Abstract
Although respiratory syncytial virus (RSV) was discovered > 40 years ago, treatment remains largely supportive. There are no safe and effective vaccines or specific treatments other than prophylaxis with passive antibody therapy (palivizumab). However, there are good reasons to think that the scene may soon change. As the pace of development of anti-viral drugs accelerates and optimism over vaccines increases, novel therapies are set to make a major impact in the management of this very common infection. The use and effect of such interventions are not easy to anticipate, but could ultimately include the interruption of RSV's transmission resulting in profound changes to the impact of RSV on human health.
Keywords: antisense RNA, anti-viral drugs, fusion inhibitors, RSV, therapeutic antibodies
1. Background
Respiratory syncytial virus (RSV) is a common cold agent and the chief worldwide viral cause of moderate-to-severe acute upper and lower respiratory tract illness in infancy. Almost all children are infected by 3 years of age [1,2], most suffering only mild symptoms with rhinorrhea, cough, fever and sometimes wheeze generally resolving in < 2 weeks. However, 25 – 40% of children develop lower respiratory signs indicative of a viral bronchiolitis or pneumonia. In most wealthy, temperate regions, ~ 1 in 40 infants is hospitalised because of RSV infection.
In the US, in 2000, it was estimated that there were ~ 86,000 RSV-related hospitalisations in a year, ~ 98% of which occurred in children < 5 years old [3].
A recently published study shows that RSV is associated with 20% of hospitalisations, 18% of emergency department visits and 15% of office visits for acute respiratory infections from November through April. Average annual hospitalisation rates were 17 per 1,000 children < 6 months of age and 3 per 1,000 children < 5 years of age [4]. The direct medical costs for all RSV infection-related hospitalisations for this age group are estimated at $US394 million in 1 year [3]; European figures are probably similar.
The risk of severe RSV infection is increased by premature birth, low birth weight or ‘small for dates’, male gender, large family size, exposure to passive smoke, lack of breast feeding and by birth ~ 4 months before the peak of the RSV season [5]. Pre-existing diseases (trisomy 21, congenital heart disease, pulmonary hypertension or immunodeficiency) and chronic pulmonary disease (especially bronchopulmonary dysplasia) pose extra risk. In countries with well-funded medical services, very few otherwise healthy children die of RSV disease. However, children who suffer from severe RSV infections in infancy are prone to recurrent wheezing and asthma diagnosis later in childhood [6] and prevention of RSV infection in the first year of life might possibly reduce the frequency of postviral wheeze in later life [7].
In elderly persons, RSV causes pneumonia, exacerbations of chronic obstructive pulmonary disease (COPD) [8] and acute deterioration in those with cardiac disease, and contributes substantially to excess deaths in the winter season. In the US, there are an estimated 14,000 – 60,000 RSV-related hospitalisations and 1,500 – 7,000 deaths in people > 65 years owing to RSV infection each year [9,10].
1.1 Animal infection
Many vertebrate species suffer from RSV disease. Bovine RSV is a significant veterinary problem, and both sheep and goats have their own strains of RSV. These do not transmit to or from man.
Mice and cotton rats are the most commonly used small animal models for anti-viral studies. Typically, anti-viral compounds or vaccines are administered before or after intranasal RSV infection with human RSV and the assessment of viral load and lung pathology is performed on day 4 and/or day 8 of infection. A variety of treatment regimes can be tested to define the best dosing option.
The cotton rat model is semi-permissive for infection with human RSV strains, but infection does not induce significant clinical signs. The virus is inoculated intranasally and viral load and pathology is tested in the lungs and upper airways. Infected cotton rats develop histological bronchiolitis and focal pneumonia. Mice require a relatively high dose of human virus to become infected, but do support viral replication in the lung and nose. By careful selection of viral strain and batch, it is possible to induce moderate-to-severe dose-related disease in mice and to reproduce virtually all the features seen after human infection (including pulmonary neutrophilia [11], bronchiolitis, lung function abnormalities and specific immune responses [12]). The fact that mice are amenable to genetic manipulation, the availability of a very wide range of reagents, relative economy and ease of handling make mice the species of first choice in many laboratories.
Other animals used for testing RSV infection include African green and Rhesus monkeys. Chimpanzees are very susceptible to infection with human strains of RSV and may exchange RSV infections from animal handlers. However, ethical concerns and high costs limit the utility of non-human primates for studies of RSV infection.
It is remarkable that all these animal species show broadly parallel augmented disease if they are vaccinated with formalin-inactivated RSV before nasal challenge.
2. Medical need
All attempts to develop safe and effective vaccine for human use have so far been unsuccessful. Formalin-inactivated vaccines tested in the US in 1960s failed to protect children against the disease and, in younger infants, tended to enhance disease during subsequent natural infection [13]. Live attenuated vaccines are being developed for human use, but tend to induce weak immunity or revert to virulence in young children. At present, palivizumab (a humanised mouse monoclonal antibody against the RSV fusion protein) is licensed for preventive use, and ribavirin has been used to treat severe infections despite limited evidence of benefit. There is a clearly and urgent need for both vaccines and effective antiviral drugs, but the needs may be tailored to specific groups (Table 1).
Table 1.
Summary of medical need.
| Infants aged 2 – 8 months during the RSV season |
Passive immunoprophylaxis; possibly vaccines, if immunogenic and safe |
| RSV-infected infants in hospital or at home |
Anti-viral drugs to limit spread and perhaps reduce disease severity |
| Care-givers of high-risk children |
Conventional vaccines |
| Elderly persons | Vaccines and perhaps anti-viral drugs |
RSV: Respiratory syncytial virus.
3. Scientific rationale
Drugs that interfere with virus attachment, fusion or intracellular replication can inhibit infection. Anti-RSV antibodies interfere with the viral lifecycle by binding free virus (neutralising antibody), attachment to host cell (antibodies to membrane-bound and secreted forms of attachment or G protein), virus–cell and cell–cell fusion (anti-RSV F Ab), inhibiting nucleoprotein (anti-RSV N Ab) or possibly by inhibiting the biological function of secreted surface glycoprotein G (anti-RSV G Ab). Two main groups of the virus, RSV-A and RSV-B, are 67% homologous at the level of nucleotides and 53% homologous at the amino acid sequence of the G protein [14,15].
The mechanism of RSV entry to a cell is not fully defined but it is thought that RSV infects by binding of fusion (F) protein to TLR4 receptor and that the G protein can act as a fractalkine receptor agonist mediating immune cell chemotaxis [16]. The G protein is not required for viral entry as recombinant viruses lacking the G protein remain infectious. After binding, the viral wall and cell membranes fuse, an effect mediated by F protein; the nucleocapsid complex then enters into the cytoplasm. The F protein is highly conserved between strains (79% nucleotide and 89% amino acid homology) [15] and anti-F antibody induced by primary RSV infection is cross-reactive between group A and B virus. Antibodies against the G protein are highly group and even subgroup specific [17,18]. The discovery of further receptors for RSV binding and uptake would be an important advance.
Various small molecules may interfere with the process of fusion and several inhibitors of fusion (for instance, benzimidazole drugs such as BMS-433771 or TMC353121) are under development. At a different level, inside a cell, viral RNA can be targeted by small interfering RNAs (siRNAs) that prevent viral protein synthesis [19] or molecules that inhibit function of key enzymes that may also be involved in replication and assembly of other viruses in addition to RSV. A good example is VX-497, an inosine monophosphate dehydrogenase (IMPDH) inhibitor, developed originally and tested in clinical trials for the HBV treatment but with broad anti-viral activity [20].
There is also evidence that RSV may cause persistent infection. Persistence has been demonstrated in guinea pigs [21], cows [22] and mice [23]. Persistent year-round RSV detection in patients with COPD is associated with airway inflammation and accelerated decline in FEV1. Those with chronic RSV infections may, therefore, benefit from anti-viral drugs able to eliminate persistence, and, therefore, alter the natural history of COPD [24]. In addition, drugs able to eliminate sources of RSV outbreaks in the community could potentially contribute to limiting the prevalence of RSV in young children.
RSV infection triggers an intense host immune response that is responsible for many of the clinical features of disease. Most viral infections induce ‘T helper 1’ type responses, characterised by high levels of IFNγ production; by contrast, asthma and atopy are characterised by ‘T helper 2’ cells producing IL-4 and IL-5 (Th2 cells). In animal models, both these patterns of cytokine production are evident depending on the conditions and previous vaccination status [12,25,26]. As it is not possible to access human samples and perform detailed, sequential, timed analysis of the relevant cells and body fluids, the extent to which human infection is mirrored by animal studies is difficult to judge. This issue is highlighted by the finding that infants with severe infections sequester RSV-specific cells in affected tissues, so that relevant cells are depleted from the peripheral blood [27].
Samples from RSV-infected children show elevated IL-4/IFNγ ratios in infants during the first week of acute bronchiolitis compared with infants with upper respiratory tract signs alone [28], consistent with excessive type 2 and/or deficient type 1 immune responses in RSV bronchiolitis [29]. In mice, RSV infection is associated with an increase in gamma-delta T cells that make a range of cytokines, and depletion of these cells greatly attenuates disease [30]. In man, RSV infection has been reported to be associated with reduced IFN-gamma production by gamma-delta T cells, compared to a control group infected with rotavirus [31].
Studies of genetic polymorphisms indicate that innate responses may be especially important in explaining the variation in severity of RSV disease [32]. In mice, macrophages play a key role in the early response to RSV infection [33]. In human cells, they have been reported to upregulate Toll-like receptor 3 and 4 expression [34,35], hence promoting sensitivity to bacterial endotoxin and other TLR4 ligands. The fact that common TLR4 polymorphisms show a significant association with RSV bronchiolitis [36], and that TLR4 expression on cells in the peripheral blood increases during bronchiolitis [37] suggests that this effect may be of considerable pathogenic significance.
IL-8 may also be important as IL-8 haplotype is closely associated with RSV disease-susceptibility [38]. Bont et al. found reduced peripheral lymphocyte function in children with severe disease, associated with raised plasma IL-8 levels [39]. IL-8 is a chemokine that promotes neutrophil chemotaxis and survival, and neutrophils are the predominant cell type present in the bronchial secretions of children with severe bronchiolitis [40], and the amount of IL-8 mRNA in the nasal aspirates of infants with bronchiolitis correlates with severity of disease [41]. CXC chemokines (including IL-8 and IP-10) are also found in large quantities in the lower airways of infants with severe RSV bronchiolitis [40].
IL-9 has been found in high concentrations in the airways of infants with severe bronchiolitis [42]. IL-9 is known to induce mucus production by bronchial epithelial cells, cause goblet cell hyperplasia and induce chemokine secretion by respiratory epithelial cells and neutrophils, suggesting that it may have a critical role in the inflammatory cascade in the airways during acute bronchiolitis.
The close involvement of the host immune response in the pathogenesis of RSV disease suggests that it might be possible to modify disease with anti-inflammatory or immunoinhibitory drugs. However, any attempt to limit the immune response would have to be accompanied by treatment with highly effective antiviral drugs so as to prevent rebound of viral replication. Despite many trials of steroid therapy, there is no clear benefit. Inhibition of other inflammatory pathways should be tested once potent antiviral drugs are available.
4. Existing treatments
Antibodies play several roles in antiviral immunity (interference with pathogen binding, opsonisation, neutralisation and elimination), thus making them attractive therapeutic agents. An initial prophylactic polyclonal RSV hyperimmune globulin (RespiGam, RSV-IGIV, MedImmune) now been superseded by Synagis® (palivizumab, MedImmune, Gaithersburg, MD, US), a humanised monoclonal Ab against RSV F protein [43,44], the only licensed prophylactic drug for RSV at present. It is administered at 15mg/kg/month i.m. during the RSV season (five doses). Palivizumab acts by preventing the spread of virus to the lower respiratory tract, thus preventing the clinical manifestations of bronchiolitis. Clinical studies showed a relative reduction in RSV-related hospital admissions of 55% in premature infants and/or bronchopulmonary dysplasia/chronic lung disease and by 45% in infants with hemodynamically significant congenital heart disease [45]. It has no significant adverse effects and there is no evidence of antibody-mediated disease enhancement [45,46]. Delaying RSV infection beyond the first year of life by prophylaxis with palivizumab may reduce the frequency of recurrent wheeze in later childhood [47]. The high cost of Synagis limits its use in many settings [48], and it is clear that even if it were widely used to prevent infection in all high-risk children, the general incidence of severe disease would be little affected, as many cases occur in otherwise normal children.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3- carboxamide, Virazole®, ICN Pharmaceuticals Ltd, Basingstoke, UK) is a nucleoside analogue and represents the only licensed anti-viral drug for treating RSV infection. It inhibits IMPDH, reducing formation of mRNA and viral polymerase [49]. It is administered in aerosol form to seriously ill infants with severe bronchiolitis and immunocompromised patients but has a limited use owing to variable efficacy and the risk of toxicity [50-53] and high cost [54]. Drug-resistant mutants have been reported, a significant risk with most single anti-viral agents [54-56].
5. Therapeutic class review
5.1 Fusion inhibitors
BMS-433771 inhibits the entry of the virus into cells through direct interaction with the F protein. It works by disruption of the conformation within the fusion hairpin structure, which is absolutely critical for the fusion [57,58]. In vitro, BMS-433771 inhibited both RSV-A and -B replication, and had an ED50 of 20 nM. In mouse and cotton rat models of RSV infection, BMS-433771 5 and 50mg/kg administered p.o., reduced viral lung titres [59,60]. Bristol-Myers Squibb has discontinued further development of this compound [61].
RFI-641 is a specific inhibitor of RSV fusion. It both blocks viral fusion to the cell membrane and prevents syncytium formation by binding to protein F [62]. In murine models of RSV infection, RFI-641 administered nasally 2 h before infection reduced viral titres in the lung by 0.63 – 1.53 log [63] and in African green monkeys, in nasal and throat samples by 2.1 and 3.1 log, respectively. In the same model, it was also effective 24 h post-infection [64]. It was tested in Phase I and II clinical trials in 2000 – 2001 [49] but Wyeth has discontinued development of RFI-641 [61].
VP-14637 is a small molecule that inhibits RSV entry into host cells designed for inhalation that could have been delivered directly to the infection site. The technology was suited for use in infants and was under development by ViroPharma. VP-14637 bound specifically to RSV-infected cells and interacted with the RSV F protein [65]. The drug was tested in an inhaled formulation in Phase I trials to evaluate the safety and pharmacokinetic profile. However, ViroPharma has discontinued development of this compound for strategic reasons [61].
JNJ-2408068 is a benzimidazole derivative [66] and has a very potent anti-RSV activity. It inhibits both virus–cell fusion and cell–cell fusion [67] of human RSV-A and -B as well as bovine RSV but it does not work against other Paramyxoviridae. In vitro cytotoxicity and antiviral effects are seen in the cotton rats, where selective anti-viral activity is evident. Significant drug levels occur in the lung and a concentration of 10 nM reduces RSV load 1,000-fold. Outside the lung, only low levels of JNJ-2408068 are found [68]. In addition, it reduces release of proinflammatory cytokines IL-6, IL-8 and RANTES from RSV-infected A549 cells [67]. TMC353121, the new morpholinopropylaminobenzimidazole derivative of JNJ-2408068 [69], has recently been synthesised as a result of molecular modelling in a lead optimisation program. A process that led to the selection of TMC353121 has been described in detail by Bonfanti et al. [69]. TMC353121 is under active preclinical evaluation by Tibotec.
5.2 Attachment inhibitor
MBX-300 (Microbiotix, Worcester, MA, US) is the compound that targets the attachment protein [70] designed for the oral treatment of RSV. It underwent in vivo efficacy studies and toxicology trials including testing in Cynomolgus monkeys and showed anti-RSV activity and a good safety profile [70,71].
5.3 IMPDH inhibitors
EICAR is a nucleoside analogue with anti-viral properties. In vitro, it inhibits replication of paramyxoviruses (parainfluenza, mumps, measles and RSV) and orthomyxovirus (influenza) with EC50s of 0.06 – 2.3 μg/ml. In HeLa, Vero, MDCK and LLCMK2 cells, even doses of 200 μg/ml are non-cytotoxic. In replicating cells, 5.6 – 12 μg/ml inhibits growth. It works by inhibiting IMPDH, leading to depletion of intracellular GTP [72]. Asahi Kasei Pharma has discontinued development of EICAR [61].
5.4 Anti-RSV morpholino oligomers
Lai et al. [73] report the effects of two antisense phosphorodiamidate morpholino oligomers (PMOs) targeting the sequence of RSV L mRNA, coupled to arginine-rich cell-penetrating peptide. Phosphorodiamidate morpholino oligomers have the same nucleobases as DNA, but a morpholine ring replaces the deoxyribose sugar and a phosphorodiamidate linkage replaces the phosphodiester [74]. Phosphorodiamidate morpholino oligomers bind the complementary RNA sequence and, therefore, have an antisense effect through steric blockage of RNA [75]. Both in vitro and in vivo, the constructs can easily enter cells where they interfere with viral protein expression. Minimal cytotoxicity was observed in human cell lines and reduction of viral titres was significant (> 2.0 log10). Intranasal treatment of BALB/c mice with peptide-bound PMO before the RSV inoculation produced a reduction in viral titre of 1.2 log10 in lung tissue at day 5 post-infection, and attenuated pulmonary inflammation at day 7 post-infection.
Collectively, these data indicate that PMOs possess potent anti-RSV activity. This strategy is worthy of further investigation as a candidate for potential therapeutic application.
6. Competitive environment
Several promising anti-RSV compounds described earlier have been at various stages of development (Table 2); however, many of the projects have been discontinued and at the moment only two are in Phase II clinical trials: ALN-RSV01 and RSV-604.
Table 2.
Examples of anti-RSV drugs.
| Compound | Company | Structure | Indication | Stage of development |
Mechanism of action |
|
|---|---|---|---|---|---|---|
| MBX-300 | Microbiotix (USA) |
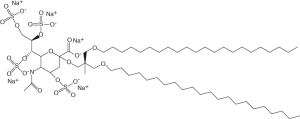
|
Infection | Discontinued | G protein inhibitor |
|
| VP-14637 | ViroPharma (USA) |
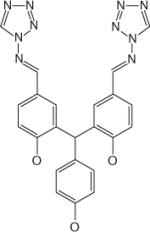
|
Infection | Discontinued | F protein inhibitor |
|
| RSV-604 | Arrow Therapeutics (Novartis, UK) |
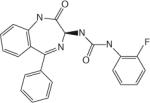
|
Phase II clinical trial |
N protein inhibitor |
||
| RFI-641 | Wyeth (USA) |
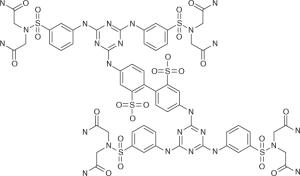
|
Infection, prophylaxis prophylaxis |
Discontinued | F protein inhibitor |
|
| LY-2 53963 | Eli Lilly (USA) |
|
Infection | Discontinued | Unspecified | |
| EICAR | Asahi Kasei Pharma |
|
Infection | Discontinued | L-polymerase inhibitor | |
| BMS-433771 | Bristol-Myers Squibb |
|
Infection | Discontinued | F protein inhibitor |
|
| ALN-RSV01 | Alnylam (USA) |
RNA | Infection, prophylaxis |
Phase II clinical trial |
Gene expression inhibitor |
|
RSV: Respiratory syncytial virus
6.1 Antisense anti-RSV agents: ALN-RSV01
RNA interference (RNAi) is a natural process that occurs in cytoplasm and leads to degradation of mRNA. Small interfering RNAs are synthesised to target any endogenous mRNA sequence in a cell or an exogenous sequence carried by a virus. While in a cell, siRNAs incorporate into a cytoplasmic protein complex, the RNA induced silencing complex (RISC). The antisense strand of the siRNA binds in a sequence-specific manner to existing mRNAs in the cytoplasm, which leads to cleavage and subsequent release of the inactivated fragments of the complementary mRNA. This process interrupts the synthesis of the corresponding protein [19,76,77].
There have been several recent reports describing a number of antiviral siRNAs tested in vitro and in vivo and where viral infections or cancers have been targeted by antisense-mediated approaches [78-80]. ALN-RSV01 (Alnylam Pharmaceuticals) is the first siRNA targeting a microbial pathogen tested in humans and the first siRNA administered to the human respiratory tract. ALN-RSV01 is designed to inhibit the replication of RSV by interrupting the synthesis of the viral nucleocapsid protein (N-protein).
Studies in mice showed that ALN-RSV01 (2 mg/kg, single dose) protected against subsequent RSV infection, and could also be used to treat an existing RSV infection, thereby decreasing viral load > 10,000-fold [81]. The results of the first two placebo-controlled healthy volunteer studies have just been released [77], demonstrating the drug is safe over a wide range after nasal delivery. Further multinational, randomised, double-blind, placebo-controlled, multidose Phase II trials with an inhaled nebuliser formulation (the expected final route of administration) are continuing. These studies may provide validation of the general strategy of siRNA delivery to the respiratory tract, regardless of the utility of this approach to treatment of RSV disease.
Antisense strategies, therefore, show great promise, but may require further improvement before they can be used clinically. Reduced cost, increased in vivo stability, increased expression levels and delivery to relevant cell populations may all represent significant hurdles for the development of this technology.
6.2 N protein inhibitors: RSV-604
RSV-604 is an oral benzodiazepine under development by Arrow Therapeutics (Novartis) [82]. Found by screening libraries of small molecules, it inhibits viral replication and the inhibitory activity is in the submicromolar range for both RSV-A and RSV-B [60]. RSV-604 represents the first in a new class of RSV inhibitors and has potential as a treatment for RSV infection. Promising preclinical data have enabled the study of the drug in Phase I trials, where volunteers have been exposed to increasing quantities of it. These studies showed that the drug was well absorbed in humans, and that one dose a day was sufficient to achieve antiviral EC90 levels [83]. It is possible that similar molecules with advantageous pharmacokinetics will be found by molecular modification.
7. Current research goals
Although generally successful, palivizumab does not always produce perfect protection and does not adequately protect from upper airway infection. To improve on palivizumab, Medimmune had developed antibodies with better binding, among them motavizumab (MEDI-524, formerly known as Numax®, Gaithersburg, MD, USA). This has 70-fold greater binding affinity to RSV F protein in vitro and a 20-fold improvement in neutralisation [44]. It not only inhibits RSV replication in lower respiratory tract, but also inhibits nasal replication. In cotton rats, it causes 100-fold greater reduction in pulmonary RSV titres compared with palivizumab [84]; in the mouse, prophylactic administration of motavizumab significantly reduces RSV replication and reduces local and systemic levels of TNF-α, IL-1α, IL-12p70, KC, IL-10 and IFNγ [85].
Motavizumab has now been tested in Phase III clinical trials in 6,635 pre-term infants at high risk of RSV infection. Its use was associated with significantly fewer hospitalisations compared with palivizumab [86]. MedImmune recently submitted a Biologics Licence Application but the licensing process has been delayed [87].
In addition to several promising small molecule antivirals, the exciting reports of the remarkable effects of anti-viral siRNA seems likely to lead to improvements in treatments for RSV infection. However, RSV infection may be difficult to control by a single drug or monoclonal. More likely, combined therapy may be required for improved therapeutic efficacy. For example, the synergistic action of several antibodies (e.g., the approach being taken by Symphogen, [88]), possibly together with an antifusion drug or compound in combination with IMPDH inhibitor, may be required to increase treatment efficacy and at the same time reduce the risk of emergence of resistant viral mutants.
8. Potential development issues
The very considerable and rising costs of developing effective anti-viral drugs for common cold agents need to be viewed in the context of the normally non-life-threatening nature of such infections. Absolute safety is paramount and is a particular concern in young children; failure to meet stringent conditions set by licensing bodies or relatively minor commercial setbacks may halt further development or lead to product withdrawal.
The convenience and practicality of different routes of drug delivery and the importance and difficulty of early diagnosis are also potential limiting issues. Specifically, it seems essential to intervene very early during infection if an anti-viral drug is to be effective. With anti-influenza drugs, it seems that treatment needs to be given in the first 48 h of illness to be of value. With RSV infections, it is possible that early treatment is also essential, and it may even be optimal to start treatment even before infection becomes clinically evident to modify disease. If this is the case, the use of anti-viral drugs would be of very limited value. The effect of delaying treatment on efficacy needs very careful study, and the results could have a major impact on the usefulness of therapy.
Approaches to treatment may need to be different in various target groups. For example, long-lived passive antibody therapy may be optimal for neonates in whom vaccines may be ineffective or dangerous and small molecule therapy impractical or potentially hazardous. Small molecule anti-viral drugs may have a role in adults, particularly those with immunosuppression, persistent infection or at risk of transmitting infection to susceptible individuals. An effective vaccine could interrupt community transmission, particularly if administered to toddlers, older children, parents and care-givers.
The fact that the epidemiology of RSV suggests community transmission can be interrupted by variations in the weather and in social interaction and that RSV strains are specific to narrow host range raises the exciting possibility that the combination of effective vaccination and anti-viral therapy targeted at community sources of winter outbreaks might conceivably lead to global elimination of RSV infection in man.
Although a distant hope, unachievable with vaccines envisaged at present and anti-viral drugs, the prospect of eventual elimination of RSV from the human population is an inspiring goal.
9. Expert opinion
Despite many attempts to develop vaccines and anti-viral drugs for RSV infection, treatment remains essentially supportive at present. The remarkable success of palivizumab has reinvigorated the field, demonstrating the potential of antibody alone to protect against RSV infection, the great medical need and the considerable commercial opportunities in this area. Although many anti-viral drugs fail during development, there are promising drugs in the pipeline. It is to be hoped that one or more will be in Phase III in the near future, establishing clinical efficacy and market niche that small molecules will occupy. Effective and safe vaccines are keenly awaited and cheaper, longer-lasting and highly effective antibody products are likely to continue to have a role in preventing disease, especially in infancy. With this range of new and effective therapies, the considerable impact of RSV infections on human health may be greatly reduced in the foreseeable future.
Acknowledgments
Declaration of interest
This paper has been supported by the Wellcome Trust and the Centre for Respiratory Infection. W Olszewska has received grant support from Tibotec Pharmaceuticals. P Openshaw has received grant support from Tibotec Pharmaceuticals and is a consultant for Kenta Biotech and Symphogen.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest •• to readers.
- 1.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368(9532):312–22. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 3.Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. 2004;22(5):275–84. doi: 10.2165/00019053-200422050-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 7.Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151(1):34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Falsey AR, Formica MA, Hennessey PA, et al. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(6):639–43. doi: 10.1164/rccm.200510-1681OC. [•This article highlights the emerging realisation that RSV infection is an important disease affecting adults and not just a paediatric problem.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–84. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179(1):25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 11.Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83(3):1492–500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Openshaw PJ, Tregoning JS. Immuneresponses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18(3):541–55. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [•This is one of several early landmark reports of vaccine-augmentation of disease.] [DOI] [PubMed] [Google Scholar]
- 14.Sullender WM, Mufson MA, Anderson LJ, Wertz GW. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J Virol. 1991;65:5425–34. doi: 10.1128/jvi.65.10.5425-5434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–9. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripp RA, Jones LP, Haynes LM, et al. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immun. 2001;2(8):732–8. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 17.Cane PA, Thomas HM, Simpson AF, et al. Analysis of the human serological immune response to a variable region of the attachment (G) protein of respiratory syncytial virus during primary infection. J Med Virol. 1996;48(3):253–61. doi: 10.1002/(SICI)1096-9071(199603)48:3<253::AID-JMV7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Hendry RM, Burns JC, Walsh EE, et al. Strain-specific serum antibody responses in infants undergoing primary infection with respiratory syncytial virus. J Infect Dis. 1988;157:640–7. doi: 10.1093/infdis/157.4.640. [DOI] [PubMed] [Google Scholar]
- 19.Tuschl T, Zamore PD, Lehmann R, et al. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999;13(24):3191–7. doi: 10.1101/gad.13.24.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markland W, McQuaid TJ, Jain J, Kwong AD. Broad-spectrum antiviral activity of the IMP dehydrogenase inhibitor VX-497: a comparison with ribavirin and demonstration of antiviral additivity with alpha interferon. Antimicrob Agents Chemother. 2000;44(4):859–66. doi: 10.1128/aac.44.4.859-866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegele RG, Hayashi S, Bramley AM, Hogg JC. Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest. 1994;105:1848–54. doi: 10.1378/chest.105.6.1848. [DOI] [PubMed] [Google Scholar]
- 22.Valarcher JF, Bourhy H, Lavenu A, et al. Persistent infection of B lymphocytes by bovine respiratory syncytial virus. Virology. 2001;291(1):55–67. doi: 10.1006/viro.2001.1083. [DOI] [PubMed] [Google Scholar]
- 23.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJ. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169(7):801–5. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson TM, Donaldson GC, Johnston SL, et al. Respiratory syncytial virus, airway inflammation, and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):871–6. doi: 10.1164/rccm.200509-1489OC. [DOI] [PubMed] [Google Scholar]
- 25.Culley FJ, Pennycook AM, Tregoning JS, et al. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J Virol. 2006;80(9):4521–7. doi: 10.1128/JVI.80.9.4521-4527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moghaddam A, Olszewska W, Wang B, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–7. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 27.Pitrez PM, Ponzi D, Machado DC, et al. Discrepancy between cytokine production from peripheral blood mononuclear cells and nasal secretions among infants with acute bronchiolitis. Ann Allergy Asthma Immunol. 2004;92(6):659–62. doi: 10.1016/S1081-1206(10)61433-0. [DOI] [PubMed] [Google Scholar]
- 28.Schauer U, Hoffjan S, Rothoeft T, et al. Severe respiratory syncytial virus infections and reduced interferon-gamma generation in vitro. Clin Exp Immunol. 2004;138(1):102–9. doi: 10.1111/j.1365-2249.2004.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legg JP, Hussain IR, Warner JA, et al. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168(6):633–9. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 30.Dodd J, Riffault S, Kodituwakku JS, et al. Pulmonary V gamma 4+ gamma delta T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182(2):1174–81. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyagi M, Shimojo N, Sekine K, et al. Respiratory syncytial virus infection suppresses IFN-gamma production of gammadelta T cells. Clin Exp Immunol. 2003;131(2):312–7. doi: 10.1046/j.1365-2249.2003.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen R, Bont L, Siezen CL, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196(6):826–34. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 33.Pribul PK, Harker J, Wang B, et al. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J Virol. 2008;82(9):4441–8. doi: 10.1128/JVI.02541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groskreutz DJ, Monick MM, Powers LS, et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176(3):1733–40. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 35.Monick MM, Yarovinsky TO, Powers LS, et al. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J Biol Chem. 2003;278(52):53035–44. doi: 10.1074/jbc.M308093200. [DOI] [PubMed] [Google Scholar]
- 36.Tal G, Mandelberg A, Dalal I, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189(11):2057–63. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 37.Gagro A, Tominac M, Krsulovic-Hresic V, et al. Increased Toll-like receptor 4 expression in infants with respiratory syncytial virus bronchiolitis. Clin Exp Immunol. 2004;135(2):267–72. doi: 10.1111/j.1365-2249.2004.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hacking D, Knight JC, Rockett K, et al. Increased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibility. Genes Immun. 2004;5(4):274–82. doi: 10.1038/sj.gene.6364067. [DOI] [PubMed] [Google Scholar]
- 39.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14(1):144–9. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 40.McNamara PS, Flanagan BF, Hart CA, Smyth RL. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2005;191(8):1225–32. doi: 10.1086/428855. [DOI] [PubMed] [Google Scholar]
- 41.Smyth RL, Mobbs KJ, O'Hea U, et al. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr Pulmonol. 2002;33(5):339–46. doi: 10.1002/ppul.10080. [DOI] [PubMed] [Google Scholar]
- 42.McNamara PS, Flanagan BF, Baldwin LM, et al. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet. 2004;363(9414):1031–7. doi: 10.1016/S0140-6736(04)15838-8. [DOI] [PubMed] [Google Scholar]
- 43.Wu H, Pfarr DS, Tang Y, et al. Ultra-potent antibodies against respiratory syncytial virus: effects of binding kinetics and binding valence on viral neutralization. J Mol Biol. 2005;350(1):126–44. doi: 10.1016/j.jmb.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol. 2008;317:103–23. doi: 10.1007/978-3-540-72146-8_4. [••This article reviews the current and future status of antibody therapy for RSV disease.] [DOI] [PubMed] [Google Scholar]
- 45.Fenton C, Scott LJ, Plosker GL. Palivizumab: a review of its use as prophylaxis for serious respiratory syncytial virus infection. Paediatr Drugs. 2004;6(3):177–97. doi: 10.2165/00148581-200406030-00004. [DOI] [PubMed] [Google Scholar]
- 46.Null D, Jr, Pollara B, Dennehy PH, et al. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr Infect Dis J. 2005;24(11):1021–3. doi: 10.1097/01.inf.0000183938.33484.bd. [DOI] [PubMed] [Google Scholar]
- 47.Simoes EA, King SJ, Lehr MV, Groothuis JR. Preterm twins and triplets. A high-risk group for severe respiratory syncytial virus infection. Am J Dis Child. 1993;147:303–6. doi: 10.1001/archpedi.1993.02160270065020. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Cummins C, Bayliss S, et al. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008;12(36):iii, ix–86. doi: 10.3310/hta12360. [DOI] [PubMed] [Google Scholar]
- 49.Sidwell RW, Barnard DL. Respiratory syncytial virus infections: recent prospects for control. Antiviral Res. 2006;71(2-3):379–90. doi: 10.1016/j.antiviral.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann SH, Wade MJ, Staffa JA, et al. Dominant lethal study of ribavirin in male rats. Mutat Res. 1987;188(1):29–34. doi: 10.1016/0165-1218(87)90111-x. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann SH, Staffa JA, Smith RA, et al. Inhalation toxicity of ribavirin in suckling ferrets. J Appl Toxicol. 1987;7(5):343–51. doi: 10.1002/jat.2550070509. [DOI] [PubMed] [Google Scholar]
- 52.Krilov LR, Mandel FS, Barone SR, Fagin JC. Follow-up of children with respiratory syncytial virus bronchiolitis in 1986 and 1987: potential effect of ribavirin on long term pulmonary function. The Bronchiolitis Study Group. Pediatr Infect Dis J. 1997;16(3):273–6. doi: 10.1097/00006454-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Krilov LR. Safety issues related to the administration of ribavirin. Pediatr Infect Dis J. 2002;21(5):479–81. doi: 10.1097/00006454-200205000-00037. [DOI] [PubMed] [Google Scholar]
- 54.Marquardt ED. Cost of ribavirin therapy for respiratory syncytial virus infection. J Pediatr. 1995;126(5 Pt 1):847. doi: 10.1016/s0022-3476(95)70434-5. [DOI] [PubMed] [Google Scholar]
- 55.Crotty S, Andino R. Implications of high RNA virus mutation rates: lethal mutagenesis and the antiviral drug ribavirin. Microbes Infect. 2002;4(13):1301–7. doi: 10.1016/s1286-4579(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 56.Crotty S, Cameron C, Andino R. Ribavirin's antiviral mechanism of action: lethal mutagenesis? J Mol Med. 2002;80(2):86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- 57.Cianci C, Meanwell N, Krystal M. Antiviral activity and molecular mechanism of an orally active respiratory syncytial virus fusion inhibitor. J Antimicrob Chemother. 2005;55(3):289–92. doi: 10.1093/jac/dkh558. [DOI] [PubMed] [Google Scholar]
- 58.Cianci C, Langley DR, Dischino DD, et al. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc Natl Acad Sci USA. 2004;101(42):15046–51. doi: 10.1073/pnas.0406696101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cianci C, Genovesi EV, Lamb L, et al. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob Agents Chemother. 2004;48(7):2448–54. doi: 10.1128/AAC.48.7.2448-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cianci C, Yu KL, Combrink K, et al. Orally active fusion inhibitor of respiratory syncytial virus. Antimicrob Agents Chemother. 2004;48(2):413–22. doi: 10.1128/AAC.48.2.413-422.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Informa UK Ltd. Pharmaprojects -Pharmaceutical Research & Development Pipeline Intelligence. 2008 [Google Scholar]
- 62.Razinkov V, Huntley C, Ellestad G, Krishnamurthy G. RSV entry inhibitors block F-protein mediated fusion with model membranes. Antiviral Res. 2002;55(1):189–200. doi: 10.1016/s0166-3542(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 63.Huntley CC, Weiss WJ, Gazumyan A, et al. RFI-641, a potent respiratory syncytial virus inhibitor. Antimicrob Agents Chemother. 2002;46(3):841–7. doi: 10.1128/AAC.46.3.841-847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss WJ, Murphy T, Lynch ME, et al. Inhalation efficacy of RFI-641 in an African green monkey model of RSV infection. J Med Primatol. 2003;32(2):82–8. doi: 10.1034/j.1600-0684.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 65.Douglas JL, Panis ML, Ho E, et al. Small molecules VP-14637 and JNJ-2408068 inhibit respiratory syncytial virus fusion by similar mechanisms. Antimicrob Agents Chemother. 2005;49(6):2460–6. doi: 10.1128/AAC.49.6.2460-2466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonfanti JF, Doublet F, Fortin J, et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate, part 1: improving the pharmacokinetic profile using the structure-property relationship. J Med Chem. 2007;50(19):4572–84. doi: 10.1021/jm070143x. [DOI] [PubMed] [Google Scholar]
- 67.Andries K, Moeremans M, Gevers T, et al. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antiviral Res. 2003;60(3):209–19. doi: 10.1016/j.antiviral.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Wyde PR, Chetty SN, Timmerman P, et al. Short duration aerosols of JNJ 2408068 (R170591) administered prophylactically or therapeutically protect cotton rats from experimental respiratory syncytial virus infection. Antiviral Res. 2003;60(3):221–31. doi: 10.1016/j.antiviral.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Bonfanti JF, Meyer C, Doublet F, et al. Selection of a respiratory syncytial virus fusion inhibitor clinical candidate. 2. Discovery of a morpholinopropylaminobenzimidazole derivative (TMC353121) J Med Chem. 2008;51(4):875–96. doi: 10.1021/jm701284j. [•This article describes the discovery process that lead to the most promising small molecule inhibitor under current development.] [DOI] [PubMed] [Google Scholar]
- 70.Kimura K, Mori S, Tomita K, et al. Antiviral activity of NMSO3 against respiratory syncytial virus infection in vitro and in vivo. Antiviral Res. 2000;47(1):41–51. doi: 10.1016/s0166-3542(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 71.Douglas JL. In search of a small-molecule inhibitor for respiratory syncytial virus. Expert Rev Anti Infect Ther. 2004;2(4):625–39. doi: 10.1586/14787210.2.4.625. [DOI] [PubMed] [Google Scholar]
- 72.Shigeta S, Mori S, Baba M, et al. Antiviral activities of ribavirin, 5-ethynyl-1-beta-D-ribofuranosylimidazole-4-carboxamide, and 6′-(R)-6′-C-methylneplanocin A against several ortho- and paramyxoviruses. Antimicrob Agents Chemother. 1992;36(2):435–9. doi: 10.1128/aac.36.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lai SH, Stein DA, Guerrero-Plata A, et al. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol Ther. 2008;16(6):1120–8. doi: 10.1038/mt.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7(3):187–95. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 75.Stein D, Foster E, Huang SB, et al. A specificity comparison of four antisense types: morpholino, 2′-O-methyl RNA, DNA, and phosphorothioate DNA. Antisense Nucleic Acid Drug Dev. 1997;7(3):151–7. doi: 10.1089/oli.1.1997.7.151. [DOI] [PubMed] [Google Scholar]
- 76.Elbashir SM, Martinez J, Patkaniowska A, et al. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20(23):6877–88. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeVincenzo J, Cehelsky JE, Alvarez R, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77(3):225–31. doi: 10.1016/j.antiviral.2007.11.009. [••The use of mucosally delivered RNAi therapy against RSV is an important proof of concept for this technology that may have wide application.] [DOI] [PubMed] [Google Scholar]
- 78.Capodici J, Kariko K, Weissman D. Inhibition of HIV-1 infection by small interfering RNA-mediated RNA interference. J Immunol. 2002;169(9):5196–201. doi: 10.4049/jimmunol.169.9.5196. [DOI] [PubMed] [Google Scholar]
- 79.Xiang TX, Li Y, Jiang Z, et al. RNA interference-mediated silencing of the Hsp70 gene inhibits human gastric cancer cell growth and induces apoptosis in vitro and in vivo. Tumori. 2008;94(4):539–50. doi: 10.1177/030089160809400416. [DOI] [PubMed] [Google Scholar]
- 80.Hamasaki K, Nakao K, Matsumoto K, et al. Short interfering RNA-directed inhibition of hepatitis B virus replication. FEBS Lett. 2003;543(1-3):51–4. doi: 10.1016/s0014-5793(03)00400-9. [DOI] [PubMed] [Google Scholar]
- 81.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11(1):50–5. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 82.Henderson EA, Alber DG, Baxter RC, et al. 1,4-benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J Med Chem. 2007;50(7):1685–92. doi: 10.1021/jm060747l. [DOI] [PubMed] [Google Scholar]
- 83.Chapman J, Abbott E, Alber DG, et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother. 2007;51(9):3346–53. doi: 10.1128/AAC.00211-07. [•This describes a possible small molecule inhibitor showing some potential.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu H, Pfarr DS, Johnson S, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652–65. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 85.Mejias A, Chavez-Bueno S, Raynor MB, et al. Motavizumab, a neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibody significantly modifies the local and systemic cytokine responses induced by RSV in the mouse model. Virol J. 2007;4:109. doi: 10.1186/1743-422X-4-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madaan A. Motavizumab: Humanized anti-RSV monoclonal antibody, prevention of RSV infection. Drugs Future. 2008;33(3):203–5. [Google Scholar]
- 87. Available from: http://www.reuters.com/article/euIpoNews/idUSLS22855520081128, [Accessed 2 April 2009]
- 88. Available from: http://www.symphogen.com/web/guest/sym003, [Accessed 20 February 2009]


