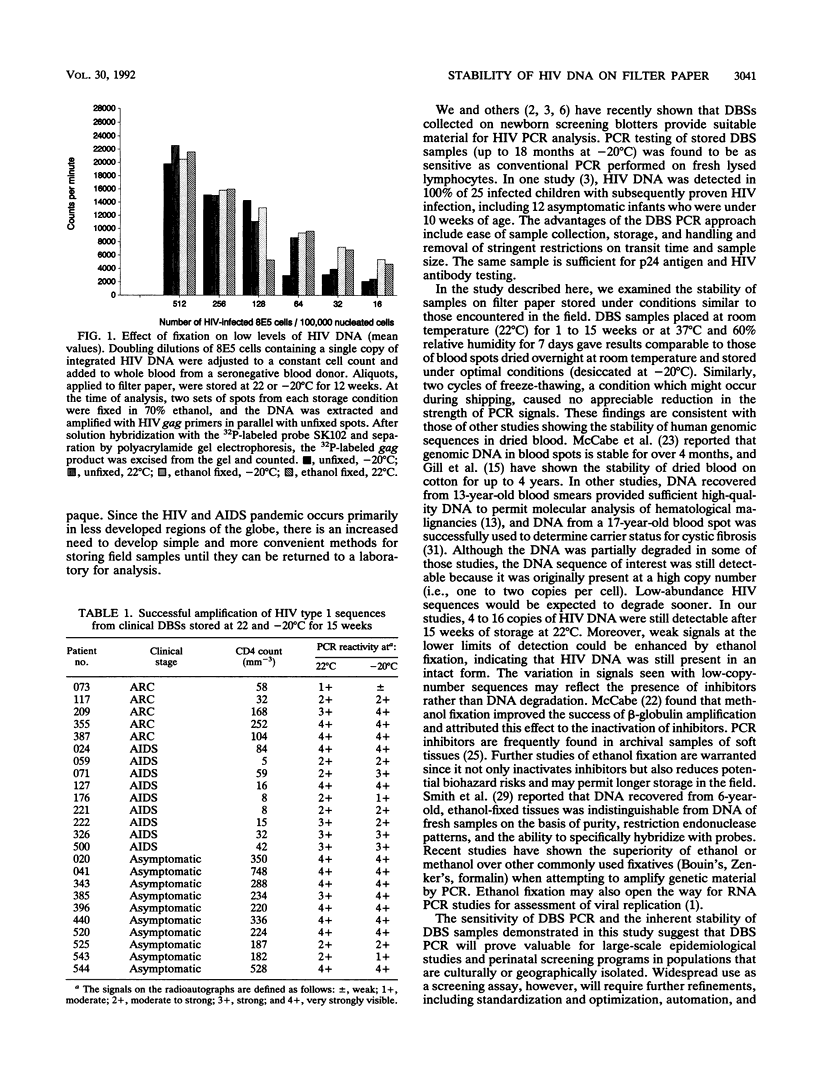
Abstract
Blood sampling on filter paper has many advantages for the detection of perinatal human immunodeficiency virus (HIV) infection by the polymerase chain reaction (PCR). However, if the method is to be widely used, an assessment of its performance under field conditions is required. To simulate conditions in the field, 50-microliters aliquots of whole blood containing low levels of HIV proviral DNA (4 to 1,024 copies per 100,000 nucleated cells) were spotted onto filter paper; dried; and subjected to heat, humidity, and prolonged storage at room temperature. After exposure, the DNA was recovered and amplified with primers to human leukocyte antigen DQ alpha- and HIV-specific sequences. Treatment at 37 degrees C and 60% humidity for 7 days, storage for 12 weeks at 22 degrees C, and freeze-thawing twice had no adverse effect on PCR reactivity when compared with the results obtained with reference spots stored at -20 degrees C. The lower limits of HIV detection in all tests ranged from 4 to 16 HIV copies per 100,000 cells. Fixation in 70% ethanol improved the amplification of low levels of HIV DNA and reduced biohazard risks. These findings suggest that dried blood spots will provide a powerful new resource for testing for HIV by PCR, especially in remote areas where refrigeration and immediate sample processing are unavailable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ezra J., Johnson D. A., Rossi J., Cook N., Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991 Mar;39(3):351–354. doi: 10.1177/39.3.1704393. [DOI] [PubMed] [Google Scholar]
- Cassol S. A., Lapointe N., Salas T., Hankins C., Arella M., Fauvel M., Delage G., Boucher M., Samson J., Charest J. Diagnosis of vertical HIV-1 transmission using the polymerase chain reaction and dried blood spot specimens. J Acquir Immune Defic Syndr. 1992;5(2):113–119. [PubMed] [Google Scholar]
- Cassol S., Salas T., Arella M., Neumann P., Schechter M. T., O'Shaughnessy M. Use of dried blood spot specimens in the detection of human immunodeficiency virus type 1 by the polymerase chain reaction. J Clin Microbiol. 1991 Apr;29(4):667–671. doi: 10.1128/jcm.29.4.667-671.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. Current and future dimensions of the HIV/AIDS pandemic in women and children. Lancet. 1990 Jul 28;336(8709):221–224. doi: 10.1016/0140-6736(90)91743-t. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Ulrich P. P., Weintrub P. S., Cowan M. J., Levy J. A., Wara D. W., Vyas G. N. Polymerase chain reaction compared with concurrent viral cultures for rapid identification of human immunodeficiency virus infection among high-risk infants and children. J Pediatr. 1989 Aug;115(2):200–203. doi: 10.1016/s0022-3476(89)80065-4. [DOI] [PubMed] [Google Scholar]
- Escaich S., Wallon M., Baginski I., Ritter J., Philippe N., Bertrand Y., Claris O., Raudrant D., Sepetjan M., Trepo C. Comparison of HIV detection by virus isolation in lymphocyte cultures and molecular amplification of HIV DNA and RNA by PCR in offspring of seropositive mothers. J Acquir Immune Defic Syndr. 1991;4(2):130–135. [PubMed] [Google Scholar]
- Evengård B., Ehrnst A., von Sydow M., Pehrson P. O., Lundbergh P., Linder E. Effect of heat on extracted HIV viral infectivity and antibody activity using the filter paper technique of blood sampling. AIDS. 1989 Sep;3(9):591–595. doi: 10.1097/00002030-198909000-00006. [DOI] [PubMed] [Google Scholar]
- Evengård B., Linder E., Lundbergh P. Standardization of a filter-paper technique for blood sampling. Ann Trop Med Parasitol. 1988 Jun;82(3):295–303. doi: 10.1080/00034983.1988.11812246. [DOI] [PubMed] [Google Scholar]
- Farzadegan H., Quinn T., Polk B. F. Detecting antibodies to human immunodeficiency virus in dried blood on filter papers. J Infect Dis. 1987 May;155(5):1073–1074. doi: 10.1093/infdis/155.5.1073. [DOI] [PubMed] [Google Scholar]
- Fey M. F., Pilkington S. P., Summers C., Wainscoat J. S. Molecular diagnosis of haematological disorders using DNA from stored bone marrow slides. Br J Haematol. 1987 Dec;67(4):489–492. doi: 10.1111/j.1365-2141.1987.tb06174.x. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P., Jeffreys A. J., Werrett D. J. Forensic application of DNA 'fingerprints'. Nature. 1985 Dec 12;318(6046):577–579. doi: 10.1038/318577a0. [DOI] [PubMed] [Google Scholar]
- Gwinn M., Pappaioanou M., George J. R., Hannon W. H., Wasser S. C., Redus M. A., Hoff R., Grady G. F., Willoughby A., Novello A. C. Prevalence of HIV infection in childbearing women in the United States. Surveillance using newborn blood samples. JAMA. 1991 Apr 3;265(13):1704–1708. [PubMed] [Google Scholar]
- Hankins C. A., Laberge C., Lapointe N., Lai Tung M. T., Racine L., O'Shaughnessy M. HIV infection among Quebec women giving birth to live infants. CMAJ. 1990 Nov 1;143(9):885–893. [PMC free article] [PubMed] [Google Scholar]
- Hoff R., Berardi V. P., Weiblen B. J., Mahoney-Trout L., Mitchell M. L., Grady G. F. Seroprevalence of human immunodeficiency virus among childbearing women. Estimation by testing samples of blood from newborns. N Engl J Med. 1988 Mar 3;318(9):525–530. doi: 10.1056/NEJM198803033180901. [DOI] [PubMed] [Google Scholar]
- Krivine A., Yakudima A., Le May M., Pena-Cruz V., Huang A. S., McIntosh K. A comparative study of virus isolation, polymerase chain reaction, and antigen detection in children of mothers infected with human immunodeficiency virus. J Pediatr. 1990 Mar;116(3):372–376. doi: 10.1016/s0022-3476(05)82823-9. [DOI] [PubMed] [Google Scholar]
- McCabe E. R., Huang S. Z., Seltzer W. K., Law M. L. DNA microextraction from dried blood spots on filter paper blotters: potential applications to newborn screening. Hum Genet. 1987 Mar;75(3):213–216. doi: 10.1007/BF00281061. [DOI] [PubMed] [Google Scholar]
- McCabe E. R. Utility of PCR for DNA analysis from dried blood spots on filter paper blotters. PCR Methods Appl. 1991 Nov;1(2):99–106. doi: 10.1101/gr.1.2.99. [DOI] [PubMed] [Google Scholar]
- Nielsen C. M., Bygbjerg I. C., Vestergaard B. F. Detection of HIV antigens in eluates from whole blood collected on filterpaper. Lancet. 1987 Mar 7;1(8532):566–567. doi: 10.1016/s0140-6736(87)90213-3. [DOI] [PubMed] [Google Scholar]
- Päbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1939–1943. doi: 10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. F., Ou C. Y., Rayfield M., Thomas P. A., Schoenbaum E. E., Abrams E., Krasinski K., Selwyn P. A., Moore J., Kaul A. Use of the polymerase chain reaction for early detection of the proviral sequences of human immunodeficiency virus in infants born to seropositive mothers. New York City Collaborative Study of Maternal HIV Transmission and Montefiore Medical Center HIV Perinatal Transmission Study Group. N Engl J Med. 1989 Jun 22;320(25):1649–1654. doi: 10.1056/NEJM198906223202503. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Nsa W., Hassig S. E., Behets F., Rayfield M., Ekungola B., Nelson A. M., Mulenda U., Francis H., Mwandagalirwa K. Perinatal transmission of the human immunodeficiency virus type 1 to infants of seropositive women in Zaire. N Engl J Med. 1989 Jun 22;320(25):1637–1642. doi: 10.1056/NEJM198906223202501. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Braylan R. C., Nutkis J. E., Edmundson K. B., Downing J. R., Wakeland E. K. Extraction of cellular DNA from human cells and tissues fixed in ethanol. Anal Biochem. 1987 Jan;160(1):135–138. doi: 10.1016/0003-2697(87)90623-3. [DOI] [PubMed] [Google Scholar]
- Weintrub P. S., Ulrich P. P., Edwards J. R., Boucher F., Levy J. A., Cowan M. J., Vyas G. N. Use of polymerase chain reaction for the early detection of HIV infection in the infants of HIV-seropositive women. AIDS. 1991 Jul;5(7):881–884. doi: 10.1097/00002030-199107000-00014. [DOI] [PubMed] [Google Scholar]
- Williams C., Weber L., Williamson R., Hjelm M. Guthrie spots for DNA-based carrier testing in cystic fibrosis. Lancet. 1988 Sep 17;2(8612):693–693. doi: 10.1016/s0140-6736(88)90512-0. [DOI] [PubMed] [Google Scholar]


