Abstract
Bdellovibrios are Gram-negative bacteria that are characterized by predatory behavior. Although Bdellovibrios exhibit an obligatory parasitic life cycle, it is possible to isolate Bdellovibrio variants that no longer require host cells for their growth. In this study, a new method for isolating Bdellovibrio bacteriovorus host-independent (HI) variants was developed. Filtered B. bacteriovorus prey cells were cultured with E. coli diaminopimelic acid (DAP) auxotrophs as host cells. Thereafter, the lysate was plated on DAP minus media, allowing only HI colonies to develop. Using this method, we have isolated numerous HI variants and demonstrated that the emergence of HI variants may be occurring at a higher frequency than was previously suggested.
Keywords: Bdellovibrio bacteriovorus, host-independent, isolation.
INTRODUCTION
Bdellovibrios are Gram-negative, motile and uniflagellated bacteria, which are characterized by predatory behavior or an obligatory parasitic life cycle. Bdellovibrios attack other Gram-negative bacteria, penetrate into their periplasm, multiply in the periplasmic space, and finally burst the cell envelope to start the cycle anew [1]. Bdellovibrios are largely found in wet, aerobic environments and were first isolated from soil, where they are commonly encountered [1].
Although Bdellovibrios exhibit an obligatory parasitic life cycle, it is possible to isolate Bdellovibrio variants that no longer require host cells for growth [2-5]. These host-independent (HI) or prey-independent variants complete the transition from attack phase to growth phase and back again on standard complex bacteriological media. Furthermore, these variants retain their ability to grow on prey and are termed “facultative”. This “facultative” predatory characteristic render HI variants more amenable to genetic manipulation than the host-dependent WT, thus making them a valuable tool in the study of Bdellovibrio biology [5-7]. The major difficulty in isolating HI variants is the need to select against the remaining host cells. To this end, a method was developed in which streptomycin resistant B. bacteriovorus were first isolated, followed by a second cultivation step on streptomycin susceptible host, and plating the lysate on streptomycin-containing medium [8]. This method, although reliable, required a relatively long incubation periods as well as manipulation of the predator in order to produce streptomycin resistant Bdellovibrio. Recently an isolation protocol was described in which HI variants are isolated from the lysate by filtration and growth on membranes, which are placed on rich media [9].
Here, we describe a rapid new method for isolating HI B. bacteriovorus directly from standard lysates that does not require filtration or initial isolation of antibiotic resistant Bdellovibrio cells.
MATERIALS AND METHODS
Bacterial Strains, Media and Culture Conditions:
B. bacteriovorus strain 109J ATCC 43826, Klebsiella pneumoniae ATCC 13883, E. coli strain S17-1 and E. coli strain WM3064, a diaminopimelic acid auxotroph, derivative of strain β2155 [10, 11] were used in this study. E. coli were grown routinely in LB media at 37˚C. Strain WM3064 media was supplemented with 0.3 mM diaminopimelic acid (DAP). HI variants were grown in peptone-yeast extract (PYE) amended with 3 mM MgCl2 and 2 mM CaCl2 at 30˚C. Cells were enumerated as colony-forming units (CFU) on agar plates. B. bacteriovorus was maintained as plaques in double-layered diluted nutrient broth (DNB) (a 1:50 dilution of nutrient broth amended with 3 mM MgCl2 and 2 mM CaCl2 [pH 7.2]) agar (0.6% agar in the top layer). B. bacteriovorus was counted as plaque forming units (PFU) using the double-layered agar method, previously described by Shilo and Bruff [12]. Standard B. bacteriovorus induced lysates were obtained by adding a plug of agar containing B. bacteriovorus plaque (about 1x106 PFU/ml) to 1x108 CFU/ml washed E. coli host cells and incubated at 30˚C, to reach a final concentration of 1x108 PFU/ml predator. To harvest B. bacteriovorus, the 18 hr lysate was passed through three separate 0.45 µm pore-size filters to remove residual prey and cell debris.
Predation Experiments:
Predation on Planktonic Cells:
Wild-type B. bacteriovorus or HI variants were grown in a standard induced lysate obtained by adding 0.5 ml of predator (1x107 PFU/ml of filtered wild-type Bdellovibrio lysate or 1x107 CFU/ml HI variant) to 5 ml (1x108 CFU/ml) of washed E. coli host cells, incubated at 30˚C in DNB on a rotary shaker. The reduction and efficiency of predation was evaluated by plating the remaining host cells on LB agar plates. Each liquid lysate test was carried out three times in triplicates.
Plaque Predation Assays:
The ability of the predator to form a lytic halo, on a relatively thin lawn of surface attached prey cells, was determined using a modification of the double-layered plaque assay [5]. K. pneumoniae was grown for 18 hr in LB. One-hundred μl of 10 times concentrated washed cells were spread on DNB media solidified with 1.5% agar. HI variants were grown as described above, centrifuged and resuspended in DNB. Twenty microliters of the predator was spotted on a lawn of host bacteria. Lytic halo assay plates were incubated at 30˚C and examined for the formation of a zone of clearing where the predator was spotted. K. pneumoniae host cells were used in this assay due to the ability of the predator to form rapid (24 hr) lytic halos, which could be easily visualized. Each plaque assay was performed at least three times with filtered B. bacteriovorus wild-type lysate or DNB serving as positive and negative controls.
RESULTS
Predation of B. bacteriovorus on E. coli Auxotrophs:
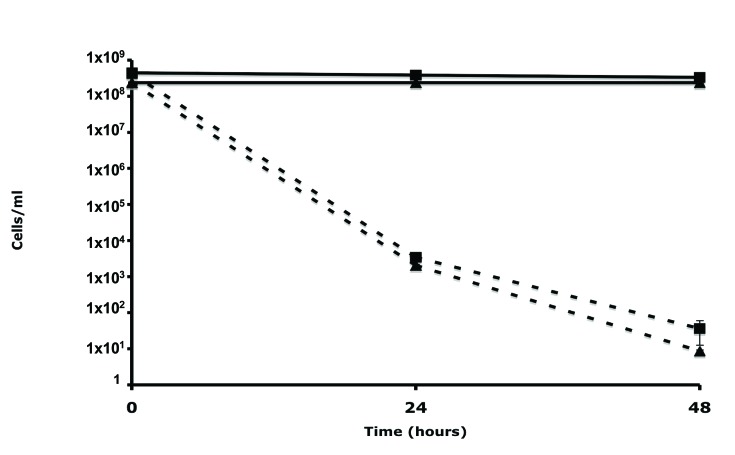
In this study we were interested in isolating Bdellovibrio HI variants using an E. coli DAP auxotroph, which could be easily selected against simply by growth on medium devoid of exogenous DAP. To confirm that B. bacteriovorus has the ability to prey on E. coli DAP auxotrophs with similar efficiency to wild-type E. coli host cells, predation experiments were conducted. E. coli strain S17-1 and WM3064 were grown for 18 hr in LB or LB+DAP respectively. The E. coli host cells were harvested by centrifugation and cultivated with filtered B. bacteriovorus 109J in DNB-DAP free media. As seen in Fig. (1), B. bacteriovorus had the ability to reduce CFU of both strains to the same extent. No reduction in CFU counts were observed in the control samples, verifying that the E. coli auxotroph can survive without DAP for extended periods of time. Furthermore, no evident difference in predation was seen when examining the two lysates by light microscopy. This preliminary experiment demonstrated that E. coli WM3064 could be utilized as prey by B. bacteriovorus using standard methods. The data provided here is in agreement with a previous study, in which E. coli WM3064 was used as a host for B. bacteriovorus [4].
Fig. (1).
Predation of E. coli WM3064 and S17-1 host cells by B. bacteriovorus. E. coli strain WM3064 (square) or S17-1 (triangle) were exposed to Wild-Type B. bacteriovorus (dotted line) or DNB control (full line). Each value represents the mean of 3 experiments. Error bars indicate standard errors.
Isolating B. bacteriovorus HI variants using E. coli Auxotrophs:
E. coli strain WM3064 was grown for 18 hr in LB+DAP media. The E. coli host cells were harvested by centrifugation, washed, and cultivated with filtered host-free B. bacteriovorus for 24 hr in DNB. Thereafter, 100 μl of serial diluted overnight lysate were spread on peptone-yeast extract (PYE) media without DAP, and incubated at 30˚C until HI colonies emerged. This method was used 19 times. In each experiment, numerous (>20 in the fourth dilution) HI yellow colonies developed. The HI colonies varied in size and pigmentation, which is characteristic of HI isolates [2]. No E. coli colonies developed on PYE-DAP minus plates. Plating a high concentration of DAP auxotroph cells directly on the PYE-DAP minus plates, also did not result in E. coli colony growth, validating that the WM3064 strain is unable to grow on media lacking DAP.
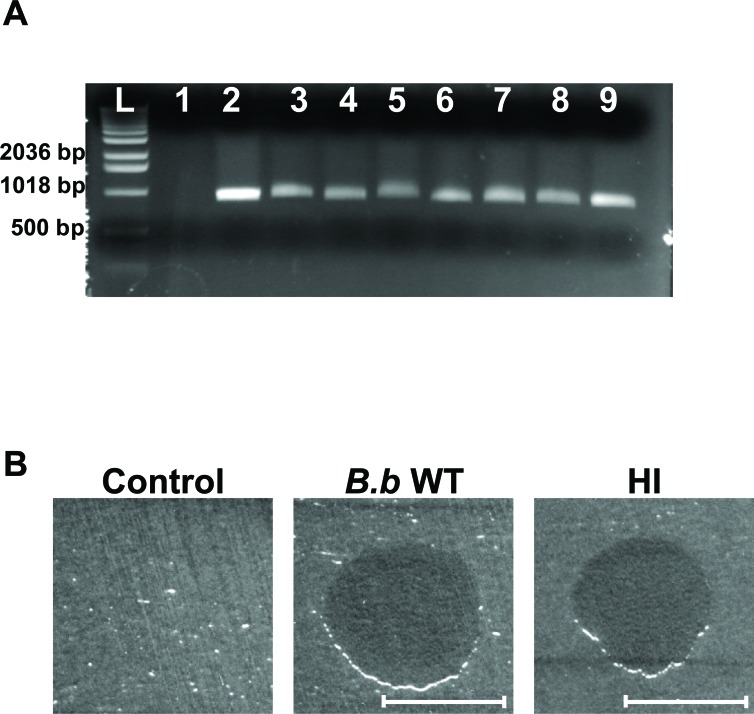
To confirm that the isolated colonies are HI derivatives of Bdellovibrio, 35 random colonies were streaked successively on PYE plates. The selected HI colonies were evaluated by PCR, using primers 3F (5′-TAGACAGATGGGATTACT-3′) and 913R (5′-GTGTGATGACGACTGTGA-3′), that amplify a ~900 bp region of B. bacteriovorus hit locus [2]. PCR reactions had confirmed that the selected HI colonies were derivatives of Bdellovibrio. A ~900 bp fragment was obtained only in the samples containing DNA template from WT B. bacteriovorus 109J and the HI isolates, with no amplification product developing when E. coli WM3064 DNA was used (Fig. 2A). Examining the HI cells by light microscopy demonstrated a strong degree of similarity to other HI variants isolates elsewhere [2, 4, 5, 8, 9]. The cells were relatively uniform in thickness, with cell length being variable from 2-60 μm. To assess the facultative predation behavior of the selected HI variants, the HI isolates were evaluated for their ability to reduce host cells. When grown in the presence of planktonic E. coli host cells, the HI isolates were able to reduce host cells CFU by ~5 logs within 48 hr of predation (from 1x108 to 1x103). Additionally, when spotted on a lawn of host cells, all of the HI isolates were able to prey and form a clear lytic halo (Fig. 2B). Thus, the genetic, phenotypic, and morphological features confirm that the isolated colonies are HI variants of Bdellovibrio.
Fig. (2).
HI variant analysis. (A) Agarose gel electrophoresis of amplified DNA with B. bacteriovorus specific primers. Lanes correspond to template E. coli strain WM3064 DNA (lane 1), template B. bacteriovorus 109J WT DNA (lane 2), template DNA from HI variants (lanes 3-9), and 1 Kb DNA ladder (Invitrogen, Carlsbad, CA) (L). A ~900 bp fragment was obtained for all of the 35 HI variants examined, with seven random HI variants shown here. (B) Plaque predation assay. Wild-Type B. bacteriovorus (B.b WT), HI variant (HI) and DNB (Control) were spotted on a thick lawn of Klebsiella pneumoniae host cells. 48 hr after inoculation a clear lytic halo formed at the point of inoculation where predation had occurred. Lytic halos appeared for all of the 35 HI isolates examined. Scale bar, 0.8 cm.
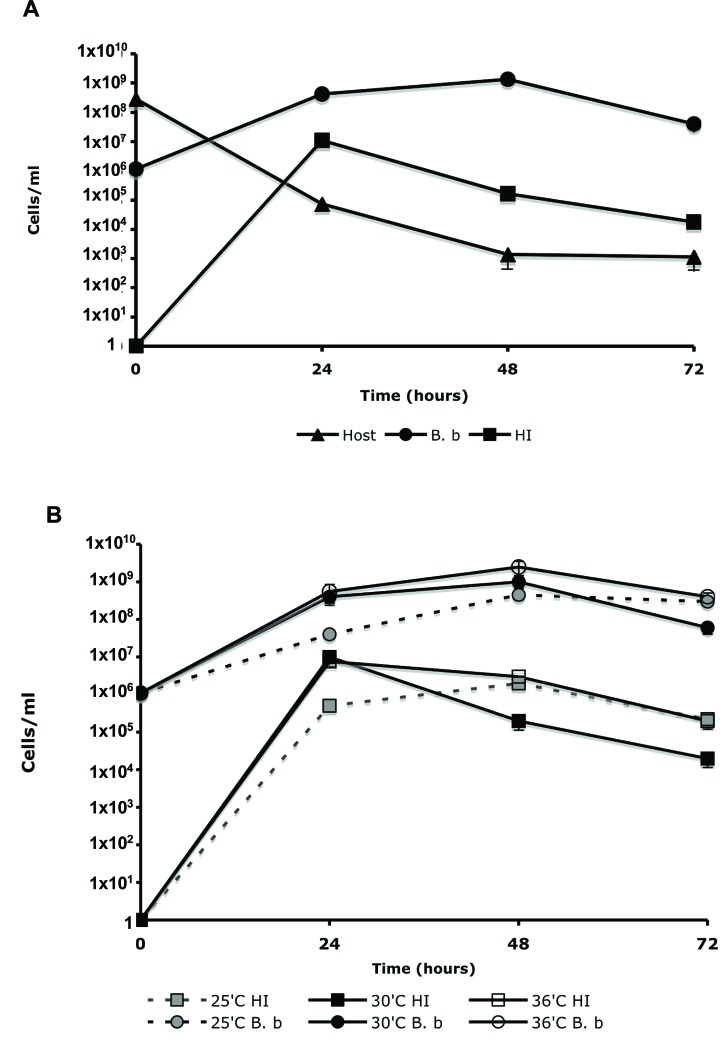
To evaluate the changes occurring in cell population, during predation, a seven-day-old standard B. bacteriovorus induced lysates was passed four times through a 0.45 µm filter, to remove any residual prey cells. The sample containing only B. bacteriovorus cells (1.1x106 PFU/ml) was added to 2.8x108 CFU/ml of E. coli WM3064 host cells, in DNB-DAP minus media, and cultured for 72 hr. The change in cell population was measured by plating 100 μl of the lysate on, LB+DAP plates (for host cell enumeration); double-layered agar (for WT Bdellovibrio); and PYE agar (for HI variants). As seen in Fig. (3A), an increase of 7 logs in HI CFU was seen during the first 24 hr of incubation, followed by a decrease in HI viability, reaching 1.8x104 CFU/ml after 72 hr. As anticipated, a 3-log increase in Bdellovibrio PFU’s and a 5-log reduction in host cell biomass were also measured. By dividing the CFU count of the HI colonies (1.1x107 CFU/ml) by the WT Bdellovibrio concentration, measured after 24 hr of incubation (4.1x108 PFU/ml), we concluded that the frequency of alteration from WT to HI to be 2.68x10-2. In order to eliminate residual HI variants in the inoculum source for WT Bdellovibrio, a seven-day-old lysate was used in this experiment since a preliminary experiment confirmed that any residual HI variants that might have developed in the initial lysate could not survive after seven days in DNB. In this experiment, HI variant was grown in PYE, washed and resuspended in DNB. The viability of the HI variant dropped from 5x108 CFU/ml to 4x104 CFU/ml after 48 hr of incubation. No HI cells survived after 120 hr of incubation in DNB. This experiment was conducted with six different HI variants; in each experiment cell viability was lost within 96 to 120 hr of incubation (data not shown). These experiments also confirmed that the HI variants are not capable of clonal growth in DNB media.
Fig. (3).
Changes in WT B. bacteriovorus, HI variants, and host cell concentration during predation. (A). WT B. bacteriovorus (B. b) was propagated on E. coli WM3064 (Host) in DNB-DAP minus media for 72 hr at 30˚C. The change in cell population was measured as described in the material and methods. (B). The influence of temperature on the emergence of HI variants. WT B. bacteriovorus (B. b) was propagated on E. coli WM3064 (Host) in DNB-DAP minus media for 72 hr at 35˚C, 30˚C, and 36˚C. Experiments were carried out three times yielding similar results. Error bars indicate standard errors.
To evaluate if the emergence of HI variants is influence by environmental conditions, three lysates were prepared as described above and incubated at 25˚C, 30˚C and 36˚C for 72 hr. As seen in Fig. (3B), the appearance of HI variants was correlated to the growth of the host dependent WT Bdellovibrio, which was slightly inhibited at 25˚C. No evident difference in the development of HI variants was seen in the lysates that were incubated at 30˚C and 36˚C. The frequency of alteration (HI CFU/WT PFU) from WT to HI was also found to be comparable between the three lysates being; 1.2x10-2, 2.5x10-2 and 2.7x10-2 for 25˚C, 30˚C, and 36˚C respectably.
DISCUSSION
In this study we have used E. coli DAP auxotrophs as prey cells in order to isolate Bdellovibrio HI variants. These host cells could be easily selected against by plating the lysate on PYE plates lacking DAP, allowing only the HI variants to grow. Microscopic evaluation and viable cell enumeration revealed that B. bacteriovorus could prey on the DAP auxotroph WM3064 strain with similar efficiency as on WT E. coli host cells (Fig. 1). Using E. coli DAP auxotrophs, we were able to rapidly isolate numerous HI variants. PCR, microscopy and predation analysis all confirmed that the isolated colonies are derivatives of HI B. bacteriovorus (Fig. 2A and 2B).
To assess the frequency of alteration from WT to HI variants within the lysate, the changes in host, WT Bdellovibrio and HI cell concentration was examined. It is interesting to note that the development of the HI variants in the system was higher than what was previously reported [8, 12]. To assure that no HI cells are carried over from the initial lysate, which was the source for the WT Bdellovibrio, we used a 7 day-old lysate. No HI cells were present in this sample (Fig. 3A and 3B). HI variants have been reported to arise at a frequency of 10-6 to 10-7 [8, 12] .Our previous work using Seidler and Starr procedure for isolating HI variants, also give rise to these variants at the same rate [4, 5]. Traditional methods used for isolating HI variants called for the removal of residual prey cells either by filtration, or by several prolonged cultivation steps. These steps were aimed at isolating antibiotic resistant Bdellovibrio mutants as well as reducing the remaining host cells population to a minimum [8, 12]. It could be assumed that the prolonged incubation periods as well as the filtration procedures, could have resulted in the lower number of HI cells recovered from the lysate. Since our procedure does not require filtration or an extensive cultivation period, more viable HI cells could supposedly be isolated.
In recent years Bdellovibrio HI variants have become an essential tool in the study of Bdellovibrio biology [5-7]. However, the factors influencing the switch from host dependency to host independency are far from being understood. Since this method allows the isolation of HI variants directly from the lysate without the need of extensive cultivation steps, filtration or total host consumption, this method could be used to study the effects of various treatments on the frequency of HI cell development. In this study we did not see any significant changes in the occurrence of HI variants when cultured at different temperatures. However, we believe that using this new isolation approach in experiments such as these will aid in the study of Bdellovibrio biology as well as shed new light on the factors contributing to HI development.
ACKNOWLEDGEMENTS
This study was supported by the Foundation of UMDNJ faculty research grant to D.E.K.
REFERENCES
- 1.Stolp H, Starr MP. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek. J Microbiol. 1963;29:217–48. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- 2.Barel G, Jurkevitch E. Analysis of phenotypic diversity among host-independent mutants of Bdellovibrio bacteriovorus 109J. Arch Microbiol. 2001;176:211–16. doi: 10.1007/s002030100312. [DOI] [PubMed] [Google Scholar]
- 3.Diedrich DL, Denny CF, Hashimoto T, Conti SF. Facultative parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970;101:989–96. doi: 10.1128/jb.101.3.989-996.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina AA, Kadouri DE. Biofilm formation of Bdellovibrio bacteriovorus host-independent derivatives. Res Microbiol. 2009 doi: 10.1016/j.resmic.2009.02.001. doi:10.1016/j.resmic.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Medina AA, Shanks RM, Kadouri DE. Development of a novel system for isolating genes involved in predator-prey interactions using host independent derivatives of Bdellovibrio bacteriovorus 109J. BMC Microbiol. 2008;8:33–43. doi: 10.1186/1471-2180-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans KJ, Lambert C, Sockett RE. Predation by Bdellovibrio bacteriovorus HD100 requires type IV pili. J Bacteriol. 2007;189:4850–59. doi: 10.1128/JB.01942-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudor JJ, Davis JJ, Panichella M, Zwolak A. Isolation of predation-deficient mutants of Bdellovibrio bacteriovorus by using transposon mutagenesis. Appl Environ Microbiol. 2008;74:5436–43. doi: 10.1128/AEM.00256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidler RJ, Starr MP. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969;100:769–85. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson MA, Schmitt JL, Sindhurakar AR, Volle CB, Nunez ME, Spain EM. Rapid isolation of host-independent Bdellovibrio bacteriovorus. J Microbiol Methods. 2008;73:279–81. doi: 10.1016/j.mimet.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Dehio C, Meyer M. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J Bacteriol. 1997;179:538–40. doi: 10.1128/jb.179.2.538-540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saltikov CS, Newman DK. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA. 2003;16:10983–88. doi: 10.1073/pnas.1834303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shilo M, Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965;40:317–28. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]