 44.Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–44. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 45.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 46.Link EM, Costa DC, Lui D, Ell PJ, Blower PJ, Spittle MF. Targeting disseminated melanoma with radiolabelled methylene blue: Comparative bio-distribution studies in man and animals. Acta Oncol. 1996;35:331–41. doi: 10.3109/02841869609101650. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, Durham S, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–21. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 48.Thevarajah S, Huston TL, Simmons RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg. 2005;189:236–9. doi: 10.1016/j.amjsurg.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 49.Shaw PM, Reiss A, Adesnik M, Nebert DW, Schembri J, Jaiswal AK. The human dioxin-inducible NAD(P)H: quinone oxidoreductase cDNA-encoded protein expressed in COS-1 cells is identical to diaphorase 4. Eur J Biochem. 1991;195:171–6. doi: 10.1111/j.1432-1033.1991.tb15691.x. [DOI] [PubMed] [Google Scholar]
- 50.Ernster L, Danielson L, Ljunggren M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962;58:171–88. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- 51.Lewis AM, Ough M, Hinkhouse MM, Tsao MS, Oberley LW, Cullen JJ. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol Carcinog. 2005;43:215–24. doi: 10.1002/mc.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beall HD, Winski SI. Mechanisms of action of quinone-containing alkylating agents. I: NQO1-directed drug development. Front Biosci. 2000;5:D639–48. doi: 10.2741/beall. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Doherty GP, Leith MK, Curphey TJ, Begleiter A. Enhanced cytotoxicity of mitomycin C in human tumour cells with inducers of DT-diaphorase. Br J Cancer. 1999;80:1223–30. doi: 10.1038/sj.bjc.6690489. [DOI] [PMC free article] [PubMed] [Google Scholar]