Abstract
The experiments explored the extent to which alterations in L-type Ca2+ channel-mediated Ca2+ entry triggers Ca2+-mediated arrhythmogenesis in Langendorff-perfused murine hearts through use of the specific L-type Ca2+ channel modulator FPL-64716 (FPL). Introduction of FPL (1 μm) resulted in a gradual development (>10 min) of diastolic electrical events and alternans in spontaneously beating hearts from which monophasic action potentials were recorded. In regularly paced hearts, they additionally led to non-sustained and sustained ventricular tachycardia (nsVT and sVT). Programmed electrical stimulation (PES) resulted in nsVT and sVT after 5–10 and >10 min perfusion, respectively. Pretreatments with nifedipine, diltiazem and cyclopiazonic acid abolished arrhythmogenic tendency induced by subsequent introduction of FPL, consistent with its dependence upon both extracellular Ca2+ entry and the degree of filling of the sarcoplasmic reticular Ca2+ store. Values for action potential duration at 90% repolarization when any of these agents were applied to FPL-treated hearts became indistinguishable from those shown by untreated control hearts, in contrast to earlier reports of their altering in long QT syndrome type 3 and hypokalaemic murine models for re-entrant arrhythmogenesis. These arrhythmic effects instead correlated with alterations in Ca2+ homeostasis at the single-cell level found in investigations of the effects of both FPL and the same agents in regularly stimulated fluo−3 loaded myocytes. These findings are compatible with a prolonged extracellular Ca2+ entry that potentially results in an intracellular Ca2+ overload and produces the cardiac arrhythmogenecity following addition of FPL.
The cardiac action potential (AP) in ventricular myocytes is initiated by extracellular Na+ entry but subsequently involves Ca2+ entry, mainly through L-type voltage-dependent Ca2+ channels (LTCCs), that both maintains the cell depolarization and initiates excitation–contraction coupling. The latter process requires a Ca2+-induced Ca2+ release (CICR) through sarcoplasmic reticular (SR) ryanodine receptor (RyR2) Ca2+ release channels (Wehrens & Marks, 2003; Jiang et al. 2004; Scoote & Williams, 2004). The consequent rise in cytosolic [Ca2+] activates systolic cardiac muscle contraction. During diastole, the extra Ca2+ is removed from the cytoplasm through SR Ca2+-ATPase (SERCA2a) into the SR or extruded from the cell via the Na+–Ca2+ exchanger (NCX; Williams et al. 2001). Abnormalities in the Ca2+ homeostatic process have been implicated in triggered arrhythmias, such as those associated with the ventricular arrhythmogenesis leading to sudden cardiac death, particularly in cardiac failure and catecholaminergic polymorphic ventricular tachycardia (Pogwizd et al. 2001; Williams et al. 2001; Jiang et al. 2002; Wehrens & Marks, 2003; Balasubramaniam et al. 2005; Goddard et al. 2008). These arrhythmias may manifest as early after-depolarizations (EADs), delayed after-depolarizations (DADs) and action potential alternans.
The present experiments, performed in both whole hearts and single myocytes, explore the extent to which alterations in LTCC-mediated entry of Ca2+ might eventually trigger mechanisms of this kind, through the use of the specific L-type calcium channel modulator FPL-64716 (FPL; Baxter et al. 1993; Triggle 2004). FPL appears to prolong both the opening of L-type Ca2+ channels during depolarization and the time course of inactivation upon repolarization (Rampe & Lacerda, 1991; Zheng et al. 1991; Kunze & Rampe, 1992; Lauven et al. 1999; Fan et al. 2000). Patch clamp studies from rat ventricular myocytes showed that FPL increased the amplitude of both Ca2+ currents and Ca2+ transients more than it increased the rate of rise of Ca2+ transients. It also slowed activation and inactivation, and enhanced the duration of tail currents upon repolarization (Fan & Palade, 2002). Our findings indeed demonstrate such arrhythmogenic effects of FPL, attributable to an enhanced Ca2+ entry, probably accompanied by consequent alterations in SR Ca2+ store.
Methods
Animals
Mice bred against a 129 genetic background (Harlan UK Ltd, Bicester, UK), housed at room temperature, fed sterile rodent chow with free access to water at all times and subjected to 12 h–12 h light–dark cycles were studied at ages between 5 and 10 months. They were killed by cervical dislocation (Schedule 1, UK Animals (Scientific Procedures) Act 1986).
Experiments on Langendorff-perfused hearts
Hearts were dissected out and immediately immersed into ice-cold bicarbonate-buffered Krebs–Henseleit (KH) buffer (containing, in mmol l−1): NaCl, 119; Na2CO3, 25; KCl, 4; KH2PO4, 1.2; MgCl2, 1.0; CaCl2, 1.8; glucose, 11; and sodium pyruvate, 2.0. The buffer was made daily and equilibrated with a 95% O2–5% CO2 gas mixture (British Oxygen Company, Manchester, UK) (Bethell et al. 1998). The electrophysiological experiments on arrhythmogenesis at the whole heart level used a Langendorff-perfused preparation adapted for the murine model (Papadatos et al. 2002; Balasubramaniam et al. 2003). The perfusate was passed through a water-jacket at a flow rate of 2.0–2.5 ml min−1 (model 505s, Watson-Marlow Berdel, Falmouth, UK). Fluid in the water-jacket was circulated with a circulator (model C−85A, Techne, Cambridge, UK) and warmed to 37°C by passing through a water-bath. The perfusate was then passed through 200 and 5 μm filters (Millipore, Watford, UK) before passing into the aorta of the heart. Hearts were thus perfused using buffer in which catecholamines were absent. They were then allowed to beat spontaneously until a steady state was reached, a time that permitted ample time for washout of any catecholamines that may have been present in the circulation at the time of killing.
The contracting heart was stimulated with a custom-made platinum stimulating electrode applied to the epicardial surface, usually for 15 min at 10 Hz, and was allowed to reach a physiologically steady state. The heart was paced from its right ventricle at three times its threshold level (between 3 and 5 V) using square-wave stimuli of 2 ms duration (Grass S48 stimulator, Grass Telefactor, Slough, UK). Monophasic action potentials (MAPs) were recorded from the epicardial basal surface of the left ventricle using a contact-type MAP electrode (Linton Instruments, Harvard Apparatus, Edenbridge, UK).
Following the equilibration period, hearts were first studied in conditions of spontaneous activity, which typically took place at a heart rate of ∼6 Hz. They were then subjected to constant ventricular pacing at cycle lengths of 8 and 10 Hz. Finally, they were subjected to programmed electrical stimulation (PES) using a variation of existing clinical techniques (Saumarez & Grace, 2000; Saumarez et al. 2003). The stimulation sequence consisted of a drive train of pacing stimuli (S1) applied with a 200 ms cycle length, with an extra stimulus (S2) inserted every eighth beat performed at cycle lengths of 8 and 10 Hz for determination of the ventricular effective refractory period (VERP).
The MAP signals were amplified, filtered (band-pass filter 0.5 Hz to 1 kHz, Gould-Nicolet Technologies, Ilford, UK) and digitized at a frequency of 5 kHz using an analog-to-digital converter (model 1401plus, Cambridge Electronic Design, Cambridge, UK). Monophasic APs that were considered for analysis were all stable for more than 30 min, and the data were manually reviewed using Spike2 software (CED, Cambridge, UK). According to accepted criteria regarding MAP morphology, MAP traces selected should have a stable baseline with a rapid upstroke without inflection or negative spikes, consistent amplitude >1 mV, and rapid first phase repolarization lacking an early plateau. Action potential duration (APD) was analysed at 30, 50, 70 and 90% repolarization (APD30, APD50, APD70 and APD90) following previous MAP analysis protocols (Gussak & Antzelevitch, 2000; Fabritz et al. 2003). Results are all expressed as means ±s.e.m.
Pharmacological agents studied were all purchased from Sigma-Aldrich, Poole, UK. The Ca2+ channel agonist FPL-64716 (FPL) was initially made up with dimethyl sulphoxide (DMSO) to make a stock concentration of 5 mm. Stock solutions of FPL were wrapped in foil because they are photosensitive and stored at −20°C. Cyclopiazonic acid (CPA) was dissolved in 96% ethanol to make an initial stock solution of 10 mm, which was stored at −20°C. Diltiazem was dissolved in distilled water to make a stock concentration of 1 mm and kept refrigerated at 4°C. Final drug solutions used were diluted with KH buffer made fresh before every experiment. The FPL was diluted to a working concentration of 1 μm. The CPA was diluted to an intermediary solution of 10 μm and then further diluted to a concentration of 150 nm. Nifedipine and diltiazem were both used at a final concentration of 100 nm.
Arrhythmogenic phenotypes in the form of after-depolarizations, delayed after-depolarizations, alternans, and monomorphic and polymorphic ventricular tachycardia were examined both before and following introduction of FPL in both control conditions and following subsequent additions of nifedipine, diltiazem and CPA. After-depolarizations were defined as depolarizing electrical events that interrupted the restitution phases of action potentials, whereas premature activations took place following full action potential recovery. Spontaneous tachycardias prolonged over more than 30 s were defined as sustained, whereas those lasting less than 30 s were defined as non-sustained.
Statistical analysis of results obtained in the different pharmacological conditions was carried out using either paired or unpaired two-way ANOVA (SPSS Inc., Chicago, IL, USA) for continuous data and Fisher's exact test for categorical data. Results are all expressed as means ±s.e.m., and differences were considered significant at a level of P < 0.05.
Measurements of myocyte [Ca2+]
Single-cell experiments used mouse ventricular myocytes isolated by adapting an established enzymatic digestion described previously (Harding et al. 1992). Following killing, hearts were excised rapidly and cannulated before being connected to a Langendorff perfusion system. The heart was then perfused for 1.5 min at 3 ml min−1 with perfusion buffer containing (mm): NaCl, 120; MgSO4, 5; KCl, 5.4; sodium pyruvate, 5; Hepes, 10; glucose, 5; taurine, 20; nitrilotriacetic acid (NTA), 5 (Sigma-Aldrich); and adjusted using NaOH to pH 7.0 at 37°C. Subsequently, the heart was perfused for 25–28 min at 3 ml min−1 with digestion buffer containing (mm): NaCl, 120; MgSO4, 5; KCl, 5.4; sodium pyruvate, 5; Hepes, 10; glucose, 5; and taurine, 20, to which was added collagenase type II (Worthington, Lakewood, NJ, USA; final concentration 1 mg ml−1), hyaluronidase (Sigma-Aldrich; final concentration 1 mg ml−1) and 200 μm Ca2+ and adjusted using NaOH to pH 6.8 at a temperature of 37°C. The pale and swollen heart was then removed and cut below the atria. The digested tissue was teased gently with fine forceps, and the dissociated cells were transferred to a conical tube containing 12.5 ml digestion buffer with 50 mg bovine serum albumin (BSA) to inactivate digestion. The resulting myocytes were subjected to further trituration using sterile plastic transfer pipettes, and allowed to sediment by gravity for 10 min.
The cell suspension was then centrifuged for 1.5 min at 100 g and the pellets resuspended twice in wash buffer containing (mm): NaCl, 119; KCl, 4.2; MgSO4, 0.94; KH2PO4, 1.2; Hepes, 20; glucose, 11.5; taurine, 20; and 1 mg ml−1 BSA, and bubbled with 95% O2–5% CO2, pH 7.4, to remove all traces of NTA, collagenase and hyaluronidase. Calcium chloride was then cautiously reintroduced by stepwise (0.2 mm) additions to reach an approximate concentration of 1.25 mm, by centrifuging the preparation at each Ca2+ reloading stage to remove dead or swollen cells. During this process, myocytes were examined periodically under the microscope to confirm a good yield of rod-shaped myocytes (better than 60%) before continuing the experiments. Cells were then transferred into a conical tube (BD Biosciences, Oxford, UK) and incubated at room temperature. The isolated myocytes were then placed in a control Hepes-buffered Krebs–Henseleit solution that was the same as the wash buffer mentioned previously without BSA and had a pH of 7.2, and transferred to a Perspex chamber 10 mm × 4 mm × 6.25 mm (length × width × depth). The myocytes spontaneously attached to the glass coverslip (3 cm × 3 cm, grade 1 coverslip (Merck, Hoddesdon, Herts, UK) forming the floor of the chamber. The myocytes were stimulated to contract using two in-built platinum field electrodes running the length of the chamber through which the periodic field stimulation was applied using a custom-built square-wave stimulator. This applied successive 40–60 V steps of duration 2.2 ms, immediately followed by a step of similar duration and amplitude but opposite polarity at a pacing frequency of 0.5 Hz. During this stimulation, cells were loaded with the acetoxymethyl (AM) ester of fluo-3 (Invitrogen, Paisley, UK; 50 μg vial diluted in 30 μl pluronic acid to give a stock concentration of 1.476 mm) by incubating the cells in a bath of volume 500 μl containing KH buffer (with 1.25 mm Ca2+) with 2 μl of fluo−3 AM solution for 15 min at room temperature in the dark. The bath with the myocytes was then transferred onto the stage of a Zeiss LSM-510 laser scanning confocal microscope system (Carl Zeiss Ltd, Welwyn Garden City, UK) with a ×20 air objective (NA 0.5; confocal aperture 1000 μm, slice thickness <42.4 μm) on a Zeiss Axiovert 100 M inverted microscope. Fluo−3 fluorescence emission was excited with a 488 nm argon laser and measured at 505–550 nm. Images were analysed using an in-house custom-written program. A series of 500 frames sampled at 98 ms per frame (128 × 128 pixels per frame) were used initially to monitor fluorescence changes over time. The appropriateness of the chosen frame capture rate was corroborated in some experiments by collecting data in the faster line-scan mode with a sampling rate of 960 μs per line. All traces had stable baselines, confirming consistent laser intensities and detector gains. The peak normalized fluorescence (F/F0) values following each response to each stimulus were calculated for each time series, and a mean was acquired for each series. Moreover, the mean peak F/F0 values as well as the baseline diastolic values were calculated. The results are expressed as mean values ±s.e.m. and compared using ANOVA (SPSS Inc.). Different solutions were perfused through the perfusion chamber as required. All confocal microscope studies were carried out at room temperature.
Results
Recordings of MAPs demonstrate arrhythmic effects of FPL
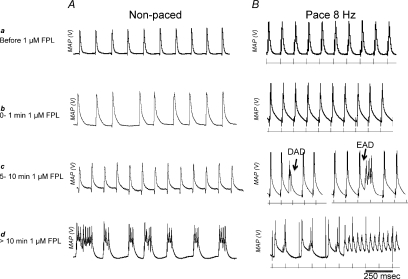
Previous work has demonstrated that FPL (1 μm) increases extracellular Ca2+ entry through L-type Ca2+ channels, thereby enhancing cytosolic Ca2+ loading (Fan & Palade, 2002). It therefore served as a useful pharmacological tool to assess the effect of the resulting alterations of cellular Ca2+ homeostasis on cardiac arrhythmogenic properties. Such experiments first compared MAP waveforms from epicardial surfaces of the left ventricles of eight Langendorff-perfused hearts during both spontaneous activity (Fig. 1A) and during regular pacing at 8 Hz (Fig. 1B) before and at different times following the introduction of FPL (1 μm) into the perfusing KH buffer solution. The traces shown in each panel exemplify results from ∼2 min of recordings in each condition. Before introduction of FPL all hearts showed normal MAP waveforms whose time courses resembled those reported on earlier occasions (Fabritz et al. 2003; Killeen et al. 2007a) and no evidence of arrhythmogenic behaviour (Fig. 1Aa and Ba).
Figure 1. Examples of monophasic action waveforms.
Epicardial monophasic action waveforms from epicardial surfaces of the left ventricles of Langendorff-perfused hearts during both non-paced activity (A) and regular pacing at 8 Hz (B) before (a) and at different times following the introduction of 1 μm FPL-64716 (FPL) into the perfusing KH buffer solution (b–d).
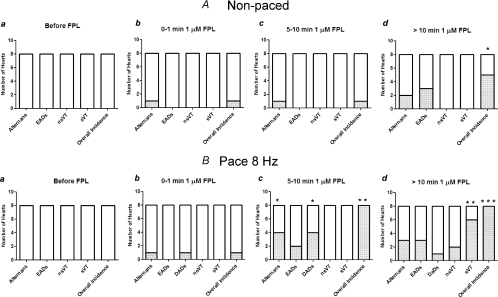
Figure 2 summarizes numbers of hearts showing each phenomenon, as well as the results of Fisher exact tests for these incidences when compared with control values obtained in the absence of FPL. Introduction of FPL then led to a progressive development, over 0–10 min, of a variety of waveform abnormalities in both non-paced and paced hearts (Figs 1Ab–d and Bb–d and 2Ab–d and Bb–d). During spontaneous activity, 1 min of exposure to FPL left MAP waveforms and rhythm relatively normal apart from a single episode of 40 pairs of action potentials showing a stable interval alternans, attributable to either ventricular or supraventricular changes, in a single heart (Figs 1Ab and 2Ab). At between 5–10 min of FPL perfusion, a single heart showed 120 pairs of action potentials in alternans (Figs 1Ac and 2Ac). At >10 min following introduction of FPL, two hearts showed 12 and 50 pairs of APs in alternans, and MAPs in three hearts showed repetitive spikes interrupting the time courses of the AP recoveries (Fig. 1Ad), resulting in a statistically significant (P= 0.009) incidence of arrhythmic events compared with control results observed before addition of FPL (Fig. 2Ad).
Figure 2. Arrhythmic phenomena in non-paced and paced hearts.
Numbers of hearts showing arrhythmic phenomena out of a total of n= 8 studied during both non-paced activity (A) and regular pacing at 8 Hz (B) before (a) and at different times following the introduction of 1 μm FPL-64716 (FPL) into the perfusing KH buffer solution (b–d). The bar graphs show incidences of hearts having one or more episodes of alternans (Ab–Ad and Bb–Bd), early and delayed after-depolarizations (EADs and DADs; Ad, Bc and Bd) or episodes of ventricular tachycardia (VT; Bd). The bar graphs also show results of Fisher's exact tests for these incidences when compared with control values obtained in the absence of FPL (*P < 0.01, **P < 0.001 and ***P < 0.0001; open bars indicate and absence or insignificant incidence of the arrhythmic phenomenon).
In paced hearts, with 1 min of exposure to FPL there were no arrhythmic episodes (Fig. 1Bb) apart from a single 40 s episode of stable alternans and one DAD in one heart (Fig. 2Bb). A total of four hearts perfused for 5–10 min with FPL exhibited episodes of alternans with a mean duration of 25 ± 7.6 s (P= 0.07, compared with control); of these, two hearts showed EADs that intercepted the recovery phase of approximately two and six out of 20 successive APs. Moreover, four hearts showed DADs, giving an overall statistically significant incidence of arrhythmic phenomena (P= 0.006) compared with control hearts (Figs 1Bc and 2Bc). With >10 min exposure to FPL, three hearts showed five episodes of stable alternans of mean duration 21 ± 6.4 s. In addition, three hearts showed EADs that interrupted six, 10 and three out of 20 successive action potentials, three hearts showed DADs, two hearts showed episodes of ventricular tachycardia (VT) lasting <30 s, and six hearts showed episodes of VT lasting >30 s (Figs 1Bd and 2Bd). The incidence of sustained VT (sVT) and the overall incidence of arrhythmic events were both significantly higher (P= 0.006, P= 0.0001) than corresponding incidences obtained before addition of FPL.
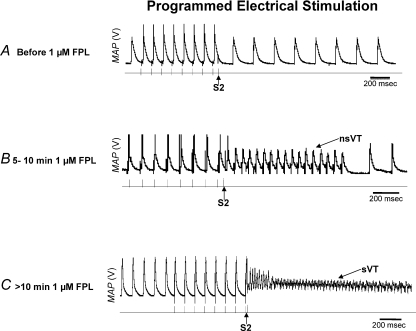
Programmed electrical stimulation procedures then provided quantifiable tests for arrhythmic substrate as employed on previous experimental and clinical occasions (Saumarez & Grace, 2000; Balasubramaniam et al. 2003). Figure 3 illustrates findings showing that FPL treatment resulted in significant arrhythmic substrate. Thus: (1) regularly paced untreated hearts showed no arrhythmic incidents (n= 8; Fig. 3A); (2) seven out of eight hearts perfused with FPL for 5–10 min showed non-sustained VT (nsVT), representing a significant increase in such arrhythmic incidence (Fig. 3B; Fisher exact test giving a two-tailed probability of P < 0.001); and (3) all eight hearts showed episodes of sVT with further perfusion with FPL for more than 10 min (Fig. 3C; P < 0.0001).
Figure 3. Monophasic action potentials recorded from the epicardium of hearts in the absence and presence of FPL.
Monophasic action potentials recorded from the epicardium of hearts before (A), 5–10 min (B) and >10 min after the addition of 1 μm FPL (C). All experiments were performed during PES at 8 Hz; the arrows indicate S2 extra-stimuli. The examples illustrated that before addition of FPL (A), isolated perfused hearts showed a persistently regular rhythm with no arrhythmogenic events during the PES procedures. The S2 extra-stimuli initiated an episode of nsVT lasting for less than 30 s in 7 out of 8 hearts perfused with FPL for 5–10 min (B). All 8 hearts showed episodes of sVT lasting for more than 30 s with further perfusion with FPL for more than 10 min (C).
Pharmacological modifications of the effects of FPL
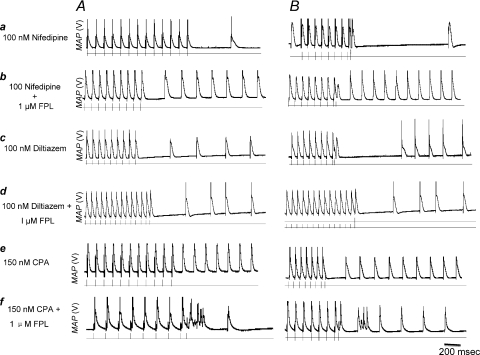
We next explored the effects of pretreatments with the dihydropyridine Ca2+ channel blocker nifedipine (100 nm), the benzothiazepine Ca2+ blocker diltiazem (100 nm) and the effects of the SERCA inhibitor CPA (150 nm) before restoring FPL to the buffer, in both regularly paced hearts (Fig. 4A) and in hearts undergoing PES (Fig. 4B). Cyclopiazonic acid (Goeger et al. 1988; Mason et al. 1989; Seidler et al. 1989) specifically inhibits SERCA activity in myocardial vesicle preparations by ∼50% at 100–200 nm and completely at 10 μm (Schwinger et al. 1997) whilst sparing Ca2+ sensitivity in the contractile myofilaments (Takahashi et al. 1995), Ca2+ currents (Bonnet et al. 1994) and NCX activity (Yard et al. 1994).
Figure 4. Epicardial monophasic action potential recordings in paced hearts and in hearts undergoing PES in the presence of various drugs.
Epicardial MAP recordings obtained from both regularly paced hearts (A) and hearts undergoing PES (B) pretreated with 100 nm nifedipine (a), 100 nm diltiazem (c) and 150 nm CPA (e) before restoring 1 μm FPL to the buffer. None of the hearts showed arrhythmic phenomena following pretreatment (a, c and e). Inclusion of either nifedipine or diltiazem during subsequent FPL treatment totally suppressed FPL-induced VT (b and d, respectively) through either pacing protocol. However, 150 nm CPA in combination with a subsequent addition of 1 μm FPL (f) abolished FPL-induced VT although it did permit EAD episodes following the shortest S2 stimuli in each of n= 4 hearts.
None of the hearts on pretreatment with nifedipine (n= 4 hearts; Fig. 4Aa and Ba), diltiazem (n= 4 hearts; Fig. 4Ac and Bc) or CPA (n= 4; Fig. 4Ae and Be) showed arrhythmic phenomena. Inclusion of either nifedipine or diltiazem during subsequent FPL treatment totally suppressed FPL-induced VT (Fig. 4Ab, Ad, Bb and Bd) through either pacing protocol in the same hearts. Treatment with CPA in combination with a subsequent addition of FPL (Fig. 4Af and Bf) abolished FPL-induced VT, although it did permit EAD episodes following the shortest S2 stimuli in all four hearts (Fisher's exact test, P= 0.002 when tested against results with FPL alone in each case).
Correlations between arrhythmic actions and AP waveform
Exposure to FPL thus produced VT in isolated murine hearts, and this occurred along with phenomena associated with triggered arrhythmogenesis such as EADs and alternans. The experiments that followed investigated the extent to which FPL produces changes in APD and refractory period, since alterations in these have been observed in previous long QT syndrome (LQTS) and hypokalaemic murine cardiac models and have been implicated in the re-entrant substrate responsible for their abnormal electrical activity (Stokoe et al. 2007a,b; Thomas et al. 2007b). Both mechanisms could co-exist. Timothy syndrome, attributed to abnormalities in the LTCC, results in a multisystem disorder that also causes LQTS (Splawski et al. 2004, 2005).
Firstly, epicardial APD values were accordingly measured at x= 30, 50, 70 and 90% recovery (APD30, APD50, APD70 and APD90) from left ventricular MAPs obtained from hearts paced at 8 Hz (Table 1). In control hearts, APD90 was 48 ± 3.5 ms (n= 6 hearts), in agreement with previous reports (e.g. Killeen et al. 2007b). We then investigated the effects of FPL (1 μm; n= 6 hearts), at different perfusion times (0–1, 5–10 and >10 min) identical to those adopted above upon values of APDx. Application of two-way ANOVA to each of the above groups demonstrated that there were no significant differences between treated or untreated groups with the exception of APD30 values obtained between 5–10 min. Therefore, FPL did not significantly alter action potential time course despite the occurrence of EADs and alternans but not sVT at times <10 min and the appearance of all three phenomena at times >10 min. This progressive development of arrhythmogenesis is therefore more likely to arise from progressive increases in intracellular Ca2+ resulting from increased net Ca2+ influx brought about by FPL. The findings complement recent reports in a murine RyR2 model whose arrhythmogenic properties were attributable to alterations in RyR2 (Goddard et al. 2008). They contrast with re-entrant arrhythmogenic properties associated with altered action potential kinetics demonstrated in models for long QT syndrome type 3 (LQT3) (Thomas et al. 2007a).
Table 1.
Left ventricular epicardial action potential durations at different repolarization times in regularly paced (8 Hz) hearts at different perfusion times in the presence and absence of 1 μm FPL-64716
| APD30 (ms) |
APD50 (ms) |
APD70 (ms) |
APD90 (ms) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.e.m. | Mean | s.e.m. | Mean | s.e.m. | Mean | s.e.m. | |
| KH buffer (n= 6) | 6 | 1.0 | 14 | 1.3 | 27 | 3.0 | 48 | 3.5 |
| 0–1 min KH buffer (n= 6) | 6 | 1.4 | 13 | 3.1 | 24 | 4.0 | 47 | 3.0 |
| 5–10 min KH buffer (n= 6) | 4 | 0.5 | 14 | 1.5 | 29 | 3.6 | 54 | 2.3 |
| >10 min KH buffer (n= 6) | 6 | 0.6 | 13 | 2.8 | 27 | 3.6 | 54 | 1.5 |
| KH buffer (n= 6) | 11 | 0.1 | 18 | 0.1 | 26 | 0.2 | 43 | 0.2 |
| 0–1 min 1 μm FPL (n= 6) | 10 | 1.3 | 20 | 1.5 | 31 | 1.7 | 49 | 1.2 |
| 5–10 min 1 μm FPL (n= 6) | 13 | 4.1 | 22 | 5.0 | 33 | 4.2 | 53 | 1.5 |
| >10 min 1 μm FPL (n= 6) | 6 | 2.2 | 11 | 4.2 | 22 | 4.5 | 60 | 2.8 |
n gives number of hearts.
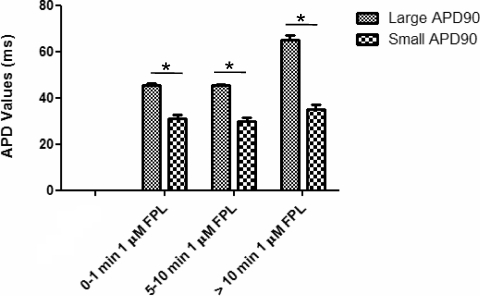
Secondly, we followed APD90 values for successive alternate MAPs during episodes of alternans in paced hearts. At 1, 5–10 and >10 min following FPL introduction, the mean duration of the shorter of each pair of MAPs was 31 ± 1.5 (n= 1 heart, 20 action potentials), 30 ± 1.5 (n= 4 hearts, 20 action potentials) and 35 ± 1.8 ms (n= 3 hearts); the corresponding value for the longer of each pair was 45 ± 0.7 (n= 1 heart), 46 ± 0.3 (n= 4 hearts) and 65 ± 2.1 ms (n= 3 hearts), respectively (Fig. 5).
Figure 5. The effect of alternans on APD90 values.
The APD90 values in alternans were calculated for alternans that appeared 1 min following introduction of FPL. However, the presence of alternans significantly (*P < 0.001) decreased APD90 values with increased FPL perfusion times after 1 min, 5–10 min and >10 min perfusion with FPL.
Thirdly, neither pretreatment with nifedipine (n= 6 hearts), diltiazem (n= 5 hearts) and CPA (n= 9 hearts) nor a further addition of FPL resulted in any significant change in APDx as indicated by ANOVA (Table 2).
Table 2.
Effects of nifedipine, diltiazem and cyclopiazonic acid on action potential duration in the presence and absence of 1 μm FPL-64716
| APD30 (ms) |
APD50 (ms) |
APD70 (ms) |
APD90 (ms) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | s.e.m. | Mean | s.e.m. | Mean | s.e.m. | Mean | s.e.m. | |
| KH buffer (n= 4) | 10 | 2.0 | 19 | 3.1 | 30 | 3.2 | 49 | 1.7 |
| 100 nm nifedipine (n= 6) | 6 | 1.7 | 19 | 1.8 | 30 | 1.6 | 49 | 1.4 |
| 100 nm nifedipine + 1 μm FPL (n= 8) | 9 | 1.4 | 17 | 1.6 | 30 | 1.5 | 49 | 1.1 |
| KH buffer (n= 5) | 7 | 1.5 | 16 | 2.2 | 30 | 2.5 | 52 | 2.5 |
| 100 nm diltiazem (n= 5) | 4 | 0.6 | 13 | 1.0 | 28 | 1.9 | 50 | 2.1 |
| 100 nm diltiazem + 1 μm FPL (n= 7) | 6 | 0.6 | 16 | 1.5 | 30 | 1.5 | 53 | 0.9 |
| KH buffer (n= 5) | 11 | 1.5 | 20 | 1.5 | 30 | 1.4 | 47 | 1.4 |
| 150 nm CPA (n= 9) | 13 | 1.6 | 20 | 2.3 | 30 | 2.9 | 49 | 3.2 |
| 150 nm CPA + 1 μm FPL (n= 7) | 10 | 1.9 | 19 | 2.1 | 29 | 2.9 | 47 | 3.7 |
n gives number of hearts.
Calcium transients in single mouse myocytes
The experiments that followed correlated these arrhythmic effects with changes in the amplitude and pattern of Ca2+ transients measured in fluo-3 loaded isolated murine cardiac myocytes. These were subjected to regular stimulation (0.5 Hz), whose timing is indicated by the dots below the F/F0 traces in Figs 6–9. Cells were studied in KH buffer under identical FPL concentrations, in the presence and absence of pretreatments with nifedipine, diltiazem and CPA. In all these experimental conditions, F/F0 traces showed stable baselines and reproducible peak responses to regular stimulation with minimal evidence of fluorophor bleaching over the adopted sampling periods as assessed by readings obtained at the beginning and end of the adopted, 50 s, sampling periods (P≫ 5%).
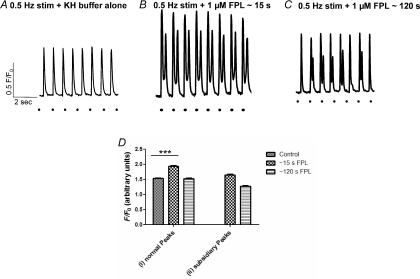
Figure 6. Fluo−3 fluorescence measurements in isolated myocytes studied using confocal microscopy.
Normalized fluorescence (F/F0) plotted against time with myocyte exposed to periodic field stimulation in perfusion buffer alone prior to any pharmacological manoeuvre (A) and records obtained following addition of 1 μm FPL to the buffer for approximately 15 s (B) and approximately 120 s (C). D shows overall mean peak F/F0 values taken from the entire set of myocytes. The overall mean peak F/F0 values are represented for the entire set of myocytes (Di) and subsidiary events (Dii).
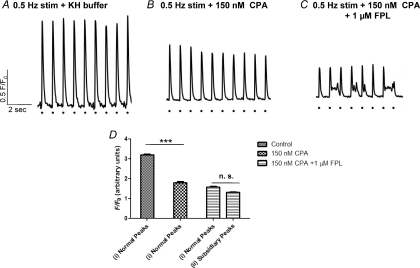
Figure 9. Calcium transients in regularly stimulated isolated myocytes in the presence of CPA and FPL.
Calcium transients in regularly stimulated (0.5 Hz) isolated myocytes before (A) and after addition of 150 nm CPA (B) and a further addition of FPL (C). D, CPA significantly (***P < 0.05; one-way ANOVA) reduced peak F/F0 from 3.20 ± 0.04 (n= 9 cells) to 1.80 ± 0.06 (n= 12 cells), but there were no further changes in peak F/F0 following introduction of FPL, which left a peak F/F0 of 1.57 ± 0.05 (n= 6 cells), but an appearance of persistent spontaneous ectopic Ca2+ peaks in the intervals between stimuli whose peak F/F0 was 1.32 ± 0.02 (n= 6 cells; C and D).
Before addition of FPL, the Ca2+ signals observed immediately following commencement of stimulation consisted of series of responses with consistent amplitudes, each directly following the individual stimuli with a peak F/F0 of 1.53 ± 0.02 (events counted over a standard sampling period of 50 s; n= 6 cells; cells from at least 2 hearts studied in each condition). The myocytes did not show spontaneous ectopic peaks in the intervals between stimuli, nor did they show any subsidiary events during the recovery phases of the evoked Ca2+ transients (Fig. 6A).
In contrast, resumption of scanning within 15 s of addition of FPL demonstrated F/F0 transients with increased amplitudes of 1.93 ± 0.03 (n= 3 cells, P < 0.001) that showed subsidiary events (1.64 ± 0.03, n= 6 cells, 2 hearts; Fig. 6B). The additional Ca2+ entry from either the extracellular space or Ca2+ stores reflected in such subsidiary events would lead to a progressive cytosolic Ca2+ loading, which, if the cause of the observed arrhythmogenesis, would account for the gradual onset of the arrhythmic phenomena described here. However, following this initial scanning, with continued stimulation for >120 s, resumption of scanning demonstrated F/F0 transients whose amplitudes were significantly lower (peak F/F0 of 1.53 ± 0.02, n= 6 cells, 2 hearts, P < 0.05) with subsidiary events of amplitude 1.27 ± 0.02 (n= 3 cells; Fig. 6C and D), a finding compatible either with photobleaching or a consequent depletion of the Ca2+ store.
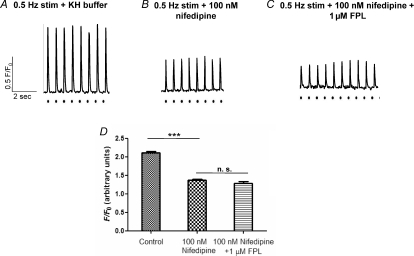
The effects of the pharmacological agents nifedipine (100 nm; Fig. 7), diltiazem (100 nm; Fig. 8) and CPA (150 nm; Fig. 9) were then compared before and 10 min after a subsequent addition of FPL in similarly stimulated cells. Despite their sharply differing sites of action in influencing Ca2+ homeostasis, such manoeuvres yielded similar results. Thus, the addition of nifedipine, diltiazem and CPA all reduced peak F/F0 in a similar manner, significantly (P < 0.05; one-way ANOVA), from 2.11 ± 0.04 (n= 6 cells), 1.77 ± 0.02 (n= 3 cells) and 3.20 ± 0.04 (n= 9 cells; Figs 7A and D, 8A and D and 9A and D) to 1.37 ± 0.02 (n= 9 cells), 1.30 ± 0.01 (n= 3 cells) and 1.80 ± 0.06 (n= 12 cells) (Figs 7B and D, 8B and D and 9B and D), respectively. These findings agree with earlier results that had used similar concentrations of nifedipine and diltiazem (Balasubramaniam et al. 2004).
Figure 7. Calcium transients in regularly stimulated isolated myocytes in the presence of nifedipine and FPL.
Calcium transients in regularly stimulated (0.5 Hz) isolated myocytes before (A) and after addition of 100 nm nifedipine (B) and a further addition of FPL (C). D, nifedipine significantly (***P < 0.05; one-way ANOVA) reduced peak F/F0 from 2.11 ± 0.04 to 1.37 ± 0.02 (n= 9 cells), but there were no further changes following introduction of FPL, which left a peak F/F0 of 1.28 ± 0.05 (n= 6 cells).
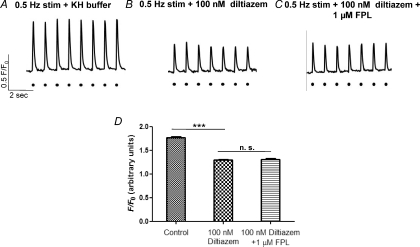
Figure 8. Calcium transients in regularly stimulated isolated myocytes in the presence of diltiazem and FPL.
Calcium transients in regularly stimulated (0.5 Hz) isolated myocytes before (A) and after addition of 100 nm diltiazem (B) and a further addition of FPL (C). D, diltiazem significantly (***P < 0.05; one-way ANOVA) reduced peak F/F0 from 1.77 ± 0.02 (n= 3 cells) to 1.30 ± 0.01 (n= 3 cells), but there were no further changes following introduction of FPL, which left a peak F/F0 of 1.31 ± 0.01 (n= 4 cells).
However, in all three cases, following washout and reintroduction of solutions that also included 1 μm FPL, giving an overall sequence of solution changes identical to that used in the corresponding experiments on whole hearts, there was no further significant change in values of F/F0, which were left at 1.28 ± 0.05 (n= 6 cells), 1.31 ± 0.01 (n= 4 cells) and 1.57 ± 0.05 (n= 6 cells; Figs 7C and D, 8C and D and 9C and D, respectively) even after 1 min following perfusion of the new agents. Furthermore, the presence of nifedipine and diltiazem abolished both ectopic and subsidiary Ca2+ release events (Figs 7C and D and 8C and D) in parallel with the similar absence of any arrhythmic phenomena in the whole hearts. In contrast, myocytes treated with both CPA and FPL showed persistent spontaneous ectopic Ca2+ peaks in the intervals between stimuli. There were also subsidiary events whose peak F/F0 (1.32 ± 0.02; n= 6 cells) was similar to those observed with FPL. Nevertheless they occurred significantly less frequently, in 5 ± 0.20 out of 23 evoked transients (n= 22 cells, 4 hearts; Fig. 9C) than in myocytes treated with FPL alone. These last findings correlate with the persistence of transient arrhythmic events observed in the whole hearts.
Discussion
The present experiments explored the extent to which alterations in LTCC-mediated entry of Ca2+ might eventually trigger Ca2+-mediated arrhythmogenesis in isolated perfused murine hearts through use of the specific L-type calcium channel modulator FPL-64716 (Baxter et al. 1993; Triggle, 2004). FPL is known to increase the contractility of both smooth and cardiac muscle (Zheng et al. 1991; Rampe & Dage, 1992; Rampe et al. 1993). Murine hearts have already successfully been used to replicate arrhythmogenic properties associated with Brugada and LQT3 syndromes in which re-entrant mechanisms have been implicated (Stokoe et al. 2007a,b; Thomas et al. 2007a,b). Recently, murine models have been used in studies of an arrhythmogenic condition related to abnormal RyR2 function (Cerrone et al. 2005; Goddard et al. 2008). Their use in the present study permitted our examination not only of the physiological effects of alterations in Ca2+ homeostasis on murine cardiac function, but also their comparison with these previously described murine models.
Treatment with FPL resulted in a gradual development of arrhythmogenic phenomena in an absence of marked alterations in action potential waveform. This would be unexpected had FPL acted as a simple Ca2+ channel agonist to increase inward Ca2+ current. However, FPL is likely to have more complex actions in additionally slowing L-type Ca2+ channel opening during depolarization and slowing inactivation upon repolarization (Rampe & Lacerda, 1991; Zheng et al. 1991; Kunze & Rampe, 1992; Lauven et al. 1999; Fan et al. 2000; Fan & Palade, 2002), features that would compromise its full agonist action, and any consequence upon subsequent NCX activity (see e.g. Noble et al. 2007) in the situation of the relatively brief, triangulated action potentials (Danik et al. 2002; Hondeghem, 2007; Milberg et al. 2007) found in murine systems.
In the Langendorff-perfused murine hearts, introduction of FPL resulted in a gradual development (over 10 min) of diastolic electrical events and alternans in non-paced hearts. In paced hearts, FPL led to an additional appearance of arrhythmic, nsVT and sVT phenomena. Finally, hearts were subjected to PES. Results from this have been shown to correlate with arrhythmogenic tendency in genetically modified hearts modelling Brugada syndrome (Stokoe et al. 2007a), LQT3 (Head et al. 2005; Stokoe et al. 2007b) and long QT syndrome type 5 (LQT5) (Balasubramaniam et al. 2003; Thomas et al. 2007b) and to provide indications of clinical arrhythmogenic risk (Saumarez & Grace, 2000; Saumarez et al. 2003; Turner et al. 2005). In the present experiments, this procedure resulted in nsVT and sVT after 5–10 and >10 min perfusion, respectively. However, there were no accompanying alterations in APD when values obtained at matched times were compared in the presence and absence of FPL. This contrasts with the increases in APD associated with re-entrant substrate in other murine models (Killeen et al. 2007a).
In contrast, diastolic events of the kind observed here have previously been attributed to a range of Ca2+ homeostatic events. Thus, EADs have been attributed to L-type Ca2+ current (ICa) reactivation during prolonged AP plateaus (Zaugg & Buser, 2001; Bers, 2002a,b, 2008; Roden, 2003; Laurita & Katra, 2005) and DADs may follow spontaneous release of intracellularly stored SR Ca2+, leading to cytosolic Ca2+ waves and oscillations and increased NCX activity (Kass & Tsien, 1982; Orchard et al. 1983; Stern et al. 1983; Wier et al. 1983; Marban et al. 1986; January et al. 1988).
The alternans that involved APD parallel similar phenomena in clinical situations, in which such beat-to-beat fluctuations in the electrocardiographic T-wave are thought to be a strong diagnostic precursor of ventricular tachycardias and ventricular fibrillation, potentially leading to sudden cardiac death (Lee et al. 1987, 1988; Bloomfield et al. 2006). They have also been observed and related to arrhythmogenic tendency in hypokalaemic murine models at high heart rates (short baseline cycle lengths; Sabir et al. 2008b). Previous studies had attributed alternans to fluctuations in cytosolic SR Ca2+ that might result from digitalis toxicity and catecholaminergic polymorphic ventricular tachycardia (Kass & Tsien, 1982; Marks et al. 2002; Bers, 2008; Sabir et al. 2008a).
The subsequent experiments in intact hearts accordingly proceeded to explore the effects of pretreatments using three different pharmacological agents, the diydropyridine nifedipine, the benzothiazipine diltiazem and the indole tetramic acid CPA on arrhythmogenic tendency induced by subsequent introduction of FPL (Saumarez & Grace, 2000; Saumarez et al. 2003). Nifedipine and diltiazem are L-type Ca2+ channel blockers, with diltiazem additionally inhibiting calcium leak through RyR2s. Previous experiments using PES protocols showed that both these agents block triggered VT induced by either caffeine or isoproterenol (Balasubramaniam et al. 2003, 2004). In contrast, CPA is a potent and specific inhibitor of SERCA-mediated Ca2+ uptake (Goeger et al. 1988; Mason et al. 1989) without affecting Ca2+ sensitivity of the contractile apparatus (Takahashi et al. 1995), Ca2+ currents (Bonnet et al. 1994; Badaoui et al. 1995) and the NCX (Yard et al. 1994). It has been used to investigate the role of SERCA in regulating the relationship between contraction, intracellular Ca2+ transients and force–frequency relationships (Goeger et al. 1988; Mason et al. 1989; Baudet et al. 1993; Schwinger et al. 1997).
These experiments demonstrated that these effects of FPL could be attributed to its enhancing extracellular Ca2+ entry; FPL-induced arrhythmogenesis was abolished in hearts subjected to pretreatment with either nifedipine or diltiazem. The effects were also dependent upon the degree of filling of the SR Ca2+ store, and accordingly abolished by CPA. Furthermore, none of these agents, whether in the presence or absence of FPL, altered APD values, in contrast with earlier reports of the actions of such agents in LQT3 (Killeen et al. 2007a; Thomas et al. 2007a).
In contrast, these arrhythmic effects correlated with alterations in Ca2+ homeostasis at the single-cell level. These experiments investigated the effects of FPL, as well as nifedipine, diltiazem and CPA, upon regularly stimulated fluo−3 loaded myocytes subjected to similar pharmacological manoeuvres to those used in the whole hearts. Thus, FPL alone resulted in an immediate increase in peak evoked F/F0 as well as an onset of diastolic Ca2+ transients in isolated myocytes subjected to regular stimulation. This change was dependent upon a Ca2+ entry enhanced by FPL. Thus, both nifedipine and diltiazem reduced such transients, whose amplitude was then not restored by a subsequent administration of FPL; in such conditions, diastolic Ca2+ transients were absent. Similarly, myocytes pretreated with CPA had reduced peak F/F0 transients. These were not restored by a subsequent addition of FPL. Nevertheless, addition of FPL resulted in an appearance of diastolic transients whose amplitude was substantially lower than diastolic transients observed with FPL alone. This is as expected for an action of FPL in activating cardiac RyR2 either by Ca2+ entry or through a direct effect reported earlier (Wasserstrom et al. 2002; McDonough et al. 2005).
The present findings are thus compatible with a major role for extracellular Ca2+ entry in this arrhythmogenecity that is attributable to a gradually developing intracellular calcium overload.
Acknowledgments
We thank the James Baird Fund, the Frank Elmore Fund, the Medical Research Council, the Wellcome Trust and the British Heart Foundation for their generous support.
References
- Badaoui A, Huchet-Cadiou C, Leoty C. Effects of cyclopiazonic acid on membrane currents, contraction and intracellular calcium transients in frog heart. J Mol Cell Cardiol. 1995;27:2495–2505. doi: 10.1006/jmcc.1995.0237. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam R, Chawla S, Grace AA, Huang CL-H. Caffeine-induced arrhythmias in murine hearts parallel changes in cellular Ca2+ homeostasis. Am J Physiol Heart Circ Physiol. 2005;289:H1584–H1593. doi: 10.1152/ajpheart.01250.2004. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam R, Chawla S, Mackenzie L, Schwiening CJ, Grace AA, Huang CL-H. Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts. Pflugers Arch. 2004;449:150–158. doi: 10.1007/s00424-004-1321-2. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam R, Grace AA, Saumarez RC, Vandenberg JI, Huang CL-H. Electrogram prolongation and nifedipine-suppressible ventricular arrhythmias in mice following targeted disruption of KCNE1. J Physiol. 2003;552:535–546. doi: 10.1113/jphysiol.2003.048249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet S, Shaoulian R, Bers DM. Effects of thapsigargin and cyclopiazonic acid on twitch force and sarcoplasmic reticulum Ca2+ content of rabbit ventricular muscle. Circ Res. 1993;73:813–819. doi: 10.1161/01.res.73.5.813. [DOI] [PubMed] [Google Scholar]
- Baxter AJ, Dixon J, Ince F, Manners CN, Teague SJ. Discovery and synthesis of methyl 2,5-dimethyl-4-[2- (phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylate (FPL 64176) and analogues: the first examples of a new class of calcium channel activator. J Med Chem. 1993;36:2739–2744. doi: 10.1021/jm00071a004. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ Res. 2002a;90:14–17. [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002b;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Bethell HW, Vandenberg JI, Smith GA, Grace AA. Changes in ventricular repolarization during acidosis and low-flow ischemia. Am J Physiol Heart Circ Physiol. 1998;275:H551–H561. doi: 10.1152/ajpheart.1998.275.2.H551. [DOI] [PubMed] [Google Scholar]
- Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM. Microvolt T−wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–463. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Bonnet V, Badaoui A, Huchet-Cadiou C, Leoty C. Potentiation of the twitch responses by inhibitors of sarcoplasmic reticulum Ca2+-ATPase in frog atrial fibres. Eur J Pharmacol. 1994;264:69–76. doi: 10.1016/0014-2999(94)90637-8. [DOI] [PubMed] [Google Scholar]
- Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- Danik S, Cabo C, Chiello C, Kang S, Wit AL, Coromilas J. Correlation of repolarization of ventricular monophasic action potential with ECG in the murine heart. Am J Physiol Heart Circ Physiol. 2002;283:H372–H381. doi: 10.1152/ajpheart.01091.2001. [DOI] [PubMed] [Google Scholar]
- Fabritz L, Kirchhof P, Franz MR, Eckardt L, Monnig G, Milberg P, Breithardt G, Haverkamp W. Prolonged action potential durations, increased dispersion of repolarization, and polymorphic ventricular tachycardia in a mouse model of proarrhythmia. Basic Res Cardiol. 2003;98:25–32. doi: 10.1007/s00395-003-0386-y. [DOI] [PubMed] [Google Scholar]
- Fan JS, Palade P. Effects of FPL 64176 on Ca transients in voltage-clamped rat ventricular myocytes. Br J Pharmacol. 2002;135:1495–1504. doi: 10.1038/sj.bjp.0704598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JS, Yuan Y, Palade P. Kinetic effects of FPL 64176 on L-type Ca2+ channels in cardiac myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:465–476. doi: 10.1007/s002100000219. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, Grace AA, Huang CL-H. Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol (Oxf) 2008;194:123–140. doi: 10.1111/j.1748-1716.2008.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeger DE, Riley RT, Dorner JW, Cole RJ. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- Harding SE, Jones SM, O’Gara P, del Monte F, Vescovo G, Poole-Wilson PA. Isolated ventricular myocytes from failing and non-failing human heart; the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992;24:549–564. doi: 10.1016/0022-2828(92)91843-t. [DOI] [PubMed] [Google Scholar]
- Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH, Grace AA, Huang CLH. Paced electrogram fractionation analysis of arrhythmogenic tendency in DeltaKPQ Scn5a mice. J Cardiovasc Electrophysiol. 2005;16:1329–1340. doi: 10.1111/j.1540-8167.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. Relative contributions of TRIaD and QT to proarrhythmia. J Cardiovasc Electrophysiol. 2007;18:655–657. doi: 10.1111/j.1540-8167.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM, Salata JJ. A model for early afterdepolarizations: induction with the Ca2+ channel agonist Bay K 8644. Circ Res. 1988;62:563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Zhang L, Chen SR. Enhanced basal activity of a cardiac Ca2+ release channel (ryanodine receptor) mutant associated with ventricular tachycardia and sudden death. Circ Res. 2002;91:218–225. doi: 10.1161/01.res.0000028455.36940.5e. [DOI] [PubMed] [Google Scholar]
- Kass RS, Tsien RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982;38:259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen MJ, Gurung IS, Thomas G, Stokoe KS, Grace AA, Huang CL-H. Separation of early afterdepolarizations from arrhythmogenic substrate in the isolated perfused hypokalaemic murine heart through modifiers of calcium homeostasis. Acta Physiol (Oxf) 2007a;191:43–58. doi: 10.1111/j.1748-1716.2007.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen MJ, Thomas G, Olesen SP, Demnitz J, Stokoe KS, Grace AA, Huang CL-H. Effects of potassium channel openers in the isolated perfused hypokalaemic murine heart. Acta Physiol (Oxf) 2007b;193:25–36. doi: 10.1111/j.1748-1716.2007.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze DL, Rampe D. Characterization of the effects of a new Ca2+ channel activator, FPL 64176, in GH3 cells. Mol Pharmacol. 1992;42:666–670. [PubMed] [Google Scholar]
- Laurita KR, Katra RP. Delayed after depolarization-mediated triggered activity associated with slow calcium sequestration near the endocardium. J Cardiovasc Electrophysiol. 2005;16:418–424. doi: 10.1046/j.1540-8167.2005.40429.x. [DOI] [PubMed] [Google Scholar]
- Lauven M, Handrock R, Muller A, Hofmann F, Herzig S. Interaction of three structurally distinct Ca2+ channel activators with single L-type Ca2+ channels. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:122–128. doi: 10.1007/s002109900059. [DOI] [PubMed] [Google Scholar]
- Lee HC, Mohabir R, Smith N, Franz MR, Clusin WT. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo-1. Correlation with monophasic action potentials and contraction. Circulation. 1988;78:1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lee HC, Smith N, Mohabir R, Clusin WT. Cytosolic calcium transients from the beating mammalian heart. Proc Natl Acad Sci USA. 1987;84:7793–7797. doi: 10.1073/pnas.84.21.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Reiken S, Marx SO. Progression of heart failure: is protein kinase A hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- Mason RP, Campbell SF, Wang SD, Herbette LG. Comparison of location and binding for the positively charged 1,4-dihydropyridine calcium channel antagonist amlodipine with uncharged drugs of this class in cardiac membranes. Mol Pharmacol. 1989;36:634–640. [PubMed] [Google Scholar]
- McDonough SI, Mori Y, Bean BP. FPL 64176 modification of CaV1.2 L-type calcium channels: dissociation of effects on ionic current and gating current. Biophys J. 2005;88:211–223. doi: 10.1529/biophysj.104.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberg P, Hilker E, Ramtin S, Cakir Y, Stypmann J, Engelen MA, Mönnig G, Osada N, Breithardt G, Haverkamp W, Eckardt L. Proarrhythmia as a class effect of quinolones: increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. J Cardiovasc Electrophysiol. 2007;18:647–654. doi: 10.1111/j.1540-8167.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Noble D, Sarai N, Noble PJ, Kobayashi T, Matsuoka S, Noma A. Resistance of cardiac cells to NCX knockout. A model study. Ann NY Acad Sci. 2007;1099:306–309. doi: 10.1196/annals.1387.018. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Papadatos GA, Wallerstein PMR, Head CEG, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AEO, Huang CL-H, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA. 2002;99:6210–6215. doi: 10.1073/pnas.082121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Rampe D, Anderson B, Rapien-Pryor V, Li T, Dage RC. Comparison of the in vitro and in vivo cardiovascular effects of two structurally distinct Ca++ channel activators, BAY K 8644 and FPL 64176. J Pharmacol Exp Ther. 1993;265:1125–1130. [PubMed] [Google Scholar]
- Rampe D, Dage RC. Functional interactions between two Ca2+ channel activators, (S)-Bay K 8644 and FPL 64176, in smooth muscle. Mol Pharmacol. 1992;41:599–602. [PubMed] [Google Scholar]
- Rampe D, Lacerda AE. A new site for the activation of cardiac calcium channels defined by the nondihydropyridine FPL 64176. J Pharmacol Exp Ther. 1991;259:982–987. [PubMed] [Google Scholar]
- Roden DM. A surprising new arrhythmia mechanism in heart failure. Circ Res. 2003;93:589–591. doi: 10.1161/01.RES.0000095382.50153.7D. [DOI] [PubMed] [Google Scholar]
- Sabir IN, Li LM, Grace AA, Huang CL-H. Restitution analysis of alternans and its relationship to arrhythmogenicity in hypokalaemic Langendorff-perfused murine hearts. Pflugers Arch. 2008a;455:653–666. doi: 10.1007/s00424-007-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabir IN, Li LM, Jones VJ, Goddard CA, Grace AA, Huang CL-H. Criteria for arrhythmogenicity in genetically-modified Langendorff-perfused murine hearts modelling the congenital long QT syndrome type 3 and the Brugada syndrome. Pflugers Arch. 2008b;455:637–651. doi: 10.1007/s00424-007-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumarez RC, Chojnowska L, Derksen R, Pytkowski M, Sterlinski M, Huang CL-H, Sadoul N, Hauer RNW, Ruzyłło W, Grace AA. Sudden death in noncoronary heart disease is associated with delayed paced ventricular activation. Circulation. 2003;107:2595–2600. doi: 10.1161/01.CIR.0000068342.96569.A1. [DOI] [PubMed] [Google Scholar]
- Saumarez RC, Grace AA. Paced ventricular electrogram fractionation and sudden death in hypertrophic cardiomyopathy and other non-coronary heart diseases. Cardiovasc Res. 2000;47:11–22. doi: 10.1016/s0008-6363(00)00096-1. [DOI] [PubMed] [Google Scholar]
- Schwinger RH, Brixius K, Bavendiek U, Hoischen S, Müller-Ehmsen J, Bolck B, Erdmann E. Effect of cyclopiazonic acid on the force-frequency relationship in human nonfailing myocardium. J Pharmacol Exp Ther. 1997;283:286–292. [PubMed] [Google Scholar]
- Scoote M, Williams AJ. Myocardial calcium signalling and arrhythmia pathogenesis. Biochem Biophys Res Commun. 2004;322:1286–1309. doi: 10.1016/j.bbrc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. discussion 8086–8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Stern MD, Kort AA, Bhatnagar GM, Lakatta EG. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca2+-dependent cellular mechanical oscillations. J Gen Physiol. 1983;82:119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe KS, Balasubramaniam R, Goddard CA, Colledge WH, Grace AA, Huang CLH. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/– murine hearts modelling the Brugada syndrome. J Physiol. 2007a;581:255–275. doi: 10.1113/jphysiol.2007.128785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe KS, Thomas G, Goddard CA, Colledge WH, Grace AA, Huang CLH. Effects of flecainide and quinidine on arrhythmogenic properties of Scn5a+/Δ murine hearts modelling long QT syndrome 3. J Physiol. 2007b;578:69–84. doi: 10.1113/jphysiol.2006.117945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Kato Y, Adachi M, Agata N, Tanaka H, Shigenobu K. Effects of cyclopiazonic acid on rat myocardium: inhibition of calcium uptake into sarcoplasmic reticulum. J Pharmacol Exp Ther. 1995;272:1095–1100. [PubMed] [Google Scholar]
- Thomas G, Gurung IS, Killeen MJ, Hakim P, Goddard CA, Mahaut-Smith MP, Colledge WH, Grace AA, Huang CL-H. Effects of L-type Ca2+ channel antagonism on ventricular arrhythmogenesis in murine hearts containing a modification in the Scn5a gene modelling human long QT syndrome 3. J Physiol. 2007a;578:85–97. doi: 10.1113/jphysiol.2006.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Killeen MJ, Gurung IS, Hakim P, Balasubramaniam R, Goddard CA, Grace AA, Huang CL-H. Mechanisms of ventricular arrhythmogenesis in mice following targeted disruption of KCNE1 modelling long QT syndrome 5. J Physiol. 2007b;578:99–114. doi: 10.1113/jphysiol.2006.118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggle DJ. Pharmacology of Cav1 (L-type) calcium channels. New York: Kluwer Academic/Plenum Publishing; 2004. [Google Scholar]
- Turner I, Huang CLH, Saumarez RC. Numerical simulation of paced electrogram fractionation: relating clinical observations to changes in fibrosis and action potential duration. J Cardiovasc Electrophysiol. 2005;16:151–161. doi: 10.1046/j.1540-8167.2005.30490.x. [DOI] [PubMed] [Google Scholar]
- Wasserstrom JA, Wasserstrom LA, Lokuta AJ, Kelly JE, Reddy ST, Frank AJ. Activation of cardiac ryanodine receptors by the calcium channel agonist FPL-64176. Am J Physiol Heart Circ Physiol. 2002;283:H331–H338. doi: 10.1152/ajpheart.00788.2001. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Marks AR. Altered function and regulation of cardiac ryanodine receptors in cardiac disease. Trends Biochem Sci. 2003;28:671–678. doi: 10.1016/j.tibs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Wier WG, Kort AA, Stern MD, Lakatta EG, Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci USA. 1983;80:7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, West DJ, Sitsapesan R. Light at the end of the Ca2+-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels. Q Rev Biophys. 2001;34:61–104. doi: 10.1017/s0033583501003675. [DOI] [PubMed] [Google Scholar]
- Yard NJ, Chiesi M, Ball HA. Effect of cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca2+-ATPase, on the frequency-dependence of the contraction-relaxation cycle of the guinea-pig isolated atrium. Br J Pharmacol. 1994;113:1001–1007. doi: 10.1111/j.1476-5381.1994.tb17092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg CE, Buser PT. When calcium turns arrhythmogenic: intracellular calcium handling during the development of hypertrophy and heart failure. Croat Med J. 2001;42:24–32. [PubMed] [Google Scholar]
- Zheng W, Rampe D, Triggle DJ. Pharmacological, radioligand binding, and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol Pharmacol. 1991;40:734–741. [PubMed] [Google Scholar]