Abstract
Spermatogenesis requires intact, fully competent Sertoli cells. Here, we investigate the functions of Dicer, an RNaseIII endonuclease required for microRNA and small interfering RNA biogenesis, in mouse Sertoli cell function. We show that selective ablation of Dicer in Sertoli cells leads to infertility due to complete absence of spermatozoa and progressive testicular degeneration. The first morphological alterations appear already at postnatal day 5 and correlate with a severe impairment of the prepubertal spermatogenic wave, due to defective Sertoli cell maturation and incapacity to properly support meiosis and spermiogenesis. Importantly, we find several key genes known to be essential for Sertoli cell function to be significantly down-regulated in neonatal testes lacking Dicer in Sertoli cells. Overall, our results reveal novel essential roles played by the Dicer-dependent pathway in mammalian reproductive function, and thus pave the way for new insights into human infertility.
Keywords: Dicer, microRNAs, Sertoli cells, germ cells, testis, spermatogenesis
Introduction
Spermatogenesis refers to the development of mature haploid spermatozoa from diploid spermatogonial germ cells and ensures continuous gamete production throughout the adult life of males. Sertoli cells (SCs), one of the somatic constituents of the testis, have long been known to play an essential role in spermatogenesis. They extend from the base to the apex of the seminiferous epithelium, and are in direct physical association with all types of germ cells. During embryonic development, SCs play a critical role in the formation of the testis (for review see (Brennan and Capel, 2004)), whereas during adulthood they are entirely committed to sustaining spermatogenesis. Adult SCs are entirely committed to sustaining spermatogenesis; they provide germ cells with structural and nutritional support, assist their movement, produce seminiferous fluid and support spermiation (reviewed in (Jegou, 1992)). Importantly, one SC can support only a finite number of germ cells, therefore the ultimate adult testis size and eventual sperm production is directly linked to the total SC number (Orth et al., 1988). The latter is already established by around P15 in mice, when, after extensive proliferative activity, SCs cease dividing and switch from a fetal, ‘immature’ to an adult, ‘mature’ state. This maturation is characterized by radical morphological and functional changes, the most characteristic being the formation of the blood-testis barrier (BTB) at the level of adjacent SC tight junctions and the commitment of SCs to sustain germ cell progression through meiosis and differentiation into spermatozoa (reviewed in (Mruk and Cheng, 2004; Sharpe et al., 2003)). Thus, the adult spermatogenic outcome is not only dependent on the SC number, but also on their functional integrity.
Regulation of spermatogenesis at the post-transcriptional level, particularly during spermiogenesis, was early shown to be of crucial importance (reviewed in (Braun, 1998)). Recently, a novel mechanism of post-transcriptional regulation mediated by microRNAs (miRNAs) has emerged (for review see (Pillai et al., 2007)). MicroRNAs are endogenous, small (19-25 nucleotides), non-coding RNAs that act as negative post-transcriptional regulators of gene expression and control diverse aspects of development in several species. In animals, the majority of miRNAs regulate their target mRNAs by inhibiting their translation; however some may regulate their targets by inducing their degradation (Lim et al., 2005). Dicer is an RNaseIII endonuclease essential for miRNA processing; its deletion, which leads to complete loss of mature miRNAs, is lethal at E7.5 in mice. Importantly, Dicer acts as a ‘transcriptional regulator’ itself, since it plays a role in the structural maintenance and silencing of centromeres in murine ES cells (Kanellopoulou et al., 2005).
The functional relevance of Dicer and miRNAs in spermatogenesis is only starting to be unraveled. Several miRNAs are specifically expressed or enriched in the testis (Ro et al., 2007a; Yan et al., 2007) and all essential members of the RNA interference (RNAi) machinery (Drosha, Dicer, Ago2) are expressed in SCs, meiotic and postmeiotic germ cells (Gonzalez-Gonzalez et al., 2008; Kotaja et al., 2006). In fact Dicer was recently reported to be required for primordial germ cell development and spermatogenesis (Hayashi et al., 2008). The purpose of our study was to investigate the role of Dicer and miRNAs in SC function, and thereby their involvement in spermatogenesis. We found that male mice in which Dicer was deleted specifically in SCs were infertile, due to defective SC function preceeded by down-regulation of SC-specific genes known to be essential for spermatogenesis. Our results demonstrate for the first time the crucial importance of Dicer –and thereby miRNAs- in SC function, and thereby unravel the existence of post-transcriptional control in the supporting cell lineage of the testis.
Materials and Methods
Animals
Dcrflox (Dcrfx) and Mis-Cre (Amh-Cre) mice were kindly provided by B. Harfe and F. Guillou respectively, and were genotyped as described (Harfe et al., 2005; Lecureuil et al., 2002). To achieve selective inactivation of Dcr in Sertoli cells, we mated transgenic MisCre female mice expressing Cre recombinase under the control of the Mis gene promoter with male mice carrying two floxed Dcr alleles in order to generate 50% Dcrfx/wt;MisCre and 50% Dcrfx/wt mice. These animals were then intercrossed to produce Dcrfx/fx;MisCre as well as control littermates, namely Dcrfx/fx and Dcrfx/wt;MisCre mice. The genetic background of these genetically modified mice is a mixed C57BL/6J and SV129. Protocols for the use of animals were approved by the Commission d’Ethique de 1’Expérimentation Animale of the University of Geneva Medical School and the Geneva Veterinarian Office.
Fertility tests and sperm analysis
Dcrfx/fx;MisCre males (n=8) or control littermates (Dcrfx/wt;MisCre, n=5 and Dcrfx/fx, n=5) were each bred with two 6-week-old wild type C57BL/6J female mice for 6 months. The number of litters and pups/litter were systematically recorded. Epididymal sperm count was performed with sperm extracted from the caudal epididymis and ductus deferens of adult (P60) male mice and was analyzed for its concentration as previously described (Guerif et al., 2002).
Histology and immunohistochemistry
Tissues were fixed overnight either in 4% paraforlmaldehyde (PFA) or in Bouin’s fixative and embedded in paraffin. Five-μm sections were stained with haematoxylin and eosin (H&E) or processed for immunohistochemistry (IHC). For IHC analysis, PFA-fixed sections were incubated overnight at 4°C with the following antibodies: anti-GATA4 (sc-9053, Santa Cruz Biotechnology, 1:50), anti-ZO1 (#61-7300, Zymed, 1:250), anti-β galactosidase (ab9361, Abcam, 1:500), anti-MVH (1:1000, gift from Toshiaki Noce) and anti-3ß-HSD (1:500, gift from Ian Mason). For fluorescent staining, Alexa-conjugated secondary antibodies (Invitrogen) were used for signal revelation, whereas for stable staining, signals were revealed with DAB (Sigma). All images were obtained with a Zeiss Axioscop microscope and processed using the AxioVision software. For X-gal coloration tissues were fixed in 4% PFA for 2 hours, immersed in PBS1x/sucrose 25% and then stained with X-gal (1M MgCl2, 10% sodium deoxycholate, 10% NP40, X-gal 20 mg/ml, 200mM potassium ferricyanide, 200mM potassium ferrocyanide) overnight at 37°.
Proliferation and apoptosis assays
Fifteen regions from 3 different animals per genotype were randomly selected to count proliferating or TUNEL (TdT-mediated X-dUTP nicked labeling)-positive cells. The proliferation assay was performed with PFA-fixed sections double stained with anti-GATA4 (sc-9053, Santa Cruz Biotechnology, 1:50) and anti-Ki67 (BD, 1:100) overnight at 4°C. Values were expressed as the percentage of proliferating SCs over the total number of SCs counted in a given region. Apoptotic assays were performed both by means of TdT-mediated X-dUTP nicked labeling (TUNEL) reaction using the In Situ Cell death kit (Roche) and double IHC using anti-cleaved caspase3 (1:200, Cell Signaling, #9661L) and anti-GCNA1 (1:50, gift from G. Enders) so as to reveal the identity of TUNEL-positive cells. The percentage of apoptotic, TUNEL positive cells within seminiferous tubules was expressed as the average number of apoptotic cells within 20 seminiferous tubes.
Microarray analysis
Total RNAs from 3 control (Dcrfx/fx) and 3 mutant (Dcrfx/fx;MisCre) P0 and P5 pairs of testes were extracted individually using the RNeasy Micro kit (Qiagen) according to the manufacturer’s protocol, and their quality was assessed using Agilent Biosizing Total RNA NanoChips. To minimize biological variability, mutant and control pups originated from the same litters. Briefly, for each of the 12 independent samples, 1μg of total RNA was reverse transcribed and amplified using the MessageAmpTM II-Biotin Enhanced Single Round aRNA Amplification Kit (#1791, Ambion). For each probe, 20 μg of the amplified biotinylated cRNA was fragmented and hybridized to Mouse Genome 430 2.0 Arrays (Affymetrix, High Wycombe, UK) as described (Cederroth et al., 2007). All microarray data are available through ArrayExpress (http://www.ebi.ac.uk/arrayexpress/, accession #E-TABM-426).
Classification and functional analysis of genes
Classification of differentially expressed genes was performed using the Ingenuity Pathways Knowledge Base (Ingenuity Systems, www.Ingenuity.com), based on their involvement in diverse biological processes. In short, a data set containing the Affymetrix gene identifiers, their corresponding expression values and p-values, was used to map to the corresponding gene object in the Ingenuity Pathways Knowledge Base. A fold-change cutoff of at least 2 was set between control and mutant testes to further filter genes whose expression was significantly altered. These genes, called “focus genes”, were used as the starting point for generating biological networks. To start building networks, the program queries the Ingenuity Pathways Knowledge Base for interactions between focus genes and all other gene objects stored in the knowledge base, and generates a set of networks. IPA then computes a score for each network based on how well it fits to the set of focus genes. The score is derived from a p-value and indicates the likelihood of the Focus Genes in a network being found together due by chance. Scores of 2 or higher represent a 99% confidence level. Biological functions are then calculated and assigned to each network.
MicroRNA expression profiling
Purification of P6 SCs, small RNA isolation and cloning were performed as described (Ro et al., 2007a). MicroRNA profiling on purified spermatogenic populations was performed using quantitative PCR as described (Ro et al., 2006). Oligonucleotides used for qRT-PCR are listed on Supplementary Table 4.
MicroRNA target recognition analysis
The prediction model employed for the identification of potential miRNA-target interactions is similar to that of Kertesz et al. (Kertesz et al., 2007). and relies on: (i) the initial identification of seeds for miRNAs, followed by (ii) the evaluation of the free energy gain (? ? G) resulting from the formation of the miRNA-target duplex, which takes into consideration the competing internal mRNA structures, and requires at least 10 nucleotides upstream and 15 downstream of the target site to be unfolded. For seed identification, we used standard parameters, requiring seed length to be 6-8 bases from position 2 of the miRNA, and not allowing mismatches except a single G:U wobble in 7-mers and 2 G:U in 8-mers. The stringency cut-off we used yields over 80% specificity as estimated from published luciferase assays.
Real-Time quantitative PCR
Total RNAs from 6 control (Dcrfx/fx) and 6 mutant (Dcrfx/fx;MisCre) testes at P0 and P5 were extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s protocol. Total RNAs for each of the 24 independent samples were reverse transcribed and 1/40th of the cDNA was used as template for PCR amplification as previously described (Cederroth et al., 2007). The statistical significance of fold-changes was determined by a paired Student’s t-test. Primers used for qRT-PCR are listed in Supplementary Table 5.
Results
Complete and specific elimination of miRNAs in Sertoli cells of Dcrfx/fx;MisCre testis
To investigate the in vivo role of Dicer in SCs, mice bearing two loxP-flanked alleles of Dicer (Dcrfx/fx) (Harfe et al., 2005) were crossed with mice carrying the Mis-Cre transgene (Lecureuil et al., 2002), and their progeny were then intercrossed to obtain males in which Dicer was specifically inactivated in SCs (Dcrfx/fx;MisCre), as well as control Dcrfx/wt;MisCre and Dcrfx/fx littermates. Mis-driven Cre recombinase has been reported to efficiently delete floxed alleles specifically in SCs from E14.5 onwards (Lecureuil et al., 2002; Vernet et al., 2006). We ourselves confirmed the specificity of Dicer ablation first by ß-gal immunohistochemistry (IHC) (Fig. 1A). To further analyze the efficiency of Dicer removal in SCs we sought to assess whether the downstream products of Dicer –microRNAs- were eliminated too. For this purpose, we first sequenced the small RNA-ome from purified mouse P6 SCs using a method previously described (Ro et al., 2007b) and identified a total of 248 miRNAs present in SCs (Supplementary Table 1), of which 5 were exclusively or predominantly expressed in SCs (miR-299, miR-376a, miR-381, miR-409-5p, miR-674*, see Fig. 1B). Real-Time PCR showed that the levels of these 5 SC-specific miRNAs were completely suppressed in P5 Dcrfxfx;MisCre testes, whereas spermatogonia-specific miRNAs were unaffected (Fig. 1B), thus further confirming the efficiency and specificity of Dcr excision. Interestingly, SC-specific miRNAs were reduced by about 2-fold in P0 Dcrfxfx;MisCre testes even though the Cre recombinase activity starts at E14.5. This suggests that pre-existing miRNAs in SCs are quite stable molecules and that they remain present within the cell for several days.
Fig. 1. Complete SC-specific Dicer ablation and subsequent SC-specific miRNA suppression using the MisCre recombinase.
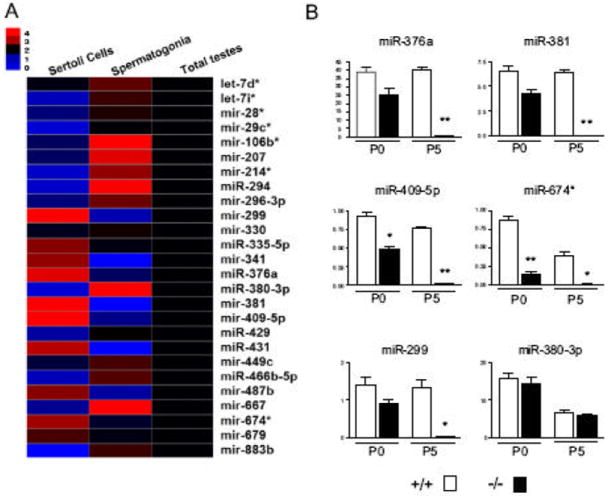
(A) Shown in this heat map are the expression levels of 26 representative miRNAs from purified Sertoli cells, primitive type A spermatogonia and total testes at P6, as determined by quantitative RT-PCR. mir-299, mir-376a, mir-381, mir-409-5p, mir-674*, mir-431, mir-341 and mir-487b appear to be predominantly expressed in P6 Sertoli cells, with the first five being exclusively expressed in SCs. (B) Expression levels of 5 Sertoli cell-specific miRNAs (miR-376a, miR-381, miR-409-5p, miR-674*, miR-299) were completely suppressed in P5 Dcrfx/fx;MisCre (-/-) testes whereas those of a spermatogonia-specific miRNA (miR-380-3p) were unaffected. *p<0.05, **p<0.01, ***p<0.001 versus controls.
Ablation of Dicer in Sertoli cells results in reduced testis size and infertility
Dcrfx/fx;MisCre males were viable, grew to adulthood normally and appeared to have normal sexual behavior and external genitalia when compared to control littermates. At P60, testes lacking Dicer in SCs showed a dramatic, 90%, mass reduction compared to control Dcrfx/wt;MisCre and Dcrfx/fx littermates (10±3 mg versus 100±20 mg and 100±20 mg respectively, Fig. 2A-D). Testicular descent had occurred normally in Dcrfx/fx;MisCre males and internal reproductive organs such as the seminal vesicles and the prostate were normally masculinized (data not shown). Dcrfx/fx;MisCre males exhibited virile, androgen-dependent behavior including normal mounting and copulatory activity (data not shown), and importantly, their testicular testosterone levels at P0, P5 and P60 were not significantly different from those in controls, although interstitial hyperplasia was observed from P5 onwards (Fig. S1). Despite this, Dcrfx/fx;MisCre males were infertile: during a 6-month mating period with wild type C57/BL6 females, they (n=8) failed to produce any offspring, whereas control littermates systematically sired offspring (n=8-12 pups/litter, 6-9 litters per mating cage). Sperm count analysis revealed that no spermatozoa were present in P60 Dcrfx/fx;MisCre caudal epididymides, whereas normal sperm counts were found for control littermates (Fig. 2G). Histological analysis confirmed the complete absence of spermatozoa in testes (Fig. 2F) and epididymal ducts (Fig. 2I) of Dcrfx/fx;MisCre males. Instead, numerous exfoliated germ cells were found in the lumen of mutant epididymal ducts (arrowhead in Fig. 2I).
Fig. 2. Dramatic size reduction and complete absence of spermatozoa in Dcrfx/fx;MisCre testes.
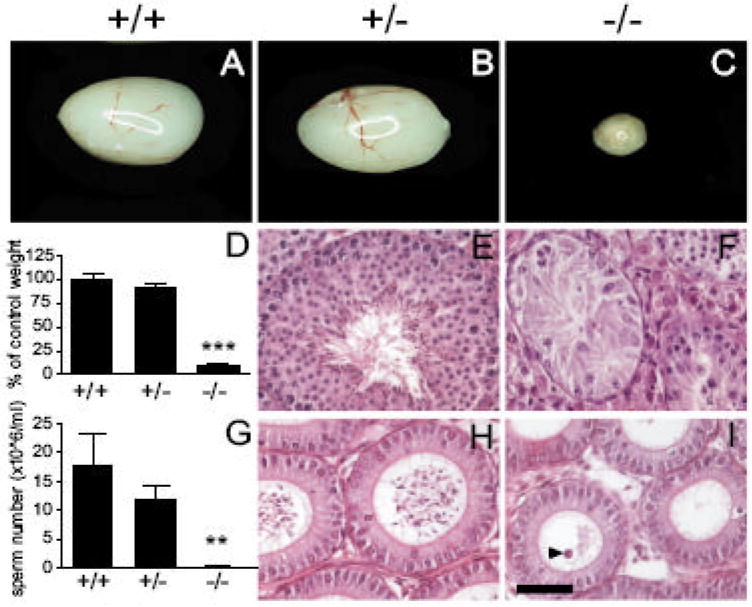
At P60, testes from Dcrfx/fx;MisCre mice (C) showed a drastic, 90% reduction (D, n=8-14 animals per genotype) in size compared to control Dcrfx/fx (A) and Dcrfx/wt;MisCre (B) littermates. H&E staining of testes (E, F) and epidydimides (H, I) revealed complete absence of mature spermatozoa. Exfoliated germ cells were present in mutant epidydimal ducts (arrowhead in I). (G) Results of sperm count analysis (n=4-9 animals per genotype). +/+, +/- and -/- are abbreviations for Dcrfx/fx, Dcrfx/wt;MisCre and Dcrfx/fx;MisCre animals respectively. Results are mean ±SEM, *p<0.05, **p<0.01, ***p<0.001 versus controls. Scale bar: 50μm.
Ablation of Dicer in SCs results in severely impaired spermatogenesis and age-dependent testis degeneration
Histological analysis revealed a severely impaired spermatogenesis and testis degeneration in P60 Dcrfx/fx;MisCre mice; with rare exceptions, elongated spermatids were completely absent. More precisely, the seminiferous epithelium of mutant testes displayed a wide range of defects, including vacuolization (Fig. 3A), presence of Sertoli-cell-only (SCO) tubes (Fig. 3B), tubes arrested at an early post-meiotic stage (Fig. 3C), as well as tubes which were not only smaller in diameter and devoid of a lumen, but also showed complete disorganization of the typical cell layering (Fig. 3D). The latter was confirmed by IHC: anti-GATA4 staining revealed the abnormal presence of SC nuclei in the center of tubes (Fig. 3F), whereas an anti-MVH antibody, which specifically labels the germ cell cytoplasm, revealed severe germ cell disorganization (Fig. 3H).
Fig. 3. Numerous spermatogenic defects in adult Dcrfx/fx;MisCre testes.
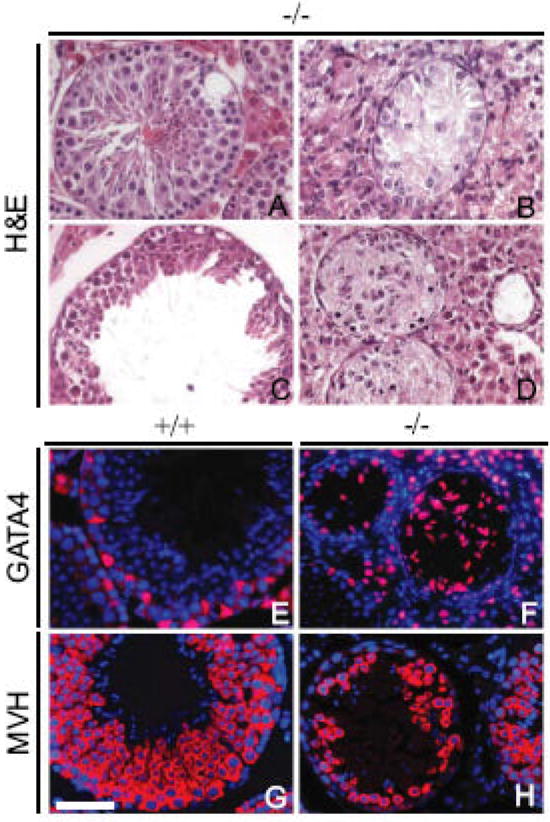
H&E staining of representative P60 Dcrfx/fx;MisCre testis sections. Spermatogenic defects included vacuolization (A), Sertoli-cell-only (SCO) tubes (B), tubes with spermatogenic arrest (C) or disorganization of the seminiferous epithelium (D). Anti-GATA4 (red) staining (E,F) revealed abnormal positioning of SC nuclei (F), whereas anti-MVH (red) staining (G,H) revealed disorganization and reduction in germ cell number (H) in Dcrfx/fx;MisCre testes. DAPI (blue) was used for nuclear staining (E-H). +/+ and -/- are abbreviations for Dcrfx/f, and Dcrfx/fx;MisCre animals respectively. Scale bar: 50μm.
By three months of age (P90), degeneration was even more severe in Dcrfx/fx;MisCre testes, with most of the tubes having formed their lumen, but with the majority of them having degenerated into SCOs with severely impaired SC morphology (Fig. 4D). In fact, staining with tight-junction-associated protein (TJP1, formerly known as zonula occludens 1, ZO-1) –a marker of SC tight junctions- was not only present at the basal lamina of mutant tubes but in the adluminal compartment too (SI Fig. 2F), suggesting defective SC cyto-architecture and polarity. By 6 months of age (P180), mutant testes’ size was reduced to 5% of that of a control (Fig. S3A) and had degenerated into a mass of interstitial cells containing only rare remaining tubes (Fig. 4E), thus showing that testis degeneration aggravates upon aging. In fact, these sparse remaining tubes were almost completely devoid of germ cells, as confirmed by anti-MVH staining (Fig. 4F). These results show that SC Dicer is required both for SC survival and their capacity to support germ cell development.
Fig. 4. Age-dependent testis degeneration in Dcrfx/fx;MisCre mice.
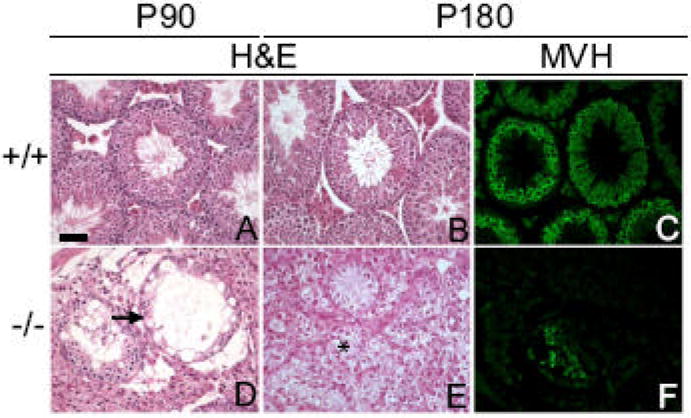
Control (A,B) and mutant (D,E) testis sections at P90 (A,D) and P180 (B,E) were stained with H&E. At P90, most tubes had become SCOs (tube pointed with arrow in D); by P180, an almost complete testicular degeneration was observed, with only 5-6 (per transverse section) tubes remaining, surrounded by a mass of interstitial cells (asterisk in E); in addition, these few remaining tubes were almost completely devoid of germ cells (F), as evidenced by anti-MVH staining (C, F). +/+ and -/- are abbreviations for Dcrfx/fx and Dcrfx/fx;MisCre animals respectively. Scale bar: 50μm.
Tubular abnormalities appear as early as P5 in Dcrfx/fx;MisCre males
To unravel the events that led to infertility, we compared development of mutant and control testes from P0 to P42, when the first spermatogenic wave is completed. At birth (P0), Dcrfx/fx;MisCre and control testes were morphologically indistinguishable (Fig. S4A-F). The first abnormalities began to appear at P5, about 8-9 days after the onset of the Mis-Cre transgene expression and when miRNAs were completely eliminated from SCs. At this stage, gonocytes resume proliferation, while moving towards the basement membrane (arrowhead in Fig. 5A). In Dcrfx/fx;MisCre testes, two major defects were observed: First, instead of lying against the basement membrane, SC nuclei were mislocalized in the center of the tubes, as confirmed by anti-GATA4 staining (arrows in Fig. 5D,E). Second, numerous pycnotic cells were present within mutant tubes (arrowheads in Fig. 5D). By P15, an 80% reduction in the size of Dcrfx/fx;MisCre testes compared to controls was observed (2.0±1 mg versus 20±2 mg respectively, p<0.0001, n=6-16 animals per genotype). Pachytene spermatocytes had not yet appeared and germ cell layering was severely perturbed (Fig. 5L). No tube had formed a lumen, and numerous pycnotic cells were found within (arrowheads in Fig. 5J). Several aspects of SC morphology suggested that these cells had remained immature: their nucleus did not display the characteristic irregular shape (compare insets in Fig. 5J and G), they were mostly mislocalized in the center of the tubes instead of the periphery (Fig. 5J-K) and TJP1 staining was more diffuse and discontinuous around the baso-lateral site of SCs compared to controls (Fig. S2D). At P21 (Fig. S4M-R), when secondary spermatocytes had appeared in control testes, and the first round spermatids were seen in some tubes, mutant testes continued to show a severe delay in meiosis and SC maturation, to harbor numerous pycnotic cells, and to display cellular disorganization. However, a few tubes had begun to form a lumen, suggesting that some SCs were partially functional. By P42, round spermatids were drastically reduced in number, and elongated spermatids were completely absent in Dcrfx/fx;MisCre testes. Tubular disorganization and vacuolization had become severe (Fig. 5P-R), germ cells showed extensive apoptosis, and most of the tubes were SCOs, although notably, those which were less severely affected displayed a lumen.
Fig. 5. Tubular defects appear as early as P5 in Dcrfx/fx;MisCre mice.
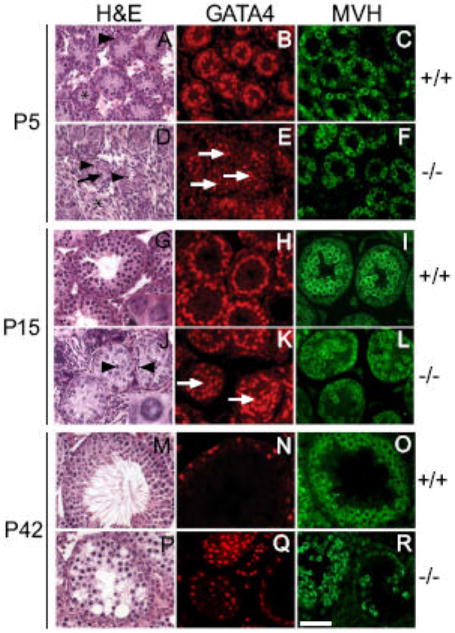
At P5, three major abnormalities were evident in Dcrfx/fx;MisCre testes: interstitial hyperplasia (asterisk in D), pycnotic cells (arrowheads in D) within tubes, SC nuclear mislocalization (arrows in D and E) with almost complete absence of cytoplasm (compare tubule structure in A and D, SC cytoplasm in A marked with asterisk). At P15, SC nuclear mislocalization persisted (arrows in K) with the majority of them having an abnormal circular (inset in J) rather than flattened triangular (inset in G) shape, and germ cell disorganization was remarkable (L). By P42 almost all tubes had become severely vacuolized (P) and germ cell loss had become striking (R). +/+ and -/- are abbreviations for Dcrfx/fx and Dcrfx/fx;MisCre animals respectively. Scale bar: 50μm.
Taken together, these data show that SC loss of Dicer severely impairs the prepubertal spermatogenic wave due to defective SC maturation, which includes dysfunctional secretory activity (absence of lumen), abnormal nuclear positioning and morphology and incapacity to properly support meiosis and spermiogenesis.
Increased SC proliferation followed by massive cell apoptosis in prepubertal Dcrfx/fx;MisCre testes
The dramatic testis size reduction we observed as early as P15 led us to the assumption that the balance between cell proliferation and death had been perturbed. Surprisingly, as evidenced by double anti-GATA4/anti-Ki67 IHC, there was a significant increase in SC proliferation of P0, P5 and P15 Dcrfx/fx;MisCre testes. At P0 and P5, we found respectively a 1.25-fold (63.9% versus 51.2%) and 1.5-fold (56.2% versus 36.4%) increase of SC proliferation in mutant testes compared to controls (Fig. 6A). At P15, when SCs normally cease dividing and become mature, we still observed a 2.6-fold (1.8% versus 4.6%) increase in proliferation in mutant testes, thus further suggesting a delayed SC maturation.
Fig. 6. Increased SC proliferation and massive cell apoptosis in prepubertal Dcrfx/fx;MisCre testes.
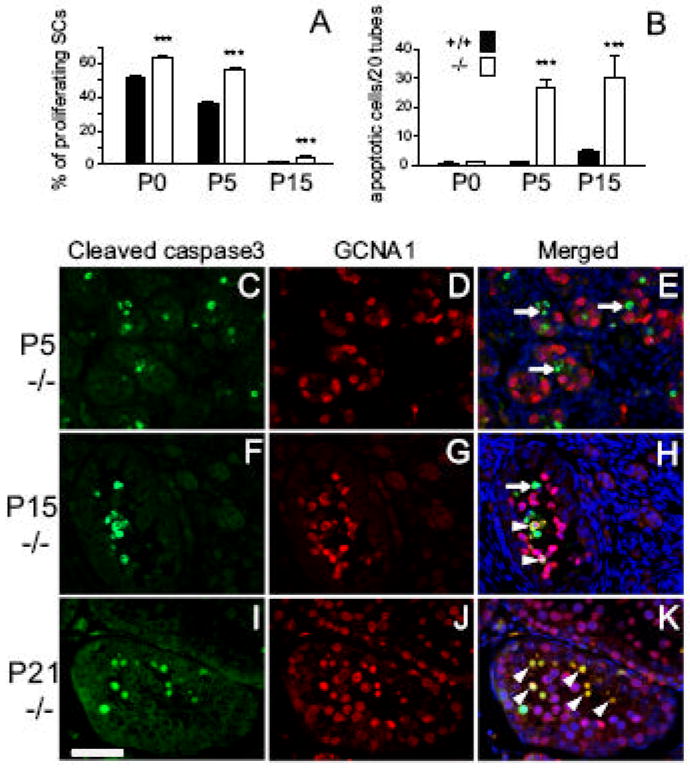
Percentages of proliferating SCs as revealed by double IHC staining with anti-GATA4 and anti-Ki67 at P0, P5 and P15 are plotted in (A). Quantifications of TUNEL labeled cells revealed a dramatic increase in apoptosis in P5 and P15 mutant testes (B). Double IHC staining (C-K) with anti-GCNA (red) and anti-cleaved caspase3 (green) antibodies was performed on control (data not shown) and mutant (-/-) testis sections at P5 (C-E), P15 (F-H) and P21 (I-K). At P5, absence of GCNA1 and cleaved caspase3 co-localization is indicative of SC apoptosis (white arrows in E), whereas at P15 and P21, double GCNA1-cleaved caspase3 cells appeared (white arrowheads), thus revealing apoptotic germ cells. DAPI (blue) was used for nuclear staining (E, H, K). +/+ and -/- are abbreviations for Dcrfx/fx and Dcrfx/fx;MisCre animals respectively. Results are mean ±SEM (n=3 animals/ genotype/ stage), *p<0.05, **p<0.01, ***p<0.001 versus controls. Scale bar: 50μm.
These results prompted us to assess cell apoptosis in mutant testes. Whereas at P0 no difference between controls and mutants was found, a 26- and 7-fold increase in apoptosis was observed in P5 and P15 mutant testes respectively, compared to controls (Fig. 6B). To reveal the identity of these apoptotic cells, we performed double anti-cleaved caspase3/anti-GCNA1 IHC, so as to label apoptotic cells and germ cell nuclei respectively. At P5, cleaved caspase-3 positive cells (Fig. 6C) were almost exclusively GCNA1 negative (Fig. 6D), suggesting that the large majority of apoptotic cells in Dcrfx/fx;MisCre testes were SCs (arrows in Fig. 6E). However at P15, both germ and SCs were found to be apoptotic (Fig. 6F-H), while at P21, apoptotic cells were almost exclusively germ cells (Fig. 6I-K). These findings suggest that the striking testis size reduction is a consequence of both the incapacity of SCs to sustain spermatogenesis and the subsequent germ cell death.
Ablation of Dicer in SCs causes alterations in the testicular transcriptome
To determine whether Dicer affects SC function at the transcriptional level, we performed a microarray analysis on control and mutant testes, just prior to (P0) and when the first morphological changes appear (P5). At these early postnatal stages, more than 80% of the tubular cells are SCs (Bellve et al., 1977), which minimizes the tissue’s heterogeneity.
Of the 29,000 probe sets defined as present in mutant or control testes at P0, 77 were up-regulated (=2 fold) and 68 were down-regulated (=-2 fold) (SI Table 2), whereas 787 and 796 probe sets were found to be up- or down-regulated respectively in P5 mutant testes (Supplementary Table 3). The variation in abundance of several key transcripts was further confirmed by quantitative RT-PCR (Fig. S7). Among the genes down-regulated in P0 mutant testes were Glial-derived nerve factor (Gdnf), mannosidase IIx (Man2a2), serpin peptidase inhibitor (Serpin5a), Claudin11 and Sox9, all of which, when inactivated in mice, lead to diverse spermatogenic defects resulting in infertility. To this group of transcripts was added another set of down-regulated genes in P5 mutant testes including Gata1, Kitl (SCF), Bclw, Dhh, Stra6, Wt1, Inhibin-β, connexin43, Jam2, Tjp2 and Amh, all known to be major regulators of testicular development or spermatogenesis (reviewed in (Matzuk and Lamb, 2002)). In contrast, when looking at the genes upregulated in mutant testes -that could be direct targets of miRNAs-, we found none that has been reported to be involved in testicular function, with the notable exception of Bcl2L11, a member of the proapoptotic Bcl-2 homology3-only protein family required for the elimination of supernumerary germ cells during the first spermatogenic wave (Coultas et al., 2005).
A hierarchical clustering (Fig. 7A) of the profile of the 145 probe sets showing a = 2 -fold change in expression in P0 mutant testes revealed that the majority of the genes downregulated at P0 remained so at P5. In contrast, the expression level of most of the genes upregulated in P0 mutant testes –with the exception of Bcl2L11- returned to normal levels at P5, a finding indicative of a transient upregulation. This also suggests that the “permanent” gene downregulation is more likely to be responsible for the observed phenotype. We therefore used the Ingenuity Pathway Analysis (IPA) software to classify the differentially expressed genes in groups of common function. We found that genes critical for “cell signaling”, “cell death”, “organ development”, “cellular development” and “tissue development” were the five most statistically significant functional groups affected by the SC loss of Dicer (Fig. 7B). Interestingly, each of these groups contained both up- and down-regulated genes –in approximately equal numbers-, suggesting that the effect of the Dicer loss is probably the result of the deregulation of both miRNAs and other factors downstream of Dicer itself.
Fig. 7. Global expression analysis of the transcriptomes of control and mutant testes at P0 and P5.
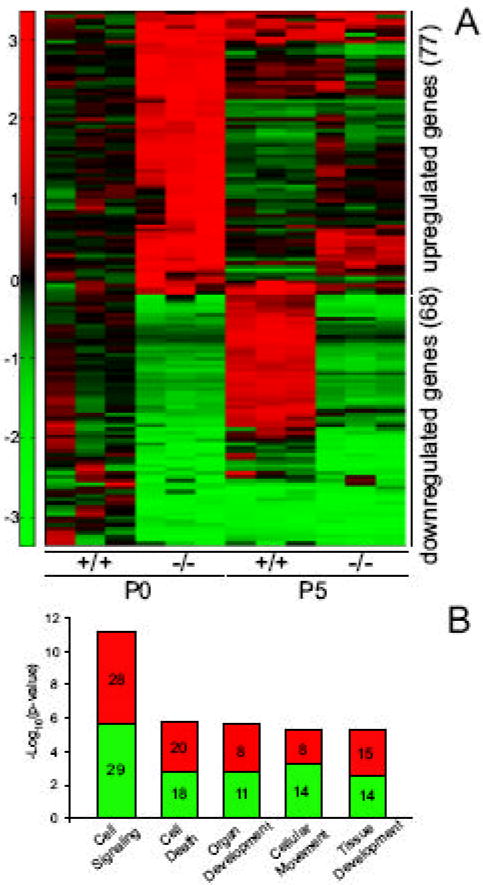
(A) Hierarchical clustering of the 145 probe-sets exhibiting a =|2| fold change in expression in P0 Dcrfx/fx;MisCre (-/-) compared to Dcrfx/fx (+/+) testes. Each line represents a probe set and each column corresponds to a specific stage and genotype as marked. Red and green colors indicate increased and decreased expression respectively. (B) IPA analysis revealed the five most statistically significant functional groups affected by the SC loss of Dicer. Each bar corresponding to a gene group is split in two (red for up-, green for down-regulated genes); numbers within are the numbers of modulated genes in each group.
Several studies have reported an enrichment of sequences complementary to miRNA seeds in the 3’-untranslated region (3’-UTR) of mRNAs that are upregulated upon Dicer -or specific miRNA- deletion. To assess if this was also the case for Dcrfx/fx;MisCre testes, we compared the distribution of predicted miRNA target sites among transcripts that were up- or down-regulated in P0 and P5 Dcrfx/fx;MisCre testes for the 248 miRNAs we cloned from purified P6 SCs. To predict likely functional miRNAs targets, we used 3 different bioinformatic approaches: when considering either all potential sequences complementary to the seed, or 1 seed per miRNA/per transcript, distributions between up- or down-regulated transcripts in P0/P5 mutant testes were not significantly different (Mann-Whitney test p-values 0.238 and 0.181 respectively). We also performed the test using a selection of potential functional seeds based on a thermodynamic model (Kertesz et al., 2007); distributions were again not significantly different (p-values of 0.155 and 0.112 respectively) although a slightly higher significance was found. These findings show that the 3’-UTR sequences of transcripts up-regulated in P0 and P5 Dcrfx/fx;MisCre testes are not significantly enriched for miRNA-binding sites, and therefore suggest that –at least in SCs- miRNAs may have limited direct effects on target gene expression at the RNA level.
Finally, we assessed the expression levels of certain repetitive elements, since there is evidence for the implication of Dicer-dependent small RNAs in the repression of repetitive parasitic sequences (Murchison et al., 2007; Svoboda et al., 2004). Quantitative RT-PCR analysis revealed no significant differences in the abundance of transcripts for MT (mouse transcript), IAP (Intracisternal A particle element), Line1 (long interspersed nuclear elements), SineB1 and SineB2 (short interspersed repetitive elements) in P0 and P5 mutant testes compared to controls (Fig. S6). Thus, the Dcrfx/fx;MisCre phenotype is probably not due to the derepression of these specific repetitive elements.
Discussion
Given the fundamental importance of gamete production for the perpetuation of a species, it comes as no surprise that spermatogenesis is a process tightly regulated at multiple levels, including the transcriptional and post-transcriptional level. An issue that has not yet been addressed is whether post-transcriptional regulation of spermatogenesis also occurs in the somatic compartment of the testis. Here, we report that Dicer, a central component of the RNAi machinery, is essential for SC maturation, function and survival. Specific deletion of Dicer in SCs leads to infertility due to absence of mature spermatozoa and testis degeneration, thus highlighting the absolute necessity of Dicer for the development of fully competent SCs.
The first histological signs of the phenotype appeared a few days after birth, and led already before puberty to a gradual degeneration of the seminiferous epithelium’s architecture. In fact, our expression profiling analyses revealed that although P0 control and mutant testes are morphologically indistinguishable, miRNA levels in SCs are reduced by approximately 50% and on the same time significant transcriptional alterations have already occurred in mutants. At P0, SC loss of Dicer affected the expression of not only numerous mRNAs specifically expressed in SCs, but also mRNAs in gonocytes (e.g. Serpin5a, TSLC1) and in LCs (e.g. Amhr2, PNMT). This suggests that despite no apparent testicular phenotype at birth, damages in SCs have already had important secondary effects on gene expression in the adjacent cell lineages, but more importantly, it confirms the long-standing notion of a close crosstalk between SCs and germ cells [reviewed in (Jegou, 1993)]. From P5 onwards, numerous testicular abnormalities appeared, the most prominent being a delay in SC maturation and a delayed entry in meiosis. Testicular degeneration worsened upon aging; by 6 months of age, mutant testes were composed almost exclusively of Leydig and fibroblast-like cells interspersed with rare –completely disrupted- tubes. Overall, our results suggest that the striking testis size reduction is very likely to have been the result of both (i) SC inability to support germ cell survival and spermatogenesis and (ii) SC death.
Increased cell death has in fact been observed in numerous conditional Dicer knockouts (Chen et al., 2008; Harfe et al., 2005; Harris et al., 2006), thereby raising the possibility that Dicer might be a “universal” regulator of cell survival. Specific ablation of Dicer in SCs was no exception since increased levels of SC apoptosis were detected as early as P5. At first glance, the dramatic phenotype of aging mutant testes could be simply explained by massive SC apoptosis leading to subsequent germ cell death. However, a closer examination of the phenotype suggests that Dicer has additional roles in regulating SC function, independent of its requirement for cell survival. Several of our findings are in support of this notion: (1) The significant alterations in gene expression at a time (P0) when histological defects and apoptosis are not yet detectable in mutant testes indicate that expression of genes essential for SC function is already affected and is not a consequence of cell death. (2) Apoptosis in mutant SCs appears gradually: some SCs die as early as P5, while some remain viable for several months and are at some level capable of supporting spermatogenesis, although they display other abnormalities (defective maturation, abnormal cellular architecture and polarization -as evidenced by TJP1 staining). Finally, (3) by 2 months of age, most remaining tubes are SCOs and composed of mutant, yet viable SCs which are Dicer-deficient, as confirmed by recombination of the Rosa26 stop LacZ marker (SI Fig. 1), thus showing that mutant SCs have lost their capacity to support germ cell survival, but remain viable themselves. These data reinforce our belief that Dicer should not be merely viewed as a global regulator of cell survival, and that the effects caused by its absence should not be interpreted solely on the base of cell death.
Among the genes whose expression was downregulated in P0 and/or P5 mutant testes, were Gdnf, Kitl, Man2a2, Gata1, Dhh, Serpin5a, Wt1, Sox9, all known to result in diverse spermatogenic defects when deleted in vivo. The significant reduction of Kitl and Gdnf levels was of particular interest; a balance between the Kitl/c-kit and GDNF/Ret signaling pathways is known to control the choice between spermatogonial differentiation and renewal (reviewed in (Wong et al., 2005)). It is thereby reasonable to assume that deregulation of this balance, notably a 5- and 3-fold reduction of Gdnf and Kitl respectively, could perturb the initial phase of spermatogenesis. It is possible that around P5, when spermatogonia resume proliferation and either renew themselves or differentiate, the reduction of Gdnf impairs their capacity to renew, whereas the reduction of Kitl negatively affects their capacity to differentiate. Defective spermatogonial renewal could lead to gradual germ cell loss, and could thereby explain the tubular degeneration we observed upon aging.
An essential question that emerges with the findings presented here is which biological activities mediated by Dicer are essential for SC function. Dicer is involved in a variety of gene-silencing phenomena at the transcriptional or translational level –through the activity of small RNAs-, but is also required for the maintenance of chromatin structure (Kanellopoulou et al., 2005). Therefore, it is likely that the Dcrfx/fx;MisCre phenotype is the result of (1) the deregulation of genetic elements that are directly under the control of Dicer itself, and/or (2) the deregulation of –direct or indirect- miRNA target genes. In the first case, loss of Dicer could lead to the up-regulation of genetic elements that are normally transcriptionally silent, which in its turn could affect the expression of other genes essential for spermatogenesis. In the second case, loss of miRNAs -subsequent to the loss of Dicer- could result in the up-regulation of direct miRNA target genes, which in their turn could deregulate the expression of other downstream factors. For the moment, our data favor the second option, since we found no significant change in the expression of a selected set of repetitive elements in mutant testes. A tempting hypothesis is raised by the fact that most genes that could actually be responsible for the observed phenotype were down-regulated in mutant testes. Taken together, our data suggest a model in which Dicer deletion leads to the gradual -but ultimately complete-disappearance of miRNAs in Sertoli cells, followed by a major transcriptome deregulation that could be the result of an alteration in the fine tuning of protein synthesis, such as the upregulation of potential transcriptional repressors. This hypothesis is supported by recent high-throughput proteomic analyses revealing that a single miRNA can repress the production of hundreds of proteins but that this repression is relatively mild, rarely exceeding 4-fold (Baek et al., 2008; Selbach et al., 2008). We therefore hypothesize that the sterility phenotype observed in Dcrfx/fx;MisCre mice is the indirect consequence of the downregulation of genes essential for Sertoli cell capacity to support germ cell survival and differentiation, such as Gdnf, Kitl, Wt1, Sox9.
In conclusion, a better understanding of spermatogenesis is essential in order to become able to treat a rapidly increasing number of cases of male infertility. By demonstrating with our study that Dicer is essential for spermatogenesis, not only do we unravel a novel role for this gene, but we also provide new insights on the mechanisms controlling SC function and germ stem cell niche regulation in mammals.
Supplementary Material
Acknowledgments
We would like to thank Françoise Kühne and Laurence Tropia for excellent technical assistance, Christelle Borel and Jean-Dominique Vassalli for critical reading of the manuscript. We are grateful to G.Enders, I. Mason and T. Noce for antibodies. S.N. has received a Swiss National Science Foundation Grant no. 3100A0-119862, and is also supported by the Prof. Dr. Max Cloëtta Foundation and the Société Académique de Genève. Wei Yan was supported in part by grants from the National Institute of Health (HD048855 and HD050281).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RE. Post-transcriptional control of gene expression during spermatogenesis. Semin Cell Dev Biol. 1998;9:483–9. doi: 10.1006/scdb.1998.0226. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–21. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S. ER{alpha} is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology. 2007;148(11):5507–19. doi: 10.1210/en.2007-0689. [DOI] [PubMed] [Google Scholar]
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–6. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, Strasser A. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. Embo J. 2005;24:3963–73. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez E, Lopez-Casas PP, Del Mazo J. The expression patterns of genes involved in the RNAi pathways are tissue-dependent and differ in the germ and somatic cells of mouse testis. Biochim Biophys Acta. 2008;1779(5):306–11. doi: 10.1016/j.bbagrm.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Guerif F, Cadoret V, Plat M, Magistrini M, Lansac J, Hochereau-De Reviers MT, Royere D. Characterization of the fertility of Kit haplodeficient male mice. Int J Androl. 2002;25:358–68. doi: 10.1046/j.1365-2605.2002.00382.x. [DOI] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci U S A. 2006;103:2208–13. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, O’Carroll D, Das PP, Tarakhovsky A, Miska EA, Surani MA. MicroRNA Biogenesis Is Required for Mouse Primordial Germ Cell Development and Spermatogenesis. PLoS ONE. 2008;3:e1738. doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegou B. The Sertoli cell. Baillieres Clin Endocrinol Metab. 1992;6:273–311. doi: 10.1016/s0950-351x(05)80151-x. [DOI] [PubMed] [Google Scholar]
- Jegou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol. 1993;147:25–96. [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39:1278–84. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- Kotaja N, Bhattacharyya SN, Jaskiewicz L, Kimmins S, Parvinen M, Filipowicz W, Sassone-Corsi P. The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc Natl Acad Sci U S A. 2006;103:2647–52. doi: 10.1073/pnas.0509333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis. 2002;33:114–8. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl):s41–9. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–93. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–94. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–26. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Ro S, Park C, Jin J, Sanders KM, Yan W. A PCR-based method for detection and quantification of small RNAs. Biochem Biophys Res Commun. 2006;351:756–63. doi: 10.1016/j.bbrc.2006.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Park C, Sanders KM, McCarrey JR, Yan W. Cloning and expression profiling of testis-expressed microRNAs. Dev Biol. 2007a;311:592–602. doi: 10.1016/j.ydbio.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W. Cloning and expression profiling of small RNAs expressed in the mouse ovary. Rna. 2007b;13:2366–80. doi: 10.1261/rna.754207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–84. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Anger M, Bernstein E, Hannon GJ, Schultz RM. RNAi and expression of retrotransposons MuERV-L and IAP in preimplantation mouse embryos. Dev Biol. 2004;269:276–85. doi: 10.1016/j.ydbio.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. Embo J. 2006;25:5816–25. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MD, Jin Z, Xie T. Molecular mechanisms of germline stem cell regulation. Annu Rev Genet. 2005;39:173–95. doi: 10.1146/annurev.genet.39.073003.105855. [DOI] [PubMed] [Google Scholar]
- Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, Ma Y. A microarray for microRNA profiling in mouse testis tissues. Reproduction. 2007;134:73–79. doi: 10.1530/REP-07-0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


