Abstract
Understanding complex diseases such as sporadic Alzheimer disease (AD) has been a major challenge. Unlike the familial forms of AD, the genetic and environmental risks factors identified for sporadic AD are extensive. MicroRNAs are one of the major noncoding RNAs that function as negative regulators to silence or suppress gene expression via translational inhibition or message degradation. Their discovery has evoked great excitement in biomedical research for their promise as potential disease biomarkers and therapeutic targets. Key microRNAs have been identified as essential for a variety of cellular events including cell lineage determination, proliferation, apoptosis, DNA repair, and cytoskeletal organization; most, if not all, acting to fine-tune gene expression at the post-transcriptional level in a host of cellular signaling networks. Dysfunctional microRNA-mediated regulation has been implicated in the pathogenesis of many disease states. Here, the current understanding of the role of miRNAs in the central nervous system is reviewed with emphasis on their impact on the etiopathogenesis of sporadic AD.
Key Words: Aging, Alzheimer disease, biomarker, microRNA, mild cognitive impairment, neurodegeneration, genomics.
INTRODUCTION
1. The Pathology and Epidemiology of Alzheimer Disease
Alzheimer disease (AD) is an aging-associated dementing disorder characterized by progressive neuronal degeneration, gliosis, and the accumulation of extracellular deposits of amyloid (senile plaques; SP) and intracellular inclusions (neurofibrillary tangles; NFT) in discrete regions of the basal forebrain, hippocampus, and association cortices [1, 2]. Rare early-onset familial forms of AD with an inheritable autosomal dominant mutation in either amyloid precursor protein (APP), presenilin-1 (PSEN1) or presenilin-2 (PSEN2) genes, account for <1% of AD cases. An unknown proportion of late-onset familial AD harbors “less aggressive” familial autosomal dominant mutations [3, 4]. The vast majority of AD cases are sporadic, with complex genetic, environmental and epigenetic risk factors [3, 5-7].
Susceptible genetic polymorphisms in over 500 genes have been proposed as risk alleles [8], but only the apolipoprotein E (APOE) ε4 allele has been validated [9-11]. In these subgroups, the APOEε4 allele acting with other genetic polymorphisms accelerates disease onset. There thus appear to exist multiple etiopathogenetic pathways which culminate in a common histopathological profile characteristic of sporadic AD. A sub-classification taking into account AD family history, age of onset, ethnicity, risk factors (i.e. education, lifestyle, vascular pathology) and possibly gender could facilitate the delineation of discrete at-risk genetic networks associated with AD.
Definitive AD diagnosis is established by the postmortem quantification of SP and NFT in affected brain regions. The amyloid and neuritic plaques arise from the accumulation of 1-40 and 1-42 amyloid beta (Aβ) peptide residues following the sequential cleavage of the APP protein by β-secretase (β-site APP cleaving enzyme 1, BACE1) and γ-secretase complex (PSEN1 and PSEN2) [12]. Increases in Aβ42 oligomers are believed to contribute to synaptic failure, oxidative stress and NFT [13]. NFT are filaments composed of a hyperphosphorylated microtubule-associated protein, tau [14].
The increases in Aβ and SP as well as NFT in the brain are aging-related phenomena and occur in the brains of the non-demented elderly. In familial AD, the ratio of Aβ42/Aβ40 specifically increases [15]. However, mutations in the presenilins and APP genes may also alter their signaling [16, 17] or transactivation functions [18, 19]. In sporadic AD, different mechanisms which alter either Aβ production or clearance have been identified. For example, increases in BACE1 expression and protein in AD brains correlate with higher Aβ production [20]. Alternatively, APP cleavage by cathepsin B followed by glutaminyl cyclase modification of the truncated Aβ (3–42) into pyroglutamate (pE)-modified Aβ3(pE)–42 may also contribute to AD pathology in other subgroups [21, 22]. Likewise, multiple pathways have been suggested to cause the polymerization of NFT, including Aβ42 [13], caspase activation [23] or decrease in insulin-like growth factor I (IGF-I) signaling [24].
Several age-related disorders that contribute to AD pathology have been described, in particular dysfunctions in antioxidant responses and mitochondrial homeostasis [25-27]. Higher oxidative damage in both peripheral and CNS tissues has been documented in AD patients, even at early stages of the disease [28]. Other abnormalities associated with AD include impairment in DNA repair [29], deregulation of calcium homeostasis and altered immune responses [30], as well as evidence of neuroinflammation [31, 32] and cerebrovascular pathology [33]. In many cases, oxidative stress is a common denominator and could be linked to underlying genetic hubs shared by these pathways. We recently identified an impaired genetic expression network in sporadic AD that may coordinate oxidative stress defense mechanisms and DNA repair responses [9].
Considerable efforts in defining surrogate biomarkers in order to facilitate the diagnosis of AD are underway (reviewed in [34]). The most robust AD biomarker at this time is the combined decrease in Aβ42 and increase in phosphorylated tau levels in cerebrospinal fluid (CSF). Unfortunately, this combination of biomarkers is not useful for the prediction of earlier stages without clinically defined dementia, such as mild cognitive impairment (MCI) [35]. Moreover, lumbar puncture for CSF examination is relatively invasive, and unsuitable for mass screening of the aging population. Readily accessible biomarkers from a minimally-invasive blood (or other peripheral) specimen would greatly facilitate the management of this condition [34].
Impairment in miRNA expression or function contributes to human diseases and has great potential as disease biomarkers [36]. Although their modes of action are complex, miRNAs generally act as post-transcriptional repressors of their target gene and/or protein [37, 38]. Major implications for miRNAs in the etiological pathways and associated risk factors may be anticipated in AD. Characterization of underlying etiopathogenetic pathways related to miRNA dysfunction holds promise for the diagnosis, prognosis and management of AD and other neurological diseases. We provided evidence of augmented miRNA expression in peripheral blood mononuclear cells (PBMC) in sporadic AD, which may shed new light on the pathogenesis of AD and provide accessible blood-based diagnostic and prognostic biomarkers for this condition [39].
THE ROLES OF MICRORNA IN THE CENTRAL NERVOUS SYSTEM
1. Small Noncoding MicroRNAs
The unprecedented discovery of small noncoding miRNAs and their implications for virtually all biological functions [40-42] is still in early stages of research with at least 1000 or more miRNAs expected to be identified in the human genome [43]. At present, 706 human miRNA genes have been registered in stem-loop form in the web-database, miRBase (Sanger Institute, version 13.0) [44]. With the exception of the founding let-7 family of microRNAs in mammals [45], their annotation is based on their cloning chronology, with family members distinguished in alphabetical and/or numerical order [46].
The great majority of miRNA genes are transcribed by RNA polymerase II under the regulation of type II promoters, with a few exceptions being constitutively expressed by type III promoters [47]. MicroRNAs are found in various inter- and intragenic locations, and at least 42% are estimated to be expressed in clusters [48]. The pri-miRNA transcripts are cleaved by the endonuclease Drosha complex into hairpin secondary structures called pre-miRNAs in the nucleus, and then exported into the cytoplasm where they are cleaved by the endonuclease Dicer into 18 to 22 nucleotide long mature miRNAs [49]. MicroRNA expression is regulated not only at their promoter, but also at post-transcriptional levels of processing [50]. In fact, Dicer is localized to the somatodendritic compartment in neurons and glia [51] and is activated to process pre-miRNA precursors following synaptic signaling [52].
The mature miRNAs are then assembled into RNA-induced silencing complexes (RISC) and bind to partially complementary sites mostly within the 3' untranslated region (UTR) of target mRNAs (reviewed in [49, 53, 54-56]). Regulatory subunits within the RISC complex and/or present on the mRNAs (i.e. cytoplasmic ribonucleoprotein complexes, mRNPs) play decisive roles in miRNA-mRNA localization, miRNA responsiveness to cellular signaling and mode of action [37, 56, 57]. The miRNP-mRNA complex may be directed to polyribosomes in sub-cellular compartments, where miRNA regulate translation. Alternatively, the complex accumulates in foci termed P-bodies and/or granules for mRNA storage or degradation [53, 56]. While the various regulatory factors and signaling effectors associated with miRNA regulation are not fully understood, an example of a stress responsive mechanism to redirect stored mRNA in P-bodies back into translation has been described for the RNA-binding protein, HuR [58]. Similar mechanisms of reversal in miRNA-mediated repressions are expected within miRNP-mRNA complexes in diverse brain and synaptic functions [56].
The principles of target recognition for miRNA:mRNA duplex formation are based on the complementarity of an eight nucleotide seed region (reviewed in [59-61]). Various algorithms are now available to predict major evolutionarily conserved targets in 3'UTR for over 60% of mRNAs [61]. One single miRNA has hundreds of potential gene targets, whereas dozens or more microRNA response elements (MRE) are predicted in the 3'UTR of individual transcripts. However, mechanisms of alternative splicing [62] and 3'UTR cleavage [63] have evolved to alter miRNA regulation in specific cellular context. Improvement of target prediction by algorithms are expected to eventually take into account such variables as cell-specific splicing, MRE accessibility within mRNA secondary structures [64], the presence of protein binding sites [65], and perhaps RNA editing [66, 67]. In fact, miRNA editing occurs at a high frequency in the brain and may serve to silence miRNAs [68]. Alternatively, miRNA editing may also reinforce and stabilize miR:mRNA duplex in order to favor target silencing by mRNA cleavage [69].
Multiple miRNAs are involved in regulating either a single gene or sets of genes in common and divergent genetic networks. Despite the extreme level of complexity in miRNA regulatory networks, one approach to facilitate the identification of biological functions is to integrate genomic (i.e. mRNA, miRNA) and proteomic expression data with miRNA target predictions in the same cellular context. This approach successfully identified target predictions from various algorithms in various species and experimental systems, some of which are validated in cell-based functional assays (Table 1A, 1B). Eventually, bioinformatics tools will assist in the delineation of regulatory networks and feedback loops, by integrating signaling and transcription factors and identifying the presence of regulatory elements and links with other miRNAs. Despite the arduous task ahead, key miRNAs and their regulatory feedback loops have been identified with specific CNS functions and neurological disorders (Table 1A, B).
Table 1A.
Identification of MicroRNA Associated with the Central Nervous System
| MicroRNA | Cell/Tissue | Target1. | Functions | Sp. | Ref.2. |
|---|---|---|---|---|---|
| Down-Regulated | |||||
| miR-9, 131 | Presenilin 1 knockout | - | Brain development | m | [72] |
| miR-130a, 206 | Mesenchymal stem cell | TAC1 | Neurotransmission | h | [73] |
| Up-Regulated | |||||
| let-7a/c/e | P19; embryo | - | Neuronal speciation | m | [50] |
| miR-7 | Vasotocinergic neuron | - | Neurosecretion | z | [74] |
| miR-9-1 | Embryo | Fgf genes | CNS development | z | [75] |
| miR-9a | Sensory organs | Senseless | Sensory neuron | d | [76] |
| let-7g, miR-21, 27a/b, 29a, 138 | SH-SY5Y | - | Neurogenesis | [77] | |
| miR-23 | NT2 | HES1 | Neurogenesis | h | [78] |
| miR-26a | Hippocampus | MAP2 | Dendrite | r | [79] |
| miR-30a-5p | Prefrontal Cortex | BDNF | Cortical development & function | h | [80] |
| miR-124 | P19; neural tube | SCP1 | CNS development | c/m | [81] |
| miR-124 | Neural tube | LAMC1 ITGB1 | Neuronal speciation | c | [82] |
| miR-124 | CAD; N2a | PTBP1 | Brain-specific alternative splicing; Neurogenesis | m | [83] |
| miR-125b | P19 | Lin-28 | Neurogenesis | m | [84] |
| miR-132 | PC12; neuron | p250GAP | Neuronal plasticity | r | [85] |
| miR-134 | Hippocampus | Limk1 | Dendrite & synapse | m/r | [86] |
| miR-184 | Cortical neurons | - | Synaptic plasticity (DNA methylation) | m | [87] |
| miR-200 family | Embryo | neuroD, foxg1, lfng, zfhx1 | Olfactory neurogenesis | m/z | [88] |
| miR-200b | Hippocampus | ZFHX1B | CNS development; TGFβ-signaling | m | [89] |
| miR-204 | Choroid plexus | TRPM3 | CSF associated | m | [90] |
| miR-430 | Embryo | - | Brain morphogenesis | z | [91] |
Reported miRNA target’s gene symbol: APP, amyloid precursor protein; BACE1, β-site APP cleaving enzyme 1; BDNF, Brain-derived neurotrophic factor; CFH, Complement factor H; fgf, fibroblast growth factor; GRIA2, Glutamate receptor subunit; HES1, Hairy and enhancer of split 1; ITGB1, Integrin β1; LAMC1, laminin γ1; Limk1, Lim-domain-containing protein kinase; MAP2, Microtubule -associated protein 2; p250GAP, p250GTPase-activating protein; PITX3, Paired-like homeodomain transcription factor Pitx3; PTBP1, Polypyrimidine tract binding protein 1, PTB/hnRNPI; SCP1, Small C-terminal domain phosphatase 1; SLITRK1, Slit and Trk-like 1; TAC1, Tachykinin; TRPM3, Transient receptor potential cation channel M3; VSNL1, Visinin-like 1; ZFHX1B, Zinc finger E-box binding homeobox 2, ZEB2.
The common or significantly altered miRNAs are reported from published microarray profiling data.
Table 1B.
Identification of MicroRNA Associated with Neurological Diseases
| MicroRNA | Cell/Tissue | Target1. | Associated Disease | Sp. | Ref.2. |
|---|---|---|---|---|---|
| Down-Regulated | |||||
| miR-8 | Adult flies | Atrophin | Behavorial defect model | d | [70] |
| let-7 | Larval | APP-like | AD model system | c.e. | [92] |
| miR-106a | HEK-293 | APP | AD functional assay | h | [93] |
| miR-106b | Cortex | APP | AD | h | [94] |
| miR-298, 328 | Brain | BACE1 | AD model system | m | [95] |
| miR-29a/b-1 | Cortex | BACE1 | AD | h | [96] |
| miR-107 | Temporal cortex | BACE1 | AD, MCI | h | [97] |
| let-7i, miR-9, 15a, 26b, 93, 181c, 210 | Cortex | - | AD | h | [96] |
| miR-124b | Hippocampus | - | AD | h | [98] |
| miR-9, 132, 146b, 210, 212, 425 | Hippocampus & FG | AD | h | [99] | |
| miR-15b, 146b, 181c, 338 | CSF | - | AD | h | [99] |
| miR-9/, miR9* | Cortex | REST/CoREST | HD | h | [100] |
| miR-29b; 124a | Cortex | - | HD | h | [100] |
| miR-132 | Cortex | p250GAP | HD | h | [101] |
| miR-10b; 132, 495 | Brainstem | - | PD | m | [102] |
| miR-133b | dopaminergic neuron | PITX3 | PD | h/m | [103] |
| miR-338-3p; 337-3p | Brain | - | Prion disease | m | [104] |
| miR-26b, 29b | Prefrontal cortex | - | Schizophrenia | h | [105] |
| miR-9, 124a, 125b | Spinal cord | - | Spina bifida model | r | [71] |
| Up-Regulated | |||||
| let-7c, miR-99a, 125b-2, 155, 802 | Fetal brain | - | DS | h | [106] |
| miR-197, 320, 511, 520h, 516-3 | Cortex | AD | h | [96] | |
| miR-9, 125b, 128, 130, 145 | Hippocampus | - | AD/ ROS generating metals | h | [98, 107] |
| miR-146a | Hippocampus & cortex | CFH | AD/ Aβ & ROS inducible | h | [108] |
| miR-27a, 34a, 92, 145, 381, 422a, 423 | Hippocampus & FG | - | AD | h | [99] |
| let-7f, miR-30, 125a, 197, 371, 517, 520a | CSF | AD | h | [99] | |
| let-7f, miR-34a, 155, 181b, 200a, 371, 517* | BMC | - | AD | h | [39] |
| miR-29a, 330 | Cortex | - | HD | h | [101] |
| miR-218; 132 | Cortex | HD | h | [100] | |
| let-7b, miR-146a; 128; 139-5p;320; 328; 342-3p | Brain | Prion disease | m | [104] | |
| miR-106b | Prefrontal cortex | - | Schizophrenia | h | [105] |
| miR-181b | Temporal cortex | VSNL1/GRIA2 | Schizophrenia | h | [109] |
2. The Identification of MicroRNAs in the CNS
The role of miRNAs in neuronal development and function was first elucidated in Caenorhabditis elegans [110]. The importance of miRNAs in CNS functions and disease is now well recognized (reviewed in [111-122]). Initially, the implication of miRNAs in mammalian CNS was determined by miRNA expression profiling [117, 123]. Among the first hundred miRNAs cloned, miR-7, -9/9*, -124a, -124b, -125a, -125b, -128, -132, -135, -137, -139, -153, -149, -183, and miR-219 were reported to be abundantly expressed in mouse and human adult brains [124]. Several more miRNAs, including let-7 family members were shown to co-purify with polysomes in neurons [125]. Similar miRNAs were also identified in rat and monkey [126].
More refined cellular specificity in their expression was reported for astrocytes (miR-23, -26, -29), neurons (miR-124, -128) [127], and hippocampal neurons (let-7 family) [50]. Cellular speciation associated with orthologue miRNAs was also reported in zebra fish for neuronal precursors (miR-92b), mature neurons (miR-124, -181, -222), motor neurons (miR-218a) and dendrites (miR-134) [128]. Likewise, in rat hippocampal neurons, miRNAs have been sub-localized within soma and dendrite compartments, including miR-124 and miR-26a respectively [79]. In human prefrontal cortex, miR-30a was shown to be enriched in pyramidal neurons [80]. A detailed comparison of miRNA expression profiles in mouse and zebra fish CNS revealed species differences [129]. Eventually, a tissue and sub-cellular cartography of microRNA levels in human CNS should contribute to a more complete understanding of their functions.
A detailed functional analysis for a human miRNA was accomplished for the predominant brain miRNA, miR-124 [130]. Direct evidence of a role for miR-124 was derived from its over-expression in HeLa cells, which shifted the gene expression pattern to that of a neuron [131]. Expression of miR-124a was shown to be under the negative control of the transcriptional repressor, REST (RE1 silencing transcription factor), a regulator of neuronal differentiation [132]. In turn, miR-124 targets the REST-binding anti-neural factor, SCP1 (Small C-terminal domain phosphatase 1), and reinforces the induction of neurogenesis [81]. Its constant expression also maintains neuronal speciation [82], in part by targeting the polypyrimidine tract binding protein 1 (PTBP1/hnRNPI), a repressor of alternative splicing expressed in non-neuronal cells [83]. Thus, miR-124 may act at different levels of gene regulation for neuronal speciation, involving complex regulatory and splicing mechanisms and negative feedback loops [133].
Another key miRNA under the regulation of REST is miR-9 [134]. This miRNA is reported to have several functions depending on its cellular context, such as in CNS development [71], midbrain-hindbrain boundary formation [75], or in oxidative stress responses [107]. In Drosophila, miR-9 regulates the transcription factor Senseless in the development of the peripheral nervous system [76]. MicroRNA miR-9 is also required for brain functions, as suggested in PSEN1 null mice, where its decrease is associated with severe brain defects [72]. The loss of PSEN1 impacts memory, synaptic plasticity and induces neurodegenerative changes [135, 136].
Also noteworthy is the let-7 family which is strongly expressed in brain and neuronal stem cells [50]. The evolutionarily conserved let-7 family members are highly edited [69], and the processing of pri-let-7 into active mature miRNAs is tightly regulated in the brain [50]. One possible post-transcriptional regulation is by the RNA binding protein, Lin28 [137], which binds to the pri-let-7 precursor in order to inhibit its processing by Drosha [138, 139]. The expression of miR-125 during neurogenesis suppresses Lin28 [84] and together with let-7 further represses Lin28 in an autoregulatory circuit [140]. Moreover, let-7 directly regulates Dicer and may have a global regulatory control on miRNA functions [141]. At this time, no specific CNS functions are described for the let-7 family members in humans; however, their roles in differentiation and as tumor suppressors have been demonstrated [142].
3. MicroRNA Associated with Neurological Diseases
The relationship between microRNA dysfunctions and neurological diseases is perhaps best illustrated with fragile X mental retardation. In these patients, the absence of Fragile X mental retardation 1 protein (FMRP) impairs Dicer and RISC functions required for miRNA-mediated synaptic plasticity and dendritic development [143]. Experimentally, altering miRNA processing by the inactivation of Dicer in Drosophila also prevents dendritic development [144] and enhances polyglutamine and tau-induced neurodegeneration [145]. Likewise, inactivation of Dicer in the CNS of mice impairs dendrite formation, neuronal survival, and gradually leads to neurodegeneration [146-148].
Dysfunction in RISC complex formation also occurs in the pathology of Huntington disease (HD). The interaction of the HD protein with one of the RISC subunit Argonaute-2 is impaired in HD, which prevents the formation of P-bodies [149]. Moreover, impairment in HD protein regulation of REST results in the repression of miRNA expression in HD brains, in particular the down-regulation of miR-132 affecting neurite outgrowth [101] and the down regulation of miR-124a and miR-9/9* affecting a double negative feedback loop regulation of REST with miR-9 [100]. This suggests that the down-regulation of key miRNAs regulated by REST predisposes to neurodegenerative disorders.
The association of miRNA with another polyglutamine disease, dentatorubral-pallidoluysian atrophy, is suggested in Drosophila; mutation in miR-8 (orthologue miR-200a/b and miR-429) causes apoptosis in the brain and behavioral defects, along with an increase histone deacetylase (HDAC) activity [70]. HDAC inhibitors have protective effects in the treatment of stroke, AD, amyotrophic lateral sclerosis (ALS) and HD [150] and alter both gene and miRNA expression [151]. This suggests that miRNAs such as the miR-200 family could be involved in HDAC deregulation in neurological diseases.
Interestingly, miR-200a was found to be increased in AD brain [99] and peripheral blood mononuclear cells (PBMC) [39]; however, its function in AD pathology is not yet established. A specific role has been described for miR-200b in the regulation of a ZFH-1 repressor (ZFHX1B), involved in the regulation of E-cadherin expression in mouse brain [89]. Importantly, E-cadherin is a substrate of PSEN1, which is related to diverse brain functions and synaptic plasticity [152]. The miR-200 family is also involved in functions of the olfactory system [88], pathways implicated in neurodegenerative conditions such as AD and Parkinson disease (PD) [153].
In PD, miR-133b is deficient in midbrain dopaminergic neurons, and may be related to an impaired feedback loop with the Paired-like homeodomain transcription factor, PITX3 [103]. Polymorphisms in PITX3 are associated with PD [154], and perhaps this impairs its capacity to induce miR-133b expression. In another study, a polymorphism associated with PD modified the microRNA binding site of miR-433 in the transcript of fibroblast growth factor 20 (FGF20), resulting in increased alpha-synuclein expression [155]. Other polymorphisms have been associated with neurological disease, such as in the noncoding genes of miR-198 and miR-206 associated with schizophrenia [156], as well as in the binding site of miR-189 in Slit and Trk-like 1 (SLITRK1) mRNA associated with Tourette syndrome [157].
Genetic defects such as trisomy 21 (Down syndrome, DS) lead to neurodegenerative pathology consistent with AD. In particular, extra copies of APP [158] and Dyrk1a [159] genes on chromosome 21 contribute, respectively, to SP and NFT, lesions typical of AD-affected brain. Moreover, extra copies of let-7c, miR-99a, -125b-2, -155, and miR-802 on chromosome 21 are also over-expressed in DS patients [106]. Interestingly, increased Dyrk1a levels may augment REST expression [160], suggesting that other microRNAs under the regulation of REST could be affected.
MICRORNAs AND ALZHEIMER DISEASE PATHWAYS
1. MicroRNA Profiling in Alzheimer Disease
The entorhinal cortex-hippocampus system, concerned with memory functions, is among the first regions of the brain to be affected by AD pathology [161, 162]. In an initial expression profiling of the 13 most abundant brain miRNAs mentioned above [124], Lukiw [98] reported the up-regulation of miR-9, miR-125b and miR-128 in AD affected hippocampus (Table 1B). Later, 328 miRNA were profiled in the cortex of sporadic AD, showing both up- and down-regulation in miRNAs [96]. Among over 400 miRNAs profiled, several up-regulated miRNA were reported in AD-affected hippocampus and medial frontal gyrus, cerebellum and CSF [99]. Likewise, we detected mostly an up-regulation of miRNA expression in PBMC of sporadic AD as compared to aged-matched normal elderly control subjects [39].
The up-regulation of miR-125b and down-regulation of miR-9 and miR-210 have been consistently reported in different studies on miRNA expression profiling of AD-affected brain (Table 2). The deregulation of miR-125b and miR-9 in AD is particularly interesting because, as described above, these key miRNAs are involved in brain development and function as well as in neurological diseases such as DS and HD. The up-regulation of miR-197 and down-regulation of miR-15, -146b, -181c, and miR-338 are commonly altered in AD brain parenchyma and CSF.
Table 2.
Similarly Altered Patterns of MicroRNA Expression in Alzheimer Disease and Aging Model Systems
| MicroRNA1 | Alzheimer Disease Affected Tissue | Aging Model | Reference | ||||
|---|---|---|---|---|---|---|---|
| Cortex | Hippocampus | Cerebellum | CSF | PBMC | |||
| Down-Regulated | |||||||
| let-7i | ↓ | ↓ | [96,169] | ||||
| miR-9 | ↓ | ↓ | ↓ | [99, 96] | |||
| miR-15 | ↓ | ↓ | [99, 96] | ||||
| miR-146b | ↓ | ↓ | ↓ | ↓ | [99] | ||
| miR-181c | ↓ | ↓ | [99, 96] | ||||
| miR-210 | ↓ | ↓ | [99, 96] | ||||
| miR-338 | ↓ | ↓ | [99, 96] | ||||
| miR-451 | ↓ | ↓ | [99, 169] | ||||
| Up-Regulated | |||||||
| let-7f | ↑ | ↑ | ↑ | [99, 39, 181] | |||
| miR-30d | ↑ | ↑ | [99, 169] | ||||
| miR-34a | ↑ | ↑ | ↑ | ↑ | ↑ | [39, 99, 169, 170] | |
| miR-125b | ↑ | ↑ | ↑ | [99, 98] | |||
| miR-197 | ↑ | ↑ | [99, 96] | ||||
| miR-200a | ↑ | ↑ | [99, 39] | ||||
| miR-371 | ↑ | ↑ | [99, 39] | ||||
| miR-517 | ↑ | ↑ | ↑ | [99, 39] | |||
| miR-520h | ↑ | ↑ | [96, 39] | ||||
MicroRNAs that show similar expression trends in the various AD tissues and aging models are reported. Distinct sets of miRNAs expressed in the different systems are not shown.
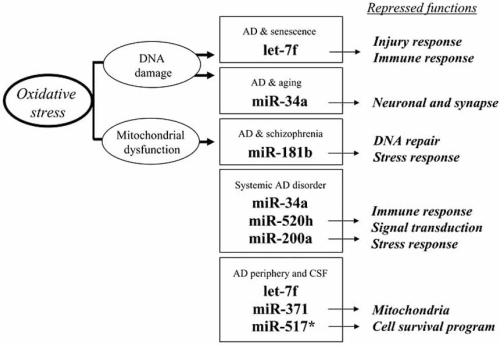
MicroRNAs similarly expressed in both AD-affected brain and PBMC are identified (Table 2; Fig. 1), including the up-regulation of miR-34a, miR-200a and miR-520h, as well as the up-regulation let-7f, miR-371 and miR-517/517* observed in the CSF and PBMC of AD patients. Of note, miR-520h is reported to be highly up-regulated in AD brain [96], and its increase in Alzheimer PBMC suggests it may have a major role in the systemic manifestations of the disease. Systemic dysfunction is also indicated by the up-regulation of miR-34a and miR-200a in AD cortex and PBMC.
Fig. (1).
Up-regulated microRNA in peripheral blood mononuclear cells (PBMC) of sporadic Alzheimer disease (AD) patients. MicroRNAs are small noncoding RNA which generally act as post-transcriptional repressors of gene and/or protein expression. Their common up-regulation in AD brain, cerebrospinal fluid (CSF) and PBMC as well as in other neurological disorders and aging model systems is shown, with implications for oxidative stress, DNA damage and mitochondrial dysfunction. Down-regulated genes in Alzheimer PBMC [163] which may be repressed by these miRNAs were identified using target predictions from miRanda algorithm [44]. Major functional categories which may be theoretically repressed in AD-affected PBMC are indicated.
We suggest here that the systemic increase in specific miRNAs may suppress various cellular functions in AD, such as redox defenses or DNA repair mechanisms, by targeting mRNA and/or protein species in brain and peripheral tissues (Fig. 1). Indeed, gene expression studies in AD have shown substantial down-regulation of various mRNA species in brain [164], PBMC [163] and lymphocytes [165] relative to non-demented controls. Likewise, impairment in protein synthesis is known to occur in AD [166] leading to diminished protein levels in the CSF [167] and plasma [168] of these patients.
For example, the up-regulation of peripheral miRNAs in AD could contribute to the diminished plasma proteins reported to be predictive biomarkers for AD [168], such as chemokine-7 (let-7f, miR-181b) and interleukin-1α (miR-181b, -200a). Moreover, in our genomics work on Alzheimer PBMC, we observed on one hand a significant number of down-regulated genes [163], while on the other, only up-regulation of miRNAs was detected [39]. Interestingly, predicted targets for up-regulated miRNAs correlated with down-regulated genes in functional categories of Synapse Activity, Transcription, and Injury/Redox Homeostasis and DNA Damage (Fig. 1). This suggests that the systemic up-regulation of miRNAs in AD may contribute not only to impaired redox homeostasis and DNA repair mechanisms, but also reflect homologous CNS synaptic dysfunction occurring in AD brains. Importantly, this supports the notion that peripheral miRNA, gene and protein expressions may serve as diagnostic and prognostic biomarkers, and possibly provide leads to the development of new therapeutic interventions.
2. MicroRNA Associated with AD Etiopathogenesis
Although disorders of APP cleavage have been implicated in the etiopathogenesis of AD, experience with Down syndrome (DS) raised the possibility that hyper-expression of the APP gene may contribute to Aβ production in some forms of sporadic AD. In this respect, there is a long list of predicted miRNAs (i.e. from DIANA-microT, miRanda, TargetScanS algorithms) that may target the 3'UTR of APP, including let-7i, miR-15, -26, -29, -93, -101, -106, and miR-181 which are reportedly down-regulated in AD brain [96, 99].
In C. elegans, the APP-like orthologue is targeted by the single let-7 supporting the notion that the let-7 family of miRNAs in humans could be involved in the regulation of APP [92], e.g. let-7i which is reported to decrease in AD brain [96]. In humans, the over-expression of miR-106a/b was shown to reduce APP protein levels in kidney cells [93, 94]. This validates this target prediction for APP, and is consistent with the fact that miR-106a/b is down-regulated in AD-affected brain [94]. The down-regulation of miR-106a/b (Fig. 2), perhaps with other down-regulated miRNAs (let-7i, miR-15, -26, -29, -93 -101), may favor higher APP levels in AD brains.
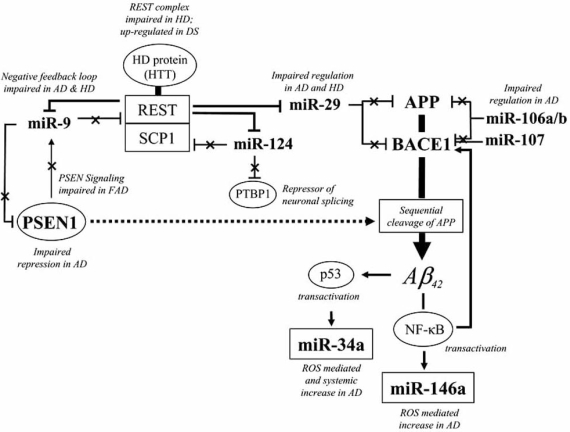
Fig. (2).
A simplified scheme of etiopathogenetic pathways associated with microRNA dyregulation in sporadic Alzheimer disease (AD). Sporadic AD may be characterized by the increase in hallmark genes (i.e. APP, BACE1, PSEN1) which contribute to amyloid beta (Aβ) production by sequential cleavage of APP. Altered miRNAs reported in AD brain which target these genes (as predicted by miRanda and TargetScan algorithms and validated in cell-based assays) are shown [93, 94, 96, 97, 108]. The REST complex negatively regulates the expression of miR-9, miR-29 and miR-124 [134]. The negative feedback loop by miR-9 which suppresses REST is impaired in AD and other neurological diseases. Familial AD (FAD) PSEN1 loss-of-function mutations are recapitulated by PSEN1 knock-out in mouse [72], leading to the decrease in miR-9 akin to that observed in sporadic AD brain. Oxidative stress and DNA damage, generated in part by elevations in amyloid beta (Aβ), iron and reactive oxygen species, induce miRNA expression as exemplified by miR-34a and miR-146a. The stress responsive transcription factor p53, which may increase in AD by Aβ transactivation [173], may further transactivate miR-34a [174]. Stress-mediated neuroinflammatory responses are represented by nuclear factor-kappa B (NF-κB) which transactivates the expression of miR-146a [108] and BACE1 [175]. Barred lines denote post-transcriptional repression of target genes and crossed lines denote de-repression associated with the down-regulation of miRNAs.
The Aβ cleavage product of APP may then arise from the increase in BACE1 and PSEN1 levels (Fig. 2). Of note, BACE1 and APP appear to be targeted by similar sets of miRNAs, including miR-9, -15, -27, -29, and miR-101. These miRNAs may therefore facilitate increases in both APP and BACE1 in AD brain. In fact, over-expression of miR-29a/b-1 reduces APP and BACE1 levels in kidney cells [96]. Another miRNA that targets BACE1 and is down-regulated in early AD and MCI-affected cortex is miR-107 [97]. In this latter example, the identification of miRNA binding sites in BACE1 was determined without stringent cross-genome conservations using the algorithm rna22 (as opposed to miRanda or TargetScan used above).
Mutations in PSEN1 and PSEN2 account for the majority of FAD cases and the products of these genes have been implicated in sporadic AD as well [135]. In PSEN1 knock-out mice, Notch signaling and transcriptional networks associated with miR-9 expression decrease and reflect to some extent the loss-of-function PSEN1 mutations associated with FAD pathology [72]. In the pathogenesis of sporadic AD, augmented PSEN1 mRNA levels [171, 172] may foster Aβ production by sequential cleavage of APP (Fig. 2). Interestingly, miR-9 is predicted to target PSEN1, suggesting that the up-regulation of PSEN1 could be associated with the decline in miR-9 levels in AD.
The down-regulation of miR-9 and miR-29 is, however, not specific to AD (Table 1B) as these miRNA species are also diminished in the brains of individuals with schizophrenia [105] and HD [100]. Conceivably, aberrant profiles of neural miRNA expression shared by various neurological disorders may give rise to common patterns of cellular dysfunction in these conditions. Moreover, deregulations in gene and protein expression may also include other mechanisms, such as long noncoding antisense BACE1 transcript which increases BACE1 expression in AD [176]. Thus, alterations in noncoding RNA expression which affect CNS functions or are associated with neurological diseases are not limited to miRNA species (review in [177]).
3. MicroRNA Associated with Oxidative Stress and AD Risk Factors
Aging remains the most robust risk factor thus far identified for sporadic AD and other human neurodegenerative conditions. Mechanisms of aging under the regulation of miRNAs may contribute to AD pathology, such as those identified in various aging model systems, including the up-regulation of let-7f, miR-30d, -34a, -432, -517 and down-regulation of let-7i and miR-451 (Table 2). These miRNA may reflect intrinsic aging mechanisms in AD [178]. In particular, there is a systemic increase in miR-34a in AD brain [99] and PBMC [39], as well as in aging mouse liver [169] and C. elegans [170]. The up-regulation miR-34a may partly result from DNA damage [179], which accumulates during aging and to an even greater extent in AD [180]. Likewise, the increase in let-7f may be related to DNA damage and cellular senescence, as it is up-regulated in replicatively senescent cells [181] and Alzheimer CSF [99] and PBMC [39]. Replicative senescence correlates with gradual telomere shortening and DNA damage, largely due to the high oxygen tension (20% O2) that exists in culture [182]. Importantly, the up-regulation of let-7 in DS and miR-34a in AD (Table 1B) also suggests that mechanisms of aging and senescence, perhaps related to DNA damage responses, are involved in degenerative processes within the brain.
Oxidative DNA damage is an early and systemic event in the pathophysiology of AD [183, 184]. In our studies on miRNA and gene expression in AD PBMC, we observed impaired DNA repair and antioxidant gene responses, which correlate with the up-regulation of miR-181b, -200a, -517* and miR-520h, and may possibly repress DNA repair and oxidative stress response mechanisms [39]. Oxidative damage in AD tissues also accrues from a gradual decline in antioxidant defenses and increasing neural levels of redox-active transition metals and other pro-oxidant species [26]. AD-associated miRNAs activated by stress-inducing agents, such as aluminum and iron sulfates, include miR-9, miR-125b and miR-128 [98, 107].
Another major source of reactive oxygen species in AD stems from infidelity of electron transport in dysfunctional mitochondria [26] which can be recapitulated using arsenic and other stressors [185, 186]. Similar to miRNA deregulation in AD, human lymphoblastoid cells up-regulate miR-34a and down-regulate miR-210 following arsenic treatment, as well as up-regulate miR-125b, -130, -145 and miR-181b under conditions of folate deficiency [187]. Folate deficiency may cause cognitive impairment and decrease DNA repair mechanisms with age [188, 189]. Analogous patterns of altered miRNA expression in AD suggest that similar mitochondrial and ROS mediated dysfuntions are associated with these miRNAs. Interestingly, miR-181b is up-regulated in schizophrenia-affected brains where mitochondrial dysfunction has been documented [190], and may warrant further investigation in AD brain in light of its up-regulation in AD PBMC.
Stress-responsive miRNAs that are implicated in cardiac hypertrophy [191] and vascular disease [192] may similarly participate in the vascular pathology of AD, pure vascular dementia and mixed dementia [193, 194]. In this context, prime examples of the latter include the up-regulation of miR-27a/b [99], miR-125b [98] and down-regulation of miR-93 [96], as observed in AD brain. It may be of particular interest to determine whether the over-expression of miR-155 in DS brain is associated with macrophage-related inflammation [195]. Also characteristic of AD-affected neural tissues are neuroinflammatory responses activated by the transcription factor, nuclear factor-kappa B (NF-κB), which induces the expression of miR-146a [108]. The expression of miR-146a was also shown to be induced by ROS-generating agents such as hydrogen peroxide and Aβ42. Taken together, these data indicate that up-regulation of miR-27a/b, -125b, -146a and down-regulation of miR-93 may contribute to the stress-related vascular and inflammatory components of AD pathology.
PERSPECTIVE
Deregulation of miRNAs in the CNS may impact a wide range of cellular functions that are not limited to the etiopathogenesis of AD. The identification of homologous patterns of miRNA expression among diverse neurological conditions may denote common pathways of neuronal dysfunction and degeneration. For example, the up-regulation of miR-181b in schizophrenia, or let-7 and miR-125b in DS, are also observed in sporadic AD and may denote common mechanisms of disease (Table 1B). If confirmed, single pharmaceuticals targeting specific miRNA regulatory pathways may prove effective in the treatment of multiple neurological afflictions. The probable decline of miR-9 in AD suggests that its target REST may induce the repression of other neuronal genes and miRNAs (i.e. miR-29a/b, -124, and -132) as reported in the hereditary neurodegenerative disorder, HD [100].
Discrepancies in miRNA expression data in AD are not unexpected and further studies will be required to determine whether disparate data are due to genetic or demographic determinants. Genetic polymorphisms in miRNA sequences, in their promoter regions or in the 3'UTR binding sites of their targets may alter miRNA expression and function. Different genetic backgrounds among familial or sporadic AD patients could well be reflected by specific miRNA expression signatures. For example, we found more robust up-regulation of miR-181b and miR-371 in Alzheimer PBMC relative to control subjects in the APOEε4-positive stratum [39]. This suggests that a higher expression of specific miRNAs in APOE4-positive carriers may contribute to earlier onset of the disease as observed in these patients. It also raises the possibility that specific PBMC gene and miRNA expression signatures may distinguish the various at-risk genetic backgrounds associated with AD.
To understand more thoroughly the role of miRNAs in diseases of the CNS, target predictions will need to be refined taking into account variables such as RNA editing, splicing and secondary structures. The elucidation of miRNA’s role in AD pathology is based on their expression in affected brain and on predictions of their targets, the latter being validated in cell-based functional assays. The fine tuning of a gene (or protein) level is not limited to one miRNA [196], and the net effect on a given target shared by concomitantly down- and up-regulated miRNAs remains unclear. The identification of up-regulated miRNAs having targets which nonetheless increase in the same AD brain tissue (i.e. APP, BACE1) may suggest loss-of-function either by genetic polymorphisms or miRNA editing.
At present, the impact of miRNA deregulation in complex regulatory networks within the brain is not known, nor how sets of miRNAs interact in the regulation of shared targets. To delineate further salient cause-effect relationships in these regulatory pathways, more in-depth characterization of the promoter elements and various transcription factors and repressors (i.e. REST, Aβ, NF-κB, p53, etc.) which alter miRNA expression is paramount. It will also be necessary to determine which sets of miRNAs are affected in different genetic backgrounds and regions of the CNS, and how they integrate within the various regulatory loops and diverging networks implicated in AD and other neurological diseases.
Future studies employing relevant transgenic mouse models should facilitate the elucidation of the beneficial or detrimental effects of specific sets of miRNA in AD pathology. As systemic oxidative damage is characteristic of the AD phenotype, the silencing of specific up-regulated miRNAs (i.e. miR-34a, -181b, -520h) which may impair stress defense and repair mechanisms could reduce ROS levels and related cellular dysfunctions in AD. Conceivably, specific sets of miRNAs will one day be used as a disease-modifying treatment of AD [197]. In the inexorable progress towards personalized medicine, the design of therapeutic miRNA regimens to modify diseased gene expression profiles may be informed by highly-individualized genomic signatures. It is also conceivable that miRNAs may provide minimally-invasive, blood-based biomarkers to facilitate early diagnosis and prognosis of MCI and AD and serve as surrogate indices of successful therapeutic interventions in these and other human CNS afflictions [198].
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution of the medical staff, neuropsychologists, nurses, patients, research coordinators and secretaries of the JGH-McGill Memory Clinic. This work was supported by an internal grant from the Sir Mortimer B. Davis-Jewish General Hospital. EW is supported in part by the Kentucky “Bucks-for-Brains” and Gheens Foundation Endowment funds. None of the authors has any disclosure concerning any actual or potential conflicts of interest.
ABBREVIATIONS
- Aβ
= Amyloid beta
- AD
= Alzheimer disease
- c
= Chick
- c.e.
= Caenorhabditis elegans
- CNS
= Central nervous system
- CSF
= Cerebrospinal fluid
- d
= Drosophila melanogaster
- DS
= Down syndrome
- FAD
= Familial Alzheimer disease
- FG
= Frontal gyrus
- h
= Human
- m
= Mouse
- miRNA
= MicroRNA
- miRNP
= MicroRNA-cytoplasmic ribonucleoprotein complex
- HD
= Huntington disease
- MCI
= Mild cognitive impairment
- MRE
= MicroRNA response element
- NFT
= Neurofibrillary tangle
- PBMC
= Peripheral blood mononuclear cell
- PD
= Parkinson disease
- r
= Rat
- RISC
= RNA-induced silencing complex
- SP
= Senile plaque
- TS
= Tourette syndrome
- UTR
= Untranslated region
- z
= Zebrafish
REFERENCES
- 1.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol. Scand. Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 2.Cummings BJ, Pike CJ, Shankle R, Cotman CW. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer's disease. Neurobiol. Aging. 1996;17:921–933. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 3.Ertekin-Taner N. Genetics of Alzheimer's disease: a centennial review. Neurol. Clin. 2007;25:611–667. doi: 10.1016/j.ncl.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi L, Tomaino C, Anfossi M, Gallo M, Geracitano S, Puccio G, Colao R, Frangipane F, Mirabelli M, Smirne N, Giovanni Maletta R, Bruni AC. Late onset familial Alzheimer's disease: novel presenilin 2 mutation and PS1 E318G polymorphism. J. Neurol. 2008;255:604–606. doi: 10.1007/s00415-008-0764-3. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis. Assoc. Disord. 2006;20:S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JA. Hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 9.Maes OC, Schipper HM, Chong G, Chertkow HM, Wang E. A GSTM3 polymorphism associated with an etiopathogenetic mechanism in Alzheimer disease. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.007. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 10.Webster JA, Myers AJ, Pearson JV, Craig DW, Hu-Lince D, Coon KD, Zismann VL, Beach T, Leung D, Bryden L, Halperin RF, Marlowe L, Kaleem M, Huentelman MJ, Joshipura K, Walker D, Heward CB, Ravid R, Rogers J, Papassotiropoulos A, Hardy J, Reiman EM, Stephan DA. Sorl1 as an Alzheimer's disease predisposition gene? Neurodegener. Dis. 2008;5:60–64. doi: 10.1159/000110789. [DOI] [PubMed] [Google Scholar]
- 11.Emahazion T, Feuk L, Jobs M, Sawyer SL, Fredman D, St Clair D, Prince JA, Brookes AJ. SNP association studies in Alzheimer's disease highlight problems for complex disease analysis. Trends Genet. 2001;17:407–413. doi: 10.1016/s0168-9525(01)02342-3. [DOI] [PubMed] [Google Scholar]
- 12.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 14.Johnson GV, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell. Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 15.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 16.Neve RL. Alzheimer's disease sends the wrong signals-a perspective. Amyloid. 2008;15:1–4. doi: 10.1080/13506120701814608. [DOI] [PubMed] [Google Scholar]
- 17.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Checler F, Sunyach C, Pardossi-Piquard R, Sevalle J, Vincent B, Kawarai T, Girardot N, St George-Hyslop P, da Costa CA. The gamma/epsilon-secretase-derived APP intracellular domain fragments regulate p53. Curr. Alzheimer Res. 2007;4:423–426. doi: 10.2174/156720507781788945. [DOI] [PubMed] [Google Scholar]
- 19.McLoughlin DM, Miller CC. The FE65 proteins and Alzheimer's disease. J. Neurosci. Res. 2008;86:744–754. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- 20.Johnston JA, Liu WW, Todd SA, Coulson DT, Murphy S, Irvine GB, Passmore AP. Expression and activity of beta-site amyloid precursor protein cleaving enzyme in Alzheimer's disease. Biochem. Soc. Trans. 2005;33:1096–1100. doi: 10.1042/BST20051096. [DOI] [PubMed] [Google Scholar]
- 21.Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer's disease-like pathology. Nat. Med. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- 22.Hook V, Schechter I, Demuth HU, Hook G. Alternative pathways for production of beta-amyloid peptides of Alzheimer's disease. Biol. Chem. 2008;38:993–1006. doi: 10.1515/BC.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J. Neuropathol. Exp. Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 24.Carro E, Torres-Aleman I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer's disease. Eur. J. Pharmacol. 2004;490:127–133. doi: 10.1016/j.ejphar.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Mancuso M, Orsucci D, Siciliano G, Murri L. Mitochondria, mitochondrial DNA and Alzheimer's disease. What comes first? Curr. Alzheimer Res. 2008;5:457–468. doi: 10.2174/156720508785908946. [DOI] [PubMed] [Google Scholar]
- 26.Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res. Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Pratico D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol. Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J. Neurosci. Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni A, Wilson 3rd DM. The involvement of DNA-damage and -repair defects in neurological dysfunction. Am. J. Hum. Genet. 2008;82:539–566. doi: 10.1016/j.ajhg.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer's disease. J. Neurovirol. 2002;8:539–550. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 31.Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ. The significance of neuroinflammation in understanding Alzheimer's disease. J. Neural. Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 32.Rogers J. The inflammatory response in Alzheimer's disease. J. Periodontol. 2008;79:1535–1543. doi: 10.1902/jop.2008.080171. [DOI] [PubMed] [Google Scholar]
- 33.Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer's disease. Exp. Physiol. 2008;93:116–120. doi: 10.1113/expphysiol.2007.038729. [DOI] [PubMed] [Google Scholar]
- 34.Schipper HM. The role of biologic markers in the diagnosis of Alzheimer's disease. Alzheimer's Dementia. 2007;3:325–332. doi: 10.1016/j.jalz.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Chertkow H, Nasreddine Z, Joanette Y, Drolet V, Kirk J, Massoud F, Belleville S, Bergman H. Mild cognitive impairment and cognitive impairment, no dementia: Part A, concept and diagnosis. Alzheimer's Dementia. 2007;3:266–282. doi: 10.1016/j.jalz.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Chang TC, Mendell JT. microRNAs in Vertebrate Physiology and Human Disease. Annu. Rev. Genomics Hum. Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 37.Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb. Symp. Quant. Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 38.Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Schipper HM, Maes OC, Chertkow HM, Wang E. MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regul. Syst. Bio. 2007;1:263–274. doi: 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 41.Ambros V. The evolution of our thinking about microRNAs. Nat. Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 42.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 43.Sheng Y, Engstrom PG, Lenhard B. Mammalian microRNA prediction through a support vector machine model of sequence and structure. PLoS ONE. 2007;2:e946. doi: 10.1371/journal.pone.0000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 46.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, reyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. Rna. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X, Ruan J, Wang G, Zhang W. Characterization and identification of microRNA core promoters in four model species. PLoS Comput. Biol. 2007;3:e37. doi: 10.1371/journal.pcbi.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 50.Wulczyn FG, Smirnova L, Rybak A, Brandt C, Kwidzinski E, Ninnemann O, Strehle M, Seiler A, Schumacher S, Nitsch R. Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB. J. 2007;21:415–426. doi: 10.1096/fj.06-6130com. [DOI] [PubMed] [Google Scholar]
- 51.Barbato C, Ciotti MT, Serafino A, Calissano P, Cogoni C. Dicer expression and localization in post-mitotic neurons. Brain Res. 2007;1175:17–27. doi: 10.1016/j.brainres.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 52.Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J. Neurochem. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007;23:243–249. doi: 10.1016/j.tig.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007;8(1):23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 55.Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in Gene Regulation: When the Smallest Governs It All. J. Biomed. Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 57.Kedde M, Agami R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle. 2008;7:899–903. doi: 10.4161/cc.7.7.5644. [DOI] [PubMed] [Google Scholar]
- 58.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Stress-induced reversal of microRNA repression and mRNA P-body localization in human cells. Cold Spring Harb. Symp. Quant. Biol. 2006;71:513–521. doi: 10.1101/sqb.2006.71.038. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe Y, Tomita M, Kanai A. Computational methods for microRNA target prediction. Methods Enzymol. 2007;427:65–86. doi: 10.1016/S0076-6879(07)27004-1. [DOI] [PubMed] [Google Scholar]
- 60.Maziere P, Enright AJ. Prediction of microRNA targets. Drug Discov. Today. 2007;12:452–458. doi: 10.1016/j.drudis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Friedman RC, Farh KK, Burge CB, Bartel D. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan S, Guo J, Huang Q, Chen X, Li-Ling J, Li Q, Ma F. Retained introns increase putative microRNA targets within 3' UTRs of human mRNA. FEBS Lett. 2007;581:1081–1086. doi: 10.1016/j.febslet.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3' untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007;39:1278–1284. doi: 10.1038/ng2135. [DOI] [PubMed] [Google Scholar]
- 65.Didiano D, Hobert O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat. Struct. Mol. Biol. 2006;13:849–851. doi: 10.1038/nsmb1138. [DOI] [PubMed] [Google Scholar]
- 66.Ohman M. A-to-I editing challenger or ally to the microRNA process. Biochimie. 2007;89:1171–1176. doi: 10.1016/j.biochi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Amariglio N, Rechavi G. A-to-I RNA editing: a new regulatory mechanism of global gene expression. Blood Cells Mol. Dis. 2007;39:151–155. doi: 10.1016/j.bcmd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Kawahara Y, Megraw M, Kreider E, Iizasa H, Valente L, Hatzigeorgiou AG, Nishikura K. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reid JG, Nagaraja AK, Lynn FC, Drabek RB, Muzny DM, Shaw CA, Weiss MK, Naghavi AO, Khan M, Zhu H, Tennakoon J, Gunaratne GH, Corry DB, Miller J, McManus MT, German MS, Gibbs RA, Matzuk MM, Gunaratne PH. Mouse let-7 miRNA populations exhibit RNA editing that is constrained in the 5'-seed/ cleavage/anchor regions and stabilize predicted mmu-let-7a:mRNA duplexes. Genome Res. 2008;18:1571–1581. doi: 10.1101/gr.078246.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA MiR-8 tunes atrophin levels to prevent neurodegeneration in drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Zhao JJ, Sun DG, Wang J, Liu SR, Zhang CY, Zhu MX, Ma X. Retinoic acid downregulates microRNAs to induce abnormal development of spinal cord in spina bifida rat model. Childs Nerv. Syst. 2008;24:485–492. doi: 10.1007/s00381-007-0520-5. [DOI] [PubMed] [Google Scholar]
- 72.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. Rna. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greco SJ, Rameshwar P. MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. PNAS. 2007;104:15484–15489. doi: 10.1073/pnas.0703037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rem-bold M, Hausen H, Arendt D. Conserved sensory-neuro-secretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 75.Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat. Neurosci. 2008;11:641–648. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 76.Li Y, Wang F, Lee JA, Gao FB. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev. 2006;20:2793–2805. doi: 10.1101/gad.1466306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukuda Y, Kawasaki H, Taira K. Exploration of human miRNA target genes in neuronal differentiation. Nucleic Acids Symp. Ser. (Oxf.) 2005;2005:341–342. doi: 10.1093/nass/49.1.341. [DOI] [PubMed] [Google Scholar]
- 78.Kawasaki H, Taira K. Hes1 is a target of microRNA-23 during retinoic-acid-induced neuronal differentiation of NT2 cells. Nature. 2003;423:838–842. doi: 10.1038/nature01730. [DOI] [PubMed] [Google Scholar]
- 79.Kye MJ, Liu T, Levy SF, Xu NL, Groves BB, Bonneau R, Lao K, Kosik KS. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. Rna. 2007;13:1224–1234. doi: 10.1261/rna.480407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum. Mol. Genet. 2008;17:3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–749. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao X, Pfaff SL, Gage FH. A functional study of miR-124 in the developing neural tube. Genes Dev. 2007;21:531–536. doi: 10.1101/gad.1519207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol. Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol. Cell. Biol. 2005;25:9198–9208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. PNAS. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 87.Nomura T, Kimura M, Horii T, Morita S, Soejima H, Kudo S, Hatada I. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum. Mol. Genet. 2008;17:1192–1199. doi: 10.1093/hmg/ddn011. [DOI] [PubMed] [Google Scholar]
- 88.Choi PS, Zakhary L, Choi WY, Caron S, Alvarez-Saavedra E, Miska EA, McManus M, Harfe B, Giraldez AJ, Horvitz RH, Schier AF, Dulac C. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christoffersen NR, Silahtaroglu A, Orom UA, Kauppinen S, Lund AH. miR-200b mediates post-transcriptional repression of ZFHX1B. Rna. 2007;13:1172–1178. doi: 10.1261/rna.586807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deo M, Yu JY, Chung KH, Tippens M, Turner DL. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev. Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- 91.Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- 92.Niwa R, Zhou F, Li C, Slack FJ. The expression of the Alzheimer's amyloid precursor protein-like gene is regulated by developmental timing microRNAs and their targets in Caenorhabditis elegans. Dev. Biol. 2008;315:418–425. doi: 10.1016/j.ydbio.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, Lee JC, Saunders AJ. MicroRNAs can regulate human APP levels. Mol. Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hebert SS, Horre K, Nicolai L, Bergmans B, Papadopoulou AS, elacourte A, De Strooper B. MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol. Dis. 2009 doi: 10.1016/j.nbd.2008.11.009. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 95.Boissonneault V, Plante I, Rivest S, Provost P. MicroRNA-298 and microRNA-328 regulate expression of mouse beta -amyloid precursor protein converting enzyme 1. J. Biol. Chem. 2009;284:1971–1981. doi: 10.1074/jbc.M807530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mande-makers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. PNAS. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang GL, Huang QW, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18:297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 99.Cogswell JP, Ward J, Taylor IA, Waters M, Shi YL, Cannon B, Kelnar K, Kemppainen D, Brown C, Chen RK, Prinjha JC, Richardson AM, Saunders AD, Roses J, Richards CA. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Disease. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 100.Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J. Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson R, Zuccato C, Belyaev ND, Guest DJ, Cattaneo E, Buckley NJ. A microRNA-based gene dysregulation pathway in Huntington's disease. Neurobiol. Dis. 2008;29:438–445. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, Hengerer B. MicroRNA and proteome expression profiling in early-symptomatic alpha-synuclein (A30P)-transgenic mice. Proteomics Clin Applications. 2008;2:697–705. doi: 10.1002/prca.200780025. [DOI] [PubMed] [Google Scholar]
- 103.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saba R, Goodman CD, Huzarewich RL, Robertson C, Booth SA. A miRNA signature of prion induced neurodegeneration. PLoS ONE. 2008;3:e3652. doi: 10.1371/journal.pone.0003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuhn DE, Nuovo GJ, Martin MM, Malana GE, Pleister AP, Jiang J, Schmittgen TD, Terry Jr AV, Gardiner K, Head E, Feldman DS, Elton TS. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem. Biophys. Res. Commun. 2008;370:473–477. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J. Inorg. Biochem. 2007;101:1265–1269. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lukiw WJ, Zhao Y, Cui JG. An NF-kB-sensitive microRNA-146a-mediated inflammatory circuit in Alzheimer's disease and in stressed human brain cells. J. Biol. Chem. 2008;283:31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I, Cairns MJ. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 110.Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- 111.Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31:20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bilen J, Liu N, Bonini NM. A new role for microRNA pathways: modulation of degeneration induced by pathogenic human disease proteins. Cell Cycle. 2006;5:2835–2838. doi: 10.4161/cc.5.24.3579. [DOI] [PubMed] [Google Scholar]
- 113.Bushati N, Cohen SM. microRNAs in neurodegeneration. Curr. Opin. Neurobiol. 2008;18:292–296. doi: 10.1016/j.conb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Nelson PT, Wang WX, Rajeev BW. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008;18:130–138. doi: 10.1111/j.1750-3639.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barbato C, Giorgi C, Catalanotto C, Cogoni C. Thinking about RNA? MicroRNAs in the brain. Mamm. Genome. 2008;19:541–551. doi: 10.1007/s00335-008-9129-6. [DOI] [PubMed] [Google Scholar]
- 116.Kosik KS. The neuronal microRNA system. Nat. Rev. Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 117.Kosik KS, Krichevsky AM. The Elegance of the MicroRNAs: A Neuronal Perspective. Neuron. 2005;47:779–782. doi: 10.1016/j.neuron.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 118.Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim. Biophys. Acta. 2008;1779:471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 119.Bicker S, Schratt G. microRNAs: Tiny Regulators of Synapse Function in Development and Disease. J. Cell. Mol. Med. 2008;12:1466–1476. doi: 10.1111/j.1582-4934.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng Y. Regulation of the mammalian nervous system by microRNAs. Mol. Pharmacol. 2009;75:259–264. doi: 10.1124/mol.108.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klein ME, Impey S, Goodman RH. Role reversal: the regulation of neuronal gene expression by microRNAs. Curr. Opin. Neurobiol. 2005;15:507–513. doi: 10.1016/j.conb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 122.Mehler MF, Mattick JS. Non-coding RNAs in the nervous system. J. Physiol. 2006;575:333–341. doi: 10.1113/jphysiol.2006.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu. Rev. Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 124.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, Krichevsky A, Grad Y, Hayes GD, Kosik KS, Church GM, Ruvkun G. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. PNAS. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 128.Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 131.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 132.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. PNAS. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J, Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen J, Kelleher RJ. The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. PNAS. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang B, Shankaranarayana Rao S, Chattarji S, Kelleher RJ, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 137.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 2008;283:21310–21314. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 139.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14:1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell. Biol. 2008;10:987–993. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 141.Tokumaru S, Suzuki M, Yamada H, Nagino M, Takahashi T. let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis. 2008;29:2073–2077. doi: 10.1093/carcin/bgn187. [DOI] [PubMed] [Google Scholar]
- 142.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell. Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nat. Cell. Biol. 2004;6:1048–10453. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- 144.Berdnik D, Fan AP, Potter CJ, Luo L. MicroRNA Processing Pathway Regulates Olfactory Neuron Morphogenesis. Curr. Biol. 2008;18:1754–1759. doi: 10.1016/j.cub.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol. Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 146.Schaefer A, O'Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, McManus MT. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. PNAS. 2008;105:5614–5619. doi: 10.1073/pnas.0801689105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Savas JN, Makusky A, Ottosen S, Baillat D, Then F, Krainc D, Shiekhattar R, Markey SP, Tanese N. Huntington's disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies. PNAS. 2008;105:10820–10825. doi: 10.1073/pnas.0800658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Targets CNS Neurol. Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 151.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–12781. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 152.Marambaud P, Robakis NK. Genetic and molecular aspects of Alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 2005;4:134–146. doi: 10.1111/j.1601-183X.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 153.Hawkes C. Olfaction in neurodegenerative disorder. Adv. Otorhinolaryngol. 2006;63:133–151. doi: 10.1159/000093759. [DOI] [PubMed] [Google Scholar]
- 154.Fuchs J, Mueller JC, Lichtner P, Schulte C, Munz M, Berg D, Wullner U, Illig T, Sharma M, Gasser T. The transcription factor PITX3 is associated with sporadic Parkinson's disease. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.08.014. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 155.Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am. J. Hum. Genet. 2008;82:283–289. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Andreassen OA, Djurovic S, Melle I, Agartz I, Hall H, Timm S, Wang AG, Werge T. Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE. 2007;2:e873. doi: 10.1371/journal.pone.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, Davis NR, Ercan-Sencicek AG, Guez DH, Spertus JA, Leckman JF, S.t. Dure L, Kurlan R, Singer HS, Gilbert DL, Farhi A, Louvi A, Lifton RP, Sestan N, State MW. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 158.Cheon MS, Dierssen M, Kim SH, Lubec G. Protein expression of BACE1, BACE2 and APP in Down syndrome brains. Amino Acids. 2008;35:339–343. doi: 10.1007/s00726-007-0618-9. [DOI] [PubMed] [Google Scholar]
- 159.Liu F, Liang Z, Wegiel J, Hwang YW, Iqbal K, Grundke-Iqbal I, Ramakrishna N, Gong CX. Overexpression of Dyrk1A contributes to neurofibrillary degeneration in Down syndrome. FASEB J. 2008;22:3224–3233. doi: 10.1096/fj.07-104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Canzonetta C, Mulligan C, Deutsch S, Ruf S, O'Doherty A, Lyle R, Borel C, Lin-Marq N, Delom F, Groet J, Schnappauf F, De Vita S, Averill S, Priestley JV, Martin JE, Shipley J, Denyer G, Epstein CJ, Fillat C, Estivill X, Tybulewicz VL, Fisher EM, Antonarakis SE, Nizetic D. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome. Am. J. Hum. Genet. 2008;83:388–400. doi: 10.1016/j.ajhg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mann DM. The pathogenesis and progression of the pathological changes of Alzheimer's disease. Ann. Med. 1989;21:133–136. doi: 10.3109/07853898909149200. [DOI] [PubMed] [Google Scholar]
- 162.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 163.Maes OC, Xu S, Yu B, Chertkow HM, Wang E, Schipper HM. Transcriptional profiling of Alzheimer blood mononuclear cells by microarray. Neurobiol. Aging. 2007;28:1795–1809. doi: 10.1016/j.neurobiolaging.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 164.Pasinetti GM. Use of cDNA Microarray in the Search for Molecular Markers Involved in the Onset of Alzheimer’s Disease Dementia. J. Neurosci. Res. 2001;65:471–476. doi: 10.1002/jnr.1176. [DOI] [PubMed] [Google Scholar]
- 165.Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ, Schaller C, Bujo H, Levey AI, Lah JJ. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 2004;61:1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 166.Keller JN. Interplay Between Oxidative Damage, Protein Synthesis, and Protein Degradation in Alzheimer’s Disease. J. Biomed. Biotech. 2006;2006:1–3. doi: 10.1155/JBB/2006/12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Puchades M, Hansson SF, Nilsson CL, Andreasen N, Blennow K, Davidsson P. Proteomic studies of potential cerebrospinal fluid protein markers for Alzheimer's disease. Brain Res. Mol. Brain Res. 2003;118:140–146. doi: 10.1016/j.molbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 168.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat. Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 169.Maes OC, An J, Sarojini H, Wang E. Murine microRNAs implicated in liver functions and aging process. Mech. Ageing Dev. 2008;129:534–541. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 170.Ibanez-Ventoso C, Yang M, Guo S, Robins H, Padgett RW, Driscoll M. Modulated microRNA expression during adult lifespan in Caenorhabditis elegans. Aging Cell. 2006;5:235–246. doi: 10.1111/j.1474-9726.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 171.Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, Irizarry MC, Hyman BT. Expression of APP pathway mRNAs and proteins in Alzheimer's disease. Brain Res. 2007;1161:116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 172.Ikeda K, Urakami K, Arai H, Wada K, Wakutani Y, Ji Y, Adachi Y, Okada A, Kowa H, Sasaki H, Ohno K, Ohtsuka Y, Ishikawa Y, Nakashima K. The expression of presenilin 1 mRNA in skin fibroblasts and brains from sporadic Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2000;11:245–250. doi: 10.1159/000017246. [DOI] [PubMed] [Google Scholar]
- 173.Ohyagi Y, Asahara H, Chui DH, Tsuruta Y, Sakae N, Miyoshi K, Yamada T, Kikuchi H, Taniwaki T, Murai H, Ikezoe K, Furuya H, Kawarabayashi T, Shoji M, Checler F, Iwaki T, Makifuchi T, Takeda K, Kira J, Tabira T. Intracellular Abeta42 activates p53 promoter: a pathway to neurodegeneration in Alzheimer's disease. FASEB J. 2005;19:255–257. doi: 10.1096/fj.04-2637fje. [DOI] [PubMed] [Google Scholar]
- 174.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buggia-Prevot V, Sevalle J, Rossner S, Checler F. NFkappaB-dependent control of BACE1 promoter transactivation by Abeta42. J. Biol. Chem. 2008;283:10037–10047. doi: 10.1074/jbc.M706579200. [DOI] [PubMed] [Google Scholar]
- 176.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent 3rd G, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Satterlee JS, Barbee S, Jin P, Krichevsky A, Salama S, Schratt G, Wu DY. Noncoding RNAs in the brain. J. Neurosci. 2007;27:11856–11859. doi: 10.1523/JNEUROSCI.3624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang E. MicroRNA, the putative molecular control for mid-life decline. Ageing Res. Rev. 2007;6:1–11. doi: 10.1016/j.arr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 179.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 181.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toussaint O, Remacle J, Dierick JF, Pascal T, Frippiat C, Royer V, Magalhacs JP, Zdanov S, Chainiaux F. Stress-induced premature senescence: from biomarkers to likeliness of in vivo occurrence. Biogerontology. 2002;3:13–17. doi: 10.1023/a:1015226524335. [DOI] [PubMed] [Google Scholar]
- 183.Migliore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, Nucciarone B, Siciliano G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol. Aging. 2005;26:567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 184.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruiz A, Cebrian ME. Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat. Res. 2008 doi: 10.1016/j.mrgentox.2008.09.021. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 186.Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–5247. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- 187.Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Res. 2006;66:10843–10848. doi: 10.1158/0008-5472.CAN-06-1894. [DOI] [PubMed] [Google Scholar]
- 188.Ross CM. Folate, mitochondria, ROS, and the aging brain. Am. J. Med. 2005;118(10):1174–1175. doi: 10.1016/j.amjmed.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 189.Reynolds EH. Folic acid, ageing, depression, and dementia. Bmj. 2002;324:1512–1515. doi: 10.1136/bmj.324.7352.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- 191.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. PNAS. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 193.Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, Casadesus G, Harris PL, Siedlak SL, Perry G. Vascular oxidative stress in Alzheimer disease. J. Neurol. Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gackowski D, Rozalski R, Siomek A, Dziaman T, Nicpon K, Klimarczyk M, Araszkiewicz A, Olinski R. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J. Neurol. Sci. 2008;266:57–62. doi: 10.1016/j.jns.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 195.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. PNAS. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kuzin A, Kundu M, Brody T, Odenwald WF. The Drosophila nerfin-1 mRNA requires multiple microRNAs to regulate its spatial and temporal translation dynamics in the developing nervous system. Dev. Biol. 2007;310:35–43. doi: 10.1016/j.ydbio.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, Gilmore BL, Burstein H, Peluso RW, Polisky B, Carter BJ, Davidson BL. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. PNAS. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maes OC, Chertkow HM, Wang E, Schipper HM. Methodology for discovery of Alzheimer disease blood-based biomarkers. J. Gerontol. A Biol. Sci. 2009 doi: 10.1093/gerona/glp045. (In press) [DOI] [PubMed] [Google Scholar]