Abstract
Women outlive men, but life expectancy is not influenced by hormone replacement (estrogen + progestin) therapy. Estrogens appear to protect brain, cardiovascular tissues, and bone from aging. Estrogens regulate genes directly through binding to estrogen receptors alpha and beta (ERα and ERβ) that are ligand-activated transcription factors and indirectly by activating plasma membrane-associated ER which, in turns, activates intracellular signaling cascades leading to altered gene expression. MicroRNAs (miRNAs) are short (19-25 nucleotides), naturally-occurring, non-coding RNA molecules that base-pair with the 3’ untranslated region of target mRNAs. This interaction either blocks translation of the mRNA or targets the mRNA transcript to be degraded. The human genome contains ~ 700-1,200 miRNAs. Aberrant patterns of miRNA expression are implicated in human diseases including breast cancer. Recent studies have identified miRNAs regulated by estrogens in human breast cancer cells, human endometrial stromal and myometrial smooth muscle cells, rat mammary gland, and mouse uterus. The decline of estradiol levels in postmenopausal women has been implicated in various age-associated disorders. The role of estrogen-regulated miRNA expression, the target genes of these miRNAs, and the role of miRNAs in aging has yet to be explored.
Key Words: MicroRNA, miRNA, estrogen, estrogen receptor.
INTRODUCTION
Women live longer than men. Estrogens (estradiol, estrone, and estriol) are steroid hormones that regulate development and homeostasis in a wide variety of tissues including the brain, reproductive tract, vasculature, and breast. Estradiol (E2) is synthesized in the ovary and is the primary estrogen in premenopausal women. Animal studies have shown that higher estrogen levels in females protect against aging by upregulating the expression of antioxidant, longevity-related genes, e.g., selenium-dependent glutathione peroxidase (GPx) and Mn-superoxide dismutase (Mn-SOD) [1], by protecting against stroke-related injury [2], by vasorelaxing effects [3], by direct myocardial protection [4], and by activating the insulin receptor substrate (IRS)-1 signaling pathway [5]. Women’s life expectancy seems not to be influenced by hormone replacement therapy (HRT = estrogens plus a progestin, usually conjugated equine estrogens and medoxyprogesterone acetate (MPA)) in postmenopausal women, but atherosclerosis and bone loss are considerably delayed. In addition, HRT protects against Alzheimer’s disease (AD) [6], perhaps by suppressing elevated gonadotropin levels, i.e., luteinizing hormone (LH), in postmenopausal women since elevated LH is thought to play a key role in AD pathogenesis [7]. Other age-associated impairments are also reduced by estrogen. For example, premenopausal women have a reduced risk for cataracts compared with men of the same age group and women in the Farmingham study who used estrogen replacement therapy (ERT) showed reduced risk for cataracts [8]. miRNAs are a class of naturally-occurring, small, non-coding RNA molecules that are related to, but distinct from, small interfering RNAs (siRNAs) which regulate mRNA translation or stability [9-11]. There are very few studies on the hormonal regulation of miRNAs expression. Select changes in microRNA (miRNA) expression correlate with diagnostic markers used in breast cancer therapies, e.g., estrogen receptor α (ERα) and tumor grade [12-22]. However, there are only 5 reports that E2 regulates miRNA expression that will be reviewed here. It is highly likely that hormones play a major role in regulating miRNAs by both genomic (transcriptional) and non-genomic mechanisms of action. Identification and characterization of estrogen-regulated miRNAs may provide new biomarkers and therapeutic targets in aging as well as in diseases including breast cancer.
Genomic ER Activities
Initiation of transcription is a complex event occurring through the cooperative interaction of multiple factors at the target gene promoter. I will use the term ER to refer to either ERα or ERβ or to both subtypes. I will refer to each subtype individually as pertinent to the known functions of these two proteins. Estrogen action is primarily mediated through binding to ER. ERα and ERβ are members of the steroid/nuclear receptor superfamily of proteins of which there are 48 members in mammals [23]. ERα and ERβ are highly conserved within the DNA binding domain (DBD, C domain), but differ in their N- and C- termini [24]. Structurally, ER has 6 domains lettered A-F from N- to C-terminus. ER is believed to be the ancestral steroid receptor originating 600-1200 million years ago, presumably because of the role of estrogens in reproduction and maturation [25, 26].
In the simplest model, the binding of estradiol (E2) via hydrogen bonding to residues within the ligand binding pocket of the ligand binding domain (LBD, E domain) results in conformational changes termed activation [27]. These conformational changes induced by E2 binding result in loosening of contact between the N-terminus and the C-terminus of ERα and exposes nuclear localization signals within the DNA binding domain (DBD, C domain) and hinge region (D domain) as well as altering structure of the LBD [28]. Crystal structure studies of the LBD of ERα excluding the F domain shows that the LBD has 12 alpha helices and E2 binding repositions helix 12 such that activation function-2 (AF-2) is exposed [29]. Helix 12 acts as a “switch” controlling accessibility of the coregulator interaction site. Ligand binding also facilitates ER dimerization (homodimerization or heterodimerization of ERα and ERβ).
E2-liganded ER interacts directly with a specific DNA sequence called the estrogen response element (ERE = 5’-AGGTCAnnnTGACCT-3’), historically located in the promoter region and currently established to be located at great distances from the transcription start site including in the 3’ flanking regions of target genes [30-34]. DNA binding increases ER interaction with basal transcription factors and coregulator proteins (reviewed in [35]). Fig. (1) depicts essential features of genomic ER action. EREs are enriched in genes upregulated by E2-ERα, at least in MCF-7 cells [36]. ERα can also be activated by phosphorylation of ser118 in the N-terminal A/B domain that activates activation function-1 (AF-1) in the absence of ligand binding [37]. At least in HeLa cells transfected with fluorescent fluorescent-tagged ERα (GFP- or CFP- ERα), E2 causes rapid intracellular and intranuclear movement of ERα to form punctuate nuclear speckles that appear to indicate ERα-nuclear matrix interaction [38-40]. In addition to direct ER-ERE binding, ER also activates transcription via a “tethering mechanism” in which ER interacts directly with transcription factors, e.g. Sp1 [41] and AP-1 [42], bound to their response elements. These DNA-protein and protein-protein interactions recruit coactivator/chromatin remodeling complexes resulting in histone modifications that lead to nucleosomal remodeling, increased accessibility to the DNA template for RNA polymerase II interaction, and increased target gene transcription (reviewed in [43-45]).
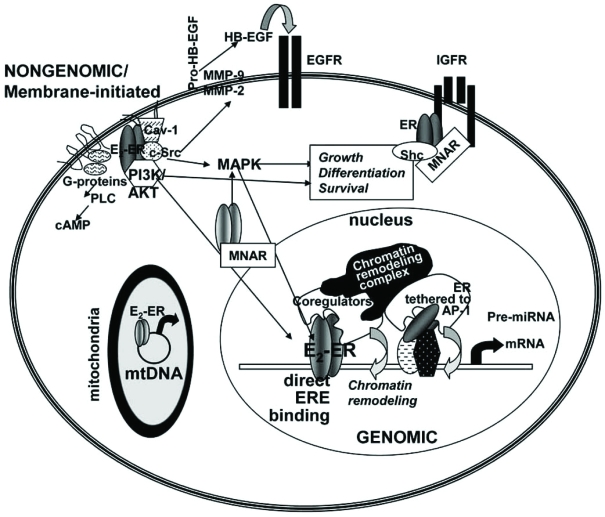
Fig. (1).
Cell model showing genomic and nongenomic activities of ERα and ERβ. ERα and ERβ are located in the cytoplasm and nucleus, bound to Caveolin-1 in caveolae in the plasma membrane and inside mitochondria. For genomic (nuclear) ER activity, E2 binds and activates ER resulting in dimerization, ERE binding or interaction with other transcription factors, e.g. AP-1 bound to DNA, coregulator and chromatin remodeling complex recruitment, chromatin remodeling, and increased transcription of target genes. For nongenomic/membrane-initiated estrogen signaling, E2 binds ERα in caveolae in the plasma membrane [112, 215]. ERα interacts with G-proteins, the p85 subunit of PI3K, c-Src, and Cav-1 to initiate PI3K/AKT and MAPK signaling cascades [61, 216]. ERα interacts with MNAR [127] and Shc [89] in the cytoplasm. ERα interacts with the EGF- and IGF-1 receptors in plasma membranes. In mitochondria, ERβ interact with the D-loop of mtDNA [217, 218].
By definition, coactivators are proteins that interact directly with transcription factors to enhance transcription [46]. It is important to note that the term coactivator or corepressor is used when referring to an ER (or other NR) coregulator is gene-, cell-type, and context- specific [47]. This indicates that proteins classified as coactivators can also repress transcription and corepressors such as SMRT are gene- and cell- specific coactivators for ER [48]. Coactivators promote the assembly of the transcription initiation complex in part by altering chromatin structure and ‘loosening’ DNA-histone interactions, facilitated by increased histone lysine residue acetylation, methylation, ubiquitination, or sumoylation [49]. Once the transcription initiation complex is complete, RNA polymerase II (RNA pol II) is recruited to the transcription start site and begins transcription. By my count, at least 60 different ER coactivators and 23 corepressors have been functionally identified (reviewed in [43, 50, 51], see also http://www.nursa.org/index.cfm). The current model predicts that ERE-bound, agonist-liganded ER recruits coactivator proteins to enhance gene expression [52]. In contrast for those genes at which tamoxifen (TAM) is an antagonist of ER transactivation, the LBD of TAM- occupied ERα does not interact with coactivators due to key conformational differences between agonist and antagonist – occupied ERα in AF-2 [53]. TAM-occupied ERα interacts with corepressors, e.g. NCoR or SMRT [54-58], that recruit histone deacetylase (HDAC) complexes thus keeping chromatin condensed and blocking transcriptional activation. Antiestrogen ICI 182,780 (Fulvestrant)-occupied ERα is targeted to the 26S proteasome for degradation [40, 59]. In contrast, although ICI 182,780 inhibits ERβ-mediated transcription, it stabilizes the ERβ protein [60].
Nongenomic Estrogen Action
In addition to its classical genomic/transcriptional effects mediated by ER-DNA interaction, described above, E2 has rapid “nongenomic, extra-nuclear, or membrane-initiated” effects that occur very rapidly, i.e., within seconds-minutes after E2 administration [61-64]. These effects are independent and distinct from the genomic, i.e., ER-mediated transcription, activities reviewed in the preceding section. Fig. (1) highlights some of the established nongenomic activities of ER. Rapid estrogen-stimulated intracellular activities are mediated by plasma membrane (PM)-associated ER and/or by an ‘orphan’ G-protein coupled receptor GPR30 [65]. Evidence of a PM-associated ER population is supported by experiments in which a cell-impermeable E2–bovine serum albumin or other E2-conjugate was shown to rapidly initiate intracellular kinase cascade activities including MAPK/ERK (p42/p44 MAPKs), endothelial nitric oxide synthase (eNOS), and PI3K/AKT [66-75]. In MCF-7 cells, E2 rapidly increased PIP2-phospholipase C activity [76], mobilized intracellular Ca2+, activated MAPK [62, 77-96], and PI3K/AKT [87, 97-102]. Immunohistochemical techniques have visualized membrane ERα in a variety of cell types including endometrial and breast cancer cells [103], pituitary cells [104], and lung cancer cells/tumors [91]. ERβ was shown to be located in the PM of primary cortical neurons in recent confocal imaging studies [105]. Overall, the PM localization of ER appears to be cell type-specific.
Premenopausal women have a lower risk of developing cardiovascular disease [106, 107] and hypertension than men or post-menopausal women and estrogens are thought to be responsible for regulating peripheral resistance [108] as well as effects in the myocardium [106]. Many of the cardioprotective activities of E2 may be mediated by nongenomic signaling. Cumulative studies show that a subpopulation of intact ERα is associated with the endothelial PM and with caveolae [61]. Recent electron microscopy studies revealed nuclear, cytoplasmic, and plasma membrane localization of both ERα and ERβ in human umbilical vein endothelial cells (HUVEC) [109]. ERα [79, 110] has been shown to interact with caveolin-1 (Cav-1) which serves as a structural core for interaction of plasma-membrane-associated proteins including the α-subunit of G-proteins, Ha-Ras, Src-kinases, eNOS, epidermal growth factor (EGF) receptors, and some protein kinase-C isoenzymes [110]. E2-ERα interaction within caveolae leads to Gαi activation, MAPK and Akt signaling, and perturbation of the local Ca+2 environment, leading to eNOS phosphorylation and NO production [61]. Endothelial PM-associated ERα is coupled via a Gαi to MAPK and eNOS [111].
The mechanism by which ERα and its splice variant ERα46 localizes to the PM involves palmitoylation [112-115]. In addition, ER interaction with intracellular proteins is important in PM association. In MCF-7 cells, the adaptor proteins Shc shuttles ERα from the nucleus to the PM where ERα interacts with the IGF-1 receptor (IGF-1R) [89, 96]. Similarly, another scaffold protein, called MNAR (modulator of nongenomic activity of ER), has been implicated in ERα-cSrc interaction and MAPK signaling [85, 95, 98, 116-128]. A role for a membrane caveolae-localized ERα46 in rapid NO release via PI3K/Akt activated eNOS has been reported in endothelial cells [112, 123, 129, 130].
In addition to PM-associated ER, GPR30 has reported to serve as a membrane estrogen receptor because it binds E2 with high affinity (Kd = 2.7nM) and activates adenylate cyclase, thus increasing cAMP levels [70, 73, 131-134]. GPR30 is distinct from ERα and ERβ in that ICI 182,780 and tamoxifen also bind GPR30 with high affinity and mimic the effects of E2 [133]. Although the role of GPR30 in MCF-7 and SKBr3 breast cancer cells has been questioned [62], it appears likely that GPR30 is a bone fide membrane estrogen receptor in some cell types [135-141].
MicroRNAs (miRNAs)
Evidence from the Encyclopedia of DNA project (ENCODE) has revealed surprising new information about the human genome. For example, although the protein coding regions account for only 2% of the total DNA in the human genome, surprisingly, 80-93 % of the genome is “expressed” [142-144]. The transcribed RNAs are largely conserved between humans and mice suggesting that these noncoding RNAs (ncRNAs) have important functions. Evidence of the importance of the various types of ncRNAs was recently reviewed [145] and includes roles in cancer, diabetes, and coronary disease, all aging-associated disorders. Among the small ncRNAs are microRNAs (miRNAs). The importance of miRNAs is highlighted by the fact that the 2008 Albert Lasker Award for Basic Medical research was awarded to Drs. Victor Ambros [146] and Gary Ruvkun [147] who discovered and characterized the first miRNAs in C. elgans and Dr. David Baulcombe who discovered let-7 miRNA in plants [148] (see also http://www.laskerfoundation.org/).
miRNAs are a class of naturally-occurring, small, non-coding RNA molecules that are related to, but distinct from, small interfering RNAs (siRNAs) [9-11]. About half of miRNAs are expressed from introns of protein-coding transcripts and miRNAs have 5' and 3' sequence features that form boundaries including transcription start sites, CpG islands, and transcription factor binding recognition elements [149]. miRNAs may be differentially processed from the sense and antisense strands of the same hairpin RNA or transcripts from the same locus, thus expanding the number of miRNAs from a single genomic locus [145].
The pathway of mature miRNA biogenesis is depicted in Fig. (2). miRNA genes are mostly transcribed by RNA polymerase II into primary-micro-RNAs (pri-miRNAs) that are capped and polyadenylated [150]. Pri-microRNAs contain self-base-pairing stem-loop structure that is necessary for critical processing within the nucleus by Drosha, an endonuclease of the RNAse III family, and its cofactor DGCR8 into short (60 to 70 nt) imperfect hairpin structure precursor-miRNAs (pre-miRNAs) [151]. These pre-miRNAs are exported from the nucleus by exportin and Ran-GTP. Pre-miRNAs are processed by the cytoplasmic RNAse II enzyme Dicer to form mature ~22 nt transiently double-stranded miRNA duplexes that are transferred to Argonaute proteins (Ago1, Ago2, Ago3, and Ago4 [152]) in the RNA-induced silencing complex (RISC), leading to unwinding of the duplexes to form single stranded microRNAs. RISC guides RNA silencing with the miRNA binding either to the 3’ untranslated region (3’ UTR) or to the open reading frame (ORF) of its target mRNA [153-156]. Most commonly, because of imperfect complimentarity of the base pairing between the miRNA and the 3’UTR, the RISC complex causes translational repression by RISC interaction with eIF6 which prevents assembly of 80S ribosomal assembly [157] or by inhibition of translation [16]. Thus, miRNA-mRNA 3’UTR interaction results in a decrease in target protein, not mRNA. The 7 to 8 nucleotide region of basepairing between the 5’ end of the mature miRNA and the mRNA is called the ‘seed sequence’. Base pairing of the miRNA-RISC complex within the ORF requires almost perfect complimentarity for its mRNA target and the mRNA is either degraded or translation is blocked [150]. The miRNA-containing ribonucleoprotein particle (miRNP)-silenced mRNA is directed to the P-bodies, where the mRNA is either released from its inhibition upon a cellular signal and/or actively degraded [158]. Comparative genomics analyses have revealed that over 45,000 miRNA binding sites within human 3'UTRs that are conserved above background levels [159]. This number was reported to indicate that more than 60% of human protein-coding genes have been under selective pressure to maintain pairing to miRNAs [159]. Recent evidence indicates that miRNAs may also increase translation of select mRNAs in a cell cycle-dependent manner [160].
Fig. (2).
Model of miRNA biogenesis and function. Primary transcripts of microRNAs (pri-miRNAs) are transcribed by RNA polymerase II, processed by the RNAse III enzyme, Drosha and its cofactor DGCR8, to precursor microRNAs (pre-miRNAs) and are then exported from the nucleus by Exportin/RAN-GTP [150]. In the cytoplasm, pre-miRNAs are processed by the RNAse III enzyme, Dicer to form mature ~22 nt transiently double-stranded miRNA duplexes that are transferred to Argonaute proteins (Ago1, Ago2, Ago3, and Ago4 [152]) in the RNA-induced silencing complex (RISC), leading to unwinding of the duplexes to form single stranded miRNAs. The mature miRNAs bind either to the 3’ untranslated region (3’ UTR) or to the open reading frame (ORF) of its target mRNA [153-156]. Binding of miRNA/RISC complex with the 3’UTR causes translational repression [16]. Thus, miRNA-mRNA 3’UTR interaction results in a decrease in target protein, not mRNA.
miRNAs have been demonstrated to play important roles in regulating various cellular processes including replication, differentiation, and apoptosis [11, 155, 161-176]. Since each of these processes play a role in aging, it is reasonable to suggest that altered expression and function of miRNAs regulate aging. The role of miRNAs in aging is completely unexplored. The human genome contains > 700-1,200 miRNAs http://microrna.sanger.ac.uk/sequences/ [149] and miRNAs are expressed in a tissue-specific manner [177]. Each miRNA targets ~ 200 transcripts directly or indirectly [178], but the bone fide physiological targets of the vast majority of miRNAs is virtually unknown.
Altered miRNA Expression in Breast Cancer
The spectrum of miRNAs expressed in solid tumors, i.e., prostate, colon, stomach, pancreas, lung, and breast, is different from normal tissues [177]. Although the precise sequence of events leading to breast tumors is not understood, lifetime exposure to estrogens is widely accepted as a major risk factor for the development of breast cancer [179]. Some investigators have documented that E2 is carcinogenic in human breast epithelial cells [180-182]. However, epidemiological evidence disputing the carcinogenicity of E2 in humans has been published [183]. Surprisingly, there are no published studies evaluating the effect of E2 on global miRNA expression in breast cancer cells.
Aberrant patterns of miRNA expression have been reported in human breast cancer [12, 13, 18, 20, 21, 151, 162, 164-170, 176-178, 184-191] and recently reviewed [150]. The first miRNA study in breast cancer indicated differential expression of miRNAs in concordance with other well-established markers of breast cancer stage and patient prognosis including ERα and PR expression, tumor stage, number of positive lymph nodes, and vascular invasion [20]. Different miRNA expression profiles were also associated with ErbB2+ versus ER+ tumors [22]. More recently, patients whose breast tumors showed reduced miR-126, miR-206, or miR-335 were found to have reduced survival, regardless of ERα or ErbB2 status [18].
A number of genes involved in breast cancer progression have been identified by in silico analysis to be targets of miRNAs that are deregulated in breast cancer [192] and some have been experimentally proven. A recent study reported that miR-21 expression was reduced in breast tumors and that antisense to miR-21 suppressed MCF-7 breast cancer cell growth in vitro and as tumor xenografts in mice by regulating Bcl-2 [13]. Interestingly, we recently reported that overexpression of miR-21 in MCF-7 cells increased soft agar colony formation, reflecting increased tumorigenicity of these cells [193]. We demonstrated that miR-21 binds to a seed element in the 3'-UTR of the programmed cell death 4 (PDCD4) gene and reduces Pdcd4 protein expression [193].
The breast cancer oncogene/coactivator AIB1/SRC-3/NCOA3 is regulated by mir-17-5p and there is a reciprocal relationship between reduced miR-17-5p and increased AIB1 in breast cancer cells [194]. Overexpression of miR-17-5p reduced E2-stimulated proliferation of MCF-7 breast cancer cells, indicating a role for deregulation of miR-17-5p in breast cancer [194]. Overexpression of miR-125a and miR-125b decreased ERBB2 and ERBB3 mRNA and protein levels, inhibited phosphorylation of ERK1/2 and AKT, and inhibited the anchorage-independent growth of ERα-negative/ErbB2-overexpressing SKBR3 breast cancer cells [195]. ERα mRNA stability is negatively regulated by miR-206 in MCF-7 cells and miR-206 expression is higher in ERα negative MDA-MB-231 cells [162].
Estrogenic Regulation of miRNA Expression
A PubMed search for estrogen AND miRNA revealed 27 papers. However, in that list and in total there are, to my knowledge, only 6 studies in which miRNA regulation by E2 has been directly examined (see below). Indeed, although a software application that will retrieve all miRNA:mRNA functional pairs in an experimentally derived set of genes was recently developed and used to identify E2-regulated mRNA genes in breast cancer [196], this paper does not experimentally address miRNA changes regulated by E2.
E2 Regulation of miRNAs in Animal Studies
The effect of E2 in miRNA expression has been examined in zebrafish [197], August Copenhagen Irish (ACI) rats [198], and mouse splenocytes [199]. A recent review of miRNA expression in female mammalian reproductive tissues described transgenic and knockout mouse models and findings related to changes in miRNAs in the ovary and uterus in response to deletion of Dicer [200], LH, and during development (immature versus mature mice) [201]. Changes in miRNA expression in mouse uterus during implantation have been cataloged [202]. Importantly, the authors of this review concluded that the expression, regulation, and function of miRNAs within specific tissues and cells still needs to be determined [201].
A study of the effect of E2 on miRNA expression in the adult (3 mos) zebrafish male (Danio rerio) identified altered expression of 38 miRNAs in the whole body homogenates [197]. E2 was added to the aquariums at a final concentration of 5 µg/liter (18 nM) and although various times of treatment were analyzed, most miRNA changes in response to E2 were observed after 12 h. miRNAs were regulated by E2 in a tissue-specific manner with E2 downregulating miRNAs in the liver and increasing miRNA expression in the skin of the zebrafish. For example, miR-122 was decreased by E2 in skin, but increased in gills, intestine. and liver. Among the most up-regulated miRNAs were miR-196b and let-7h, and miR-130c and miR-101a were the most down-regulated. The authors identified Hoxb8a as a target of miR-196b and showed that E2, by increasing miR-196b, decreased Hoxb8a [197]. The authors concluded that miR-196b may serve as “a biomarker of exposure to environmental estrogens and endocrine-disrupting chemicals that fish may encounter in their aquatic environment” [197].
In another study, miRNA expression was analyzed after 6, 12, and 18 weeks of E2-induced mammary carcinogenesis in female ACI rats [198]. After 6 and 12 wks of E2 exposure, 15 miRNAs were down-regulated, e.g., miR-22, miR-99a, miR-106a, miR-127, miR-499, and 19 miRNAs were-up-regulated, e.g., miR-17-5p, miR-20a, miR-21, miR-129-3p, miR-106a, miR-22, and miR-127. By 18 wks of E2 treatment, the mammary glands were characterized by lobular involution and hyperplasia, and only 1 miRNA was down-regulated (miR-139) and 5 miRNAs were up-regulated (miR-20b, miR-21, miR-103, mir-107, miR-129-3p, and miR-148a). Genes targeted by three of the altered miRNAs were examined: miR-20a regulates E2F1, miR-106a regulates RBI, and miR-127 regulates BCL6. Western blot of mammary gland lysates after 12 wks of E2 showed that levels of RBI and E2F1 were decreased and BCL6 protein was increased, data that are in agreement with the increase miR-20a and miR-106a and the decrease in miR-127 detected [198].
E2 decreased miR-146a, miR 125a, miR-125b, let-7e, miR-126, miR-145, and miR-143 and increased miR-223, miR-451, miR-486, miR-148a, miR-18a, and miR-708 expression in mouse splenic lymphocytes [199]. Notably, transfection of cells with miR-146a decreased LPS-induced IFNγ. AS to miR-223 blocked LPS-induced IFNγ secretion in splenocytes from E2 treated mice. This is the first report on E2 regulation of miRNA expression in immune cells [199], but provided no mechanism by which E2 regulated these changes.
E2 Regulation of miRNAs in Human Cell Lines
E2 and the ERα-selective agonist 4,4',4''-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) [203] decreased miR-206 expression in MCF-7 cells whereas 2,3-bis(4-hydroxy-phenyl)propionitrile (DPN), an ERβ-selective agonist [204], increased miR-206, pointing to a regulatory loop [162]. Interestingly, miR-206 also reduced β-actin. The authors of this report called miR-206 a “tumor suppressor” and found that miR-206 was higher in ERα-negative MDA-MB-231 cells [162], offering a mechanism, in addition to ERα promoter methylation [205-209], for reducing ERα expression in MDA-MB-231 cells.
A study identifying miRNAs expressed in myometrial and leiomyoma smooth muscle cells (MSMC and LSMC) using microarray and real time PCR reported that E2 inhibited the expression of miR-21 in MSMC and LSMC, whereas E2 increased and inhibited miR-26a in MSMC and LSMC, respectively [210]. In contrast, ICI 182,780 increased the expression of miR-20a and miR-21 in MSMC and LSMC, and miR-26a in MSMC, while inhibiting the expression of miR-26a in LSMC [210]. No mechanistic studies or mRNA target gene studies were performed to identify the mechanism(s) involved in these cell-specific differences in miRNA regulation by E2 and ICI or their downstream targets.
To identify E2 regulated miRNAs in a classical estrogen-responsive human breast cancer cell line, we treated ERα-positive MCF-7 cells with 10nM E2 or EtOH (vehicle control) for 6 h to identify primary E2 target miRNAs. RNA was harvested, labeled either with Cy3 or Cy5, and hybridized with two identical, dual-color miRNA microarrays from LC Sciences. This array contained probes to detect mature miRNA sequences as well as precursor (pre)-miRNAs in the Sanger miRNA registry 7.0 (http://microrna.sanger.ac.uk/ sequences/). The differentially expressed transcripts that were consistent on both chips are summarized in Table 1. 38 miRNA genes were regulated by E2: 9 were reduced and 29 were increased. A summary about what is known about each of these E2-responsive miRNAs in terms of breast cancer and estrogen-responsiveness is included in Table 1.
Table 1. Comparison of miRNA Expression in MCF-7 Cells Treated for 6 h with E2Versus EtOH.
MCF-7 cells were grown in dextran-coated charcoal-stripped, phenol red free IMEM medium for 48 h prior to a 6 h treatment with ethanol (EtOH, vehicle control) or 10 nM E2. RNA was isolated using miRVana and sent to LC Sciences for miRNA microarray analysis. All miRNA gene changes included in this table are statistically significant as analyzed by LC Sciences. Negative values indicate decreased expression and positive values indicate increased expression with E2. NS = no significant change in miR gene expression with E2-treatment.
| miRNA | Log 2 (E2/EtOH) | Comments Regarding the Possible Connection of the Identified miRNA Gene with Breast Cancer and/or Estrogenic Responses. Bone Fide Targets of miRNAs are Indicated |
|---|---|---|
| let-7a | -0.3 | Expressed in ERα positive human breast tumors [22]. Expression was higher in ERα+ than ERα- tumors [12]. Expressed in ZR-75, MCF-7, BT474, SK-BR-3, and MDA-MB-231 breast cancer cells [219]. Expression of Let-7 family members is reduced in ‘breast cancer stem cells’(CD44+CD24−/low) [220]. Targets of let 7 are Ras [170] and Caspase-3 [221]. |
| let-7cc | 0.3 | Expression was higher in PR+ versus PR- human breast tumors [22] and in ERα+ than ERα- tumors [12]. let-7c increased TRAIL-induced caspase 3 activation in MDA-MB-453 breast cancer cells and let-7c was predicted to target CD95L [222]. |
| let-7dd | 0.25 | let-7d was increased in acute promyelocytic leukemia patients[223]. |
| let-7f | -0.25 | let-7f was > in node negative versus positive human breast tumors [22] and higher in ERα+ tumors [12]. let-7f in mammary gland was reduced by E2 treatment of female ACI rats [198]. |
| let-7g | 1.66 | let-7g was expressed in ErbB2 positive human breast tumors [22]. |
| let-7i | 1.34 | |
| miR-106b | 1.14 | Higher in PR+ than PR– tumors, but higher in ERα- than ERα+ breast tumors [12]. miR-106b is overexpressed in breast tumors compared to normal breast and miR-106b reduced p21 mRNA and protein and thus stimulates G1-S cell cycle progression in human mammary epithelial cells [224]. miR-106b in mammary gland was increased by 6 wks of E2 treatment of female ACI rats [198]. |
| miR-149 | -3.17 | miR-149 in mammary gland was increased by 6 wks of E2 treatment of female ACI rats [198]. |
| miR-15a | 2.32 | Higher in low versus high tumor stage in human breast tumors [20] and greater in ERα+ than ERα- tumors [22]. Higher in PR+ than PR- tumors [22]. miR-15a is a tumor suppressor [225]. miR-15a negatively regulates Bcl2 at a posttranscriptional level [226]. |
| miR-15b | 1.13 | |
| miR-151 | 0.27 | Higher in ERα+/lymph node negative breast tumors from patients with a short time to distant metastasis (TDM) versus those with a long TDM [15]. |
| miR-16 | 0.79 | Higher in ERα+ than ERα- tumors [22]. miR-16-1 negatively regulates Bcl2 at a posttranscriptional level [226]. The miR-16 family negatively regulates cell cycle progression by inducing G0/G1-cell accumulation [227] by reducing CCND1 (cyclin D1), CCND3, CCNE1, and CDK6 [228]. |
| miR-182 | 0.83 | Higher in ERα+ than ERα- human breast tumors and not significantly higher in ErbB2 –vs. positive tumors [22] significantly higher in PR+ vs. PR– tumors [22]. miR-182 inhibited TRAIL-induced caspase 3 activation in MDA-MB-453 breast cancer cells and miR-182 was predicted to target caspase 3 and FADD [222]. |
| miR-183 | 0.98 | |
| miR-195 | 2 | Higher in ErbB2- vs. ErbB2 positive tumors [22], but not significantly > in ERα+ than ERα- or PR+ vs. PR– tumors [22]. miR-195 expression was increased by hypoxia in MCF-7 cells [229]. miR-195 inhibits CCND1,CCND3, CCNE1, and CDK6 protein expression [228]. |
| miR-200a | 2.58 | Correlated with ERα status in human breast tumors [22] and was significantly > in ERα+ than ERα- and PR+ than PRbreast tumors [22]. miR-200a is expressed in MCF-7 and other epithelial breast cancer cell lines [230]. miR-200 expression was reduced in tamoxifen-resistant MCF-7 cells [17]. |
| miR-200b | 0.7 | miR-200b is expressed in MCF-7 and other epithelial breast cancer cell lines [230]. |
| miR-200c | -0.42 | miR-200c is expressed in MCF-7 cells [166] and is higher than miR-200a or -200b in MCF-7 [230]. |
| miR-203 | 1.84 | Expression is increased in ovarian, breast and melanoma cancers [178]. Higher xpression in high versus low tumor stage in human breast tumors [20] and increased in tamoxifen-resistant MCF-7 cells [17]. |
| miR-20a | 0.83 | Increased in lung, breast, stomach, prostate, colon, and pancreatic tumors [177]. miR-20a expression was low in MCF-7 and other breast cancer cell lines and overexpression of the miR-17/20 locus in MCF-7 inhibited cell proliferation and cyclin D1 expression [19]. miR-20a was increased in mammary gland after 6 or 12 wks of E2 treatment of female ACI rats [198]. Both E2 and ICI decreased miR-20a in human endometrial stromal cells [231]. Targets of miR-20a are PCAF, RUNX1, and TGFBR2 [232]. |
| miR-21 | -0.14 | Significantly up-regulated in tissues or cell lines of breast cancer [20]. overexpressed in all solid tumors (lung, breast, stomach, prostate, colon, and pancreatic) [177]. significantly higher in ERα+ than ERα-, in ErbB2 - vs. ErbB2 +, and in PR+ vs. PR– breast tumors [22]. Expression was higher in breast tumor compared to adjacent normal breast tissue [233]. miR-21 expression was increased by hypoxia in MCF-7 [229]. miR-21 in mammary gland was increased after 18 wks of E2 treatment of female ACI rats [198]. Both E2 and ICI decreased miR-21 in human endometrial stromal and glandular epithelial cells, but when combined, miR-21 expression returned to basal [231]. E2 suppressed and ICI increased miR-21 in human human myometrial smooth muscle cells [210]. E2 inhibited the ICI-induced increase in miR-21 in these cells [210]. E2 (75% decrease) and Progesterone (41% decrease) reduced miR-21 expression on the uterus of ovex mice [202]. miR-21 expression was significantly reduced in tamoxifen-resistant MCF-7 cells [17]. |
| miR-23a | 0.31 | Increased by hypoxia in MCF-7 cells [229]; reduced in tamoxifen-resistant MCF-7 cells [17]. |
| miR-23b | 0.32 | Increased by hypoxia in MCF-7 cells [229]. |
| miR-25 | 1.6 | Higher in ERα+ than ERα- breast tumors [12] and PR+ vs. PR– breast tumors [22], but ot significantly higher in ErbB2-negative vs. positive breast tumors [22]. increased in ovarian, breast and melanoma cancers [178]. miR-25 was increased in mammary gland after 6 wks of E2 treatment of female ACI rats [198]. |
| miR-26a | 0.87 | Significantly > in ERα+ than ERα- breast tumors [20, 22]. significantly higher in ErbB2 – vs. ErbB2-positive breast tumors [22] and PR+ than PR- tumors [20]. miR-26a expression was increased by hypoxia in MCF-7 cells [229]. Both E2 and ICI increased miR-26a in human endometrial glandular epithelial cells, but when combined, miR-26a expression was suppressed below basal [231]. E2 and ICI increased miR-26a in human myometrial smooth muscle cells, but each inhibited miR-26a in human leiosarcoma cells [210]. |
| miR-26b | 2.07 | Higher in ERα+ than ERα- breast tumors [22], but mot significantly higher PR+ vs. PR–tumors [20]. Higher in ErbB2 –ve vs. positive breast tumors [22]. miR-26b expression was increased by hypoxia in MCF-7 cells [229]. |
| miR-27a | 1.73 | Higher in ErbB2- vs ErbB2+ and PR+ vs. PR- breast tumors [22], but not significantly higher in ER+ vs. ER– tumors [20]. miR-27a expression was increased by hypoxia in MCF-7 cells [229]. |
| miR-27b | 1.94 | Higher in ERα+ than ERα- breast tumors [22], in ErbB2-vs ErbB2+ tumors [22], and in PR+ vs. PR– tumors [22]. |
| miR-30b | 2.17 | Higher in node negative versus positive human breast tumors [20], higher in ERα+ than ERα-, in ErbB2 - vs ErbB+ and in PR+ vs. PR– breast tumors [22]. miR-30b expression was increased by hypoxia in MCF-7 cells [229]. |
| miR-320 | -0.84 | |
| miR-328 | -3.92 | Overexpression of miR-328 in A431 human epithelial carcinoma cells reduced cell adhesion, aggregation, and migration by repressing CD44 expression [234]. |
| miR-342 | -0.26 | Higher in ERα+ than ERα- ,in ErbB2 – vs. ErbB2+ , and in PR+ vs. PR– tumors [22]. miR-342 expression was reduced in tamoxifen-resistant MCF-7 cells [17]. |
| miR-365 | 1.47 | Decreased by E2 treatment in female ACI rat mammary gland [198]. |
| miR-423 | -1.49 | |
| miR-489 | 0.59 | Metastasis suppressor: decreased expression in metastatic sublines of MDA-MB-231[18]. miR-489 expression was reduced in tamoxifen-resistant MCF-7 cells [17]. |
| miR-7 | 1.84 | |
| miR-92 | 0.45 | Higher in ERα+ than ERα- and in PR+ vs. PR– breast tumors, but not higher in ErbB2- vs. ErbB2+ tumors [22]. miR-92 was increased in mammary gland after 6 wks of E2 treatment of female ACI rats [198]. miR-92 is in the miR-17/20 cluster and overexpression of the miR-17/20 cluster in MCF-7 cells inhibited basal and E2-stimulated cell proliferation and cyclin D1 transcription [19]. |
| miR-98 | 1.55 | Overexpressed in breast tumor compared to adjacent normal breast tissue [233]. |
miRNAs Regulating ER Expression or Activity
miRNAs can influence estrogen-regulated gene expression by directly reducing ERα mRNA stability or translation. Four miRNAs have been reported to reduce ERα protein levels (Fig. 3). Two miR-206 recognition sites were identified in the 3’UTR of ERα and transfection of an expression vector for miR-206 in MCF-7 cells reduced both mRNA and protein levels of ERα [162]. Treatment of MCF-7 cells with 1nM E2 or the ERα agonist PPT (10nM) reduced miR-206 levels by ~ 80%. In contrast the ERβ agonist DPN (10nM) increased miR-206 expression by ~ 60%. Interestingly, the investigators found that miR-206 levels were significantly higher in ERα-negative MDA-MB-231 cells than in MCF-7 cells, suggesting a mechanism for miR-206 in repressing ERα protein levels in MDA-MB-231 cells. The authors suggested that miR-206 may function in a mutually negative feedback loop to temporally regulate ERα expression and ductal/lobuloalveolar proliferation [162]. More recent studies showed that miR-206 is inversely correlated with ERα expression, but not ERβ, in human breast tumors [211]. Further, transfection of MCF-7 human breast cancer cells with an expression plasmid for pre-miR-206 reduced ERα mRNA expression ~ 25%, reduced the basal expression levels of PR, cyclin D1, and pS2 (all well-established ERα-regulated genes), and inhibited cell proliferation with or without E2 [211].
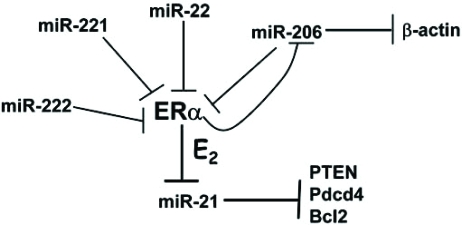
Fig. (3).
MiRNA regulation of ERα and ERα regulation of miRNA expression. MiRNAs that inhibit ERα protein expression are shown. E2-ER represses miR-206 [162] and miR-21 [202, 210] expression. Target genes of miR-21 are PTEN, Pdcd4, and Bcl2 [193]. MiR-206 also represses β-actin expression [162].
miR-221/222 was recently reported to be higher in ERα negative than ERα positive breast cancer cell lines and human breast tumors [212]. Two miR-221 and miR-222 seed elements were identified in the 3’UTR of ERα and transfection of miR-221 and miR-222 suppressed ERα protein, but not mRNA in ERα positive MCF-7 and T47D cells. Conversely, knockdown of miR-221 and miR-22 in ERα-negative MDA-MB-468 partially restored ERα protein expression and increased tamoxifen-induced apoptosis [212].
miR-22 regulates ERα protein expression in a pancreatic cancer cell line [213]. In a study to identify curcumin gene targets, curcumin increased miR-22 by 65% in BxPC-3 human pancreatic carcinoma cells [213]. One of the predicted 3’UTR gene targets of miR-22 was ESR1 (ERα) [213]. Follow-up studies showed that curcumin reduced ERα protein expression in BxPC-3 cells and that transfection of an antisense RNA oligonucleotide of miRNA-22 into BxPC-3 cells increased ERα protein by ~ 1.9-fold. Thus, miR-22 regulates ERα protein levels and the authors suggest a role for ERα as anti-tumorigenic in pancreatic cancer.
miRNAs can affect estrogen-regulated gene expression by reducing the expression of the coactivator SRC-3/AIB1/ NCOA3. miR-17-5p was demonstrated to inhibit translation of SRC-3/AIB1/NCOA3 [194]. Transfection of CHO-K1 cells with ERα and miR-17-5p inhibited E2-stimulated ERE-driven luciferase reporter activity by 50%. This report also demonstrated that transfection of MCF-7 cells (which do not express miR-17-5p) with miR-17-5p reduced E2-induced proliferation and E2-induced endogenous cyclin D1 transcription [194].
E2 Regulation of Ago2 and ERα in Human Breast Cancer Cell Lines
Argonaut-2 (Ago2), the catalytic subunit of the RISC complex that mediates miRNA-dependent cleavage/degradation in mammals [154, 170, 214], expression is higher in ERα-negative, HER2-positive (basal) than ERα-positive/HER2 negative (luminal) human breast cancer cell lines and tumors [14]. E2 and the ERα-agonist PPT, but not the ERβ-agonist DPN, increased Ago2 protein expression in MCF-7 cells [14]. Further studies showed that EGF acts through the MAPK pathway to increase Ago2 protein stability, but there were no studies examining the mechanism by which E2 and PPT, presumably through ERα, increase Ago2 protein levels. Surprisingly, Ago2 overexpression in MCF-7 cells increased ERα protein levels by 3-fold, despite also increasing miR-206 that reduces ERα. The authors concluded that this “discordant” finding indicates that there is a greater concentration of miRNAs than target proteins involved in ERα suppression than those that target ERα itself” [14].
CONCLUSION
Estrogen signaling plays a critical role in regulating reproduction, lactation, bone density, cardiovascular function, neuronal signaling, immune function, and homeostasis in a wide variety of tissues. The reduction in serum E2 in postmenopausal women is involved in a number of age-associated disorders. Research on the mechanisms by which E2 and other estrogens regulate diverse physiological effects has established both genomic and nongenomic mechanisms involving ERα, ERβ, and GPR30 in signal transduction (Fig. 1). miRNAs are small, non-coding RNAs that bind to the 3’ UTR of target mRNAs and either block the translation of the message or bind the ORF and target the mRNA transcript to be degraded. Although there are a number of studies identifying miRNA changes in breast tumors and comparing ERα-positive versus ERα-negative miRNA signatures for their potential use as biomarkers, there are few studies identifying E2-responsive miRNAs in any normal or neoplastic tissues or cell models. In those few studies that have identified E2-induced alterations in miRNA expression, there is little, if any, mechanistic detail elaborated for the E2 effect (s) on miRNA expression. Further, it appears that E2 regulates miRNA expression in a cell-type-dependent manner. Thus, identification of E2-regulated miRNAs and the function of miRNAs within specific tissues and cells still remains to be determined.
ACKNOWLEDGEMENT
Thanks to Dr. Nalinie S. Wickramasinghe who treated the MCF-7 cells and isolated the RNA for the miRNA microarray study included in Table 1. This study was supported by NIH R21 CA124811 to CMK.
REFERENCES
- 1.Vina J, Sastre J, Pallardo FV, Gambini J, Borras C. Modulation of longevity-associated genes by estrogens or phytoestrogens. Biol. Chem. 2008;389:273–277. doi: 10.1515/BC.2008.027. [DOI] [PubMed] [Google Scholar]
- 2.Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 3.Wynne FL, Payne JA, Cain AE, Reckelhoff JF, Khalil RA. Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats. Hypertension. 2004;43:405–412. doi: 10.1161/01.HYP.0000111833.82664.0c. [DOI] [PubMed] [Google Scholar]
- 4.Moolman JA. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc. Res. 2006;69:777–780. doi: 10.1016/j.cardiores.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Alonso A, Moreno M, Ordonez P, Fernandez R, Perez C, Diaz F, Navarro A, Tolivia J, Gonzalez C. Chronic estradiol treatment improves brain homeostasis during aging in female rats. Endocrinology. 2008;149:57–72. doi: 10.1210/en.2007-0627. [DOI] [PubMed] [Google Scholar]
- 6.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 7.Casadesus G, Rolston RK, Webber KM, Atwood CS, Bowen RL, Perry G, Smith MA. Menopause, estrogen, and gonadotropins in alzheimer's disease. Adv. Clin. Chem. 2008;45:139–153. doi: 10.1016/s0065-2423(07)00006-6. [DOI] [PubMed] [Google Scholar]
- 8.Worzala K, Hiller R, Sperduto RD, Mutalik K, Murabito JM, Moskowitz M, D'Agostino RB, Wilson PWF. Postmenopausal estrogen use, type of menopause, and lens opacities: the framingham studies. Arch. Intern. Med. 2001;161:1448–1454. doi: 10.1001/archinte.161.11.1448. [DOI] [PubMed] [Google Scholar]
- 9.Zamore PD, Haley B. Ribo-gnome: the big world of small rnas. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y. Principles of micro-rna production and maturation. Oncogene. 2006;25:6156–6162. doi: 10.1038/sj.onc.1209908. [DOI] [PubMed] [Google Scholar]
- 11.Couzin J. Genetics.Erasing microRNAs reveals their powerful punch. Science. 2007;316:530. doi: 10.1126/science.316.5824.530. [DOI] [PubMed] [Google Scholar]
- 12.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavare S, Caldas C, Miska EA. Microrna expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. Mir-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 14.Adams BD, Claffey KP, White BA. Argonaute-2 expression is regulated by EGFR/MAPK signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology. 2009;150(1):14–23. doi: 10.1210/en.2008-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foekens JA, Sieuwerts AM, Smid M, Look MP, de Weerd V, Boersma AWM, Klijn JGM, Wiemer EAC, Martens JWM. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc. Natl. Acad. Sci. USA. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowery AJ, Miller N, McNeill RE, Kerin MJ. MicroRNAs as prognostic indicators and therapeutic targets: potential effect on breast cancer management. Clin. Cancer Res. 2008;14:360–365. doi: 10.1158/1078-0432.CCR-07-0992. [DOI] [PubMed] [Google Scholar]
- 17.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27(kip1) J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z, Wang C, Wang M, Li Z, Casimiro MC, Liu M, Wu K, Whittle J, Ju X, Hyslop T, McCue P, Pestell RG. A cyclin d1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 2008;182:509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iorio MV, Ferracin M, Liu C-G, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol. Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT. Comparison of complete nuclear receptor sets from the human, caenorhabditis elegans and drosophila genomes. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-8-research0029. RESEARCH0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29:2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc. Natl. Acad. Sci. USA. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- 27.Tanenbaum DM, Wang Y, Williams SP, Sigler PB. Crystallographic comparison of the estrogen and progesterone receptor's ligand binding domains. Proc. Natl. Acad. Sci. USA. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metivier R, Stark A, Flouriot G, Hubner MR, Brand H, Penot G, Manu D, Denger S, Reid G, Kos M, Russell RB, Kah O, Pakdel F, Gannon F. A dynamic structural model for estrogen receptor-alpha activation by ligands, emphasizing the role of interactions between distant a and e domains. Mol. Cell. 2002;10:1019–1032. doi: 10.1016/s1097-2765(02)00746-3. [DOI] [PubMed] [Google Scholar]
- 29.Ruff M, Gangloff M, Wurtz JM, Moras D. Estrogen receptor transcription and transactivation: structure-function relationship in DNA- and ligand-binding domains of estrogen receptors. Breast Cancer Res. 2000;2:353–359. doi: 10.1186/bcr80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat. Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 31.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol. Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson J-A, Dahlman-Wright K. The genome landscape of er{alpha}- and er{beta}-binding DNA regions. Proceedings of the National Academy of Sciences. 2008. 0712085105. [DOI] [PMC free article] [PubMed]
- 33.Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon Y-S, Garcia-Bassets I, Hutt KR, Cheng CS, Jin M, Liu D, Benner C, Wang D, Ye Z, Bibikova M, Fan J-B, Duan L, Glass CK, Rosenfeld MG, Fu X-D. Sensitive chip-dsl technology reveals an extensive estrogen receptor {alpha}-binding program on human gene promoters. Proc. Natl. Acad. Sci. USA. 2007;104:4852–4857. doi: 10.1073/pnas.0700715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: Cellular and molecular biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 36.Bourdeau V, Deschenes J, Laperriere D, Aid M, White JH, Mader S. Mechanisms of primary and secondary estrogen target gene regulation in breast cancer cells. Nucleic Acids Res. 2008;36:76–93. doi: 10.1093/nar/gkm945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 38.Stenoien DL, Mancini MG, Patel K, Allegretto EA, Smith CL, Mancini MA. Subnuclear trafficking of estrogen receptor-alpha and steroid receptor coactivator-1. Mol. Endocrinol. 2000;14:518–534. doi: 10.1210/mend.14.4.0436. [DOI] [PubMed] [Google Scholar]
- 39.Stenoien DL, Nye AC, Mancini MG, Patel K, Dutertre M, O'Malley BW, Smith CL, Belmont AS, Mancini MA. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor alpha-coactivator complexes in living cells. Mol. Cell. Biol. 2001;21:4404–4412. doi: 10.1128/MCB.21.13.4404-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenoien DL, Patel K, Mancini MG, Dutertre M, Smith CL, O'Malley BW, Mancini MA. Frap reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat. Cell. Biol. 2001;3:15–23. doi: 10.1038/35050515. [DOI] [PubMed] [Google Scholar]
- 41.Porter W, Wang F, Wang W, Duan R, Safe S. Role of estrogen receptor/sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol. Endocrinol. 1996;10:1371–1378. doi: 10.1210/mend.10.11.8923463. [DOI] [PubMed] [Google Scholar]
- 42.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors eralpha and erbeta at ap1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 43.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 44.Lonard DM, O'Malley BW. Nuclear receptor coregulators: Judges, juries, and executioners of cellular regulation. Mol. Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz K, Jackson T, Bain D, Richer J, Takimoto G, Tung L. Nuclear receptor coactivators and corepressors. Mol. Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 47.O'Malley BW, McKenna NJ. Editorial: coactivators and corepressors: what's in a name? Mol. Endocrinol. 2008;22:2213–2214. doi: 10.1210/me.2008-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL. The silencing mediator of retinoic acid and thyroid hormone receptor (smrt) corepressor is required for full estrogen receptor {alpha} transcriptional activity. Mol. Cell. Biol. 2007;27:5933–5948. doi: 10.1128/MCB.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang XJ, Seto E. Hats and hdacs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 50.Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu. Rev. Physiol. 2004;66:315–360. doi: 10.1146/annurev.physiol.66.032802.155556. [DOI] [PubMed] [Google Scholar]
- 51.Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 52.Beato M, Candau R, Chavez S, Mows C, Truss M. Interaction of steroid hormone receptors with transcription factors involves chromatin remodelling. J. Steroid Biochem. Mol. Biol. 1996;56:47–59. doi: 10.1016/0960-0760(95)00223-5. [DOI] [PubMed] [Google Scholar]
- 53.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 54.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 55.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 56.Jung DJ, Lee SK, Lee JW. Agonist-dependent repression mediated by mutant estrogen receptor alpha that lacks the activation function 2 core domain. J. Biol. Chem. 2001;276:37280–37283. doi: 10.1074/jbc.M106860200. [DOI] [PubMed] [Google Scholar]
- 57.Shang Y, Brown M. Molecular determinants for the tissue specificity of serms. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 58.Huang HJ, Norris JD, McDonnell DP. Identification of a negative regulatory surface within estrogen receptor alpha provides evidence in support of a role for corepressors in regulating cellular responses to agonists and antagonists. Mol. Endocrinol. 2002;16:1778–1792. doi: 10.1210/me.2002-0089. [DOI] [PubMed] [Google Scholar]
- 59.Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- 60.Peekhaus N, Chang T, Hayes E, Wilkinson H, Mitra S, Schaeffer J, Rohrer S. Distinct effects of the antiestrogen faslodex on the stability of estrogen receptors-alpha and -beta in the breast cancer cell line mcf-7. J. Mol. Endocrinol. 2004;32:987–995. doi: 10.1677/jme.0.0320987. [DOI] [PubMed] [Google Scholar]
- 61.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 62.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 63.Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucleic Recept. Signal. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol. Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson CS, Alyea RA, Jeng YJ, Kochukov MY. Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol. Cell. Endocrinol. 2007;274:1–7. doi: 10.1016/j.mce.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Razandi M, Pedram A, Levin ER. Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol. Endocrinol. 2000;14:1434–1447. doi: 10.1210/mend.14.9.0526. [DOI] [PubMed] [Google Scholar]
- 67.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. Estrogen induces the akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J. Biol. Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- 68.Monje P, Boland R. Expression and cellular localization of naturally occurring beta estrogen receptors in uterine and mammary cell lines. J. Cell. Biochem. 2002;86:136–144. doi: 10.1002/jcb.10193. [DOI] [PubMed] [Google Scholar]
- 69.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 70.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a g protein- and protein kinase a-dependent mechanism and intracellular activation of protein phosphatase 2a. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 71.Mhyre AJ, Shapiro RA, Dorsa DM. Estradiol reduces non-classical transcription at cyclic adenosine 3',5'-monophosphate response elements in glioma cells expressing estrogen receptor alpha. Endocrinology. 2006;147:1796–1804. doi: 10.1210/en.2005-1316. [DOI] [PubMed] [Google Scholar]
- 72.Simoncini T, Scorticati C, Mannella P, Fadiel A, Giretti MS, Fu X-D, Baldacci C, Garibaldi S, Caruso A, Fornari L, Naftolin F, Genazzani AR. Estrogen receptor {alpha} interacts with g{alpha}13 to drive actin remodeling and endothelial cell migration via the rhoa/rho kinase/moesin pathway. Mol. Endocrinol. 2006;20:1756–1771. doi: 10.1210/me.2005-0259. [DOI] [PubMed] [Google Scholar]
- 73.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor g protein-coupled receptor 30 (gpr30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 74.Jaubert A-M, Mehebik-Mojaat N, Lacasa D, Sabourault D, Giudicelli Y, Ribiere C. Nongenomic estrogen effects on nitric oxide synthase activity in rat adipocytes. Endocrinology. 2007;148:2444–2452. doi: 10.1210/en.2006-1329. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Prossnitz ER, Roy SK. G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008;149:4452–4461. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graber R, Sumida C, Vallette G, Nunez EA. Rapid and long-term effects of 17 beta-estradiol on pip2-phospholipase c-specific activity of mcf-7 cells. Cell Signal. 1993;5:181–186. doi: 10.1016/0898-6568(93)90069-x. [DOI] [PubMed] [Google Scholar]
- 77.Improta-Brears T, Whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc. Natl. Acad. Sci. USA. 1999;96:4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 79.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. Ers associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol. Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 80.Song RX-D, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to mapk activation by er{alpha}-shc association and shc pathway activation. Mol. Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 81.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol. Cell. Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 83.Bulayeva NN, Gametchu B, Watson CS. Quantitative measurement of estrogen-induced erk 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69:181–192. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernando RI, Wimalasena J. Estradiol abrogates apoptosis in breast cancer cells through inactivation of bad: ras-dependent nongenomic pathways requiring signaling through erk and akt. Mol. Biol. Cell. 2004;15:3266–3284. doi: 10.1091/mbc.E03-11-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol. Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 86.Razandi M, Pedram A, Rosen EM, Levin ER. Brca1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol. Cell. Biol. 2004;24:5900–5913. doi: 10.1128/MCB.24.13.5900-5913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santen RJ, Song RX, Zhang Z, Yue W, Kumar R. Adaptive hypersensitivity to estrogen: mechanism for sequential responses to hormonal therapy in breast cancer. Clin. Cancer Res. 2004;10:337S–345S. doi: 10.1158/1078-0432.ccr-031207. [DOI] [PubMed] [Google Scholar]
- 88.Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, Baldacci C, Genazzani AR. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145:5745–5756. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- 89.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor {alpha} to the plasma membrane. Proc. Natl. Acad Sci. USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toran-Allerand CD. Minireview: a plethora of estrogen receptors in the brain: Where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 91.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 92.Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Signaling from the membrane via membrane estrogen receptor-[alpha]: Estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70:364–371. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 93.Cui Y, Parra I, Zhang M, Hilsenbeck SG, Tsimelzon A, Furukawa T, Horii A, Zhang Z-Y, Nicholson RI, Fuqua SAW. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: a mechanism of tamoxifen resistance. Cancer Res. 2006;66:5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, Donelan SS, Owen KA, Gibson MA, Shupnik MA, Silva CM, Parsons SJ, Clarke R, Bouton AH. Physical and functional interactions between cas and c-src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–7015. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- 95.Greger JG, Fursov N, Cooch N, McLarney S, Freedman LP, Edwards DP, Cheskis BJ. Phosphorylation of mnar promotes estrogen activation of phosphatidylinositol 3-kinase. Mol. Cell. Biol. 2007;27:1904–1913. doi: 10.1128/MCB.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Song RXD, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor i receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in mcf-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stoica GE, Franke TF, Wellstein A, Czubayko F, List HJ, Reiter R, Morgan E, Martin MB, Stoica A. Estradiol rapidly activates akt via the erbb2 signaling pathway. Mol. Endocrinol. 2003;17:818–830. doi: 10.1210/me.2002-0330. [DOI] [PubMed] [Google Scholar]
- 98.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic potential of the nuclear receptor coregulator proline-, glutamic acid-, leucine-rich protein 1/modulator of the nongenomic actions of the estrogen receptor. Cancer Res. 2007;67:5505–5512. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghisletti S, Meda C, Maggi A, Vegeto E. 17{beta}-estradiol inhibits inflammatory gene expression by controlling nf-{kappa}b intracellular localization. Mol. Cell. Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu Q, Pallas DC, Surks HK, Baur WE, Mendelsohn ME, Karas RH. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial no synthase by estrogen receptor {alpha} Proc. Natl. Acad Sci. USA. 2004 doi: 10.1073/pnas.0407492101. 0407492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/akt pathway by androgen through interaction of p85alpha, androgen receptor, and src. J. Biol. Chem. 2003;278:42992–43000. doi: 10.1074/jbc.M306295200. [DOI] [PubMed] [Google Scholar]
- 102.Kinoshita Y, Chen S. Induction of aromatase (cyp19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res. 2003;63:3546–3555. [PubMed] [Google Scholar]
- 103.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 104.Watson CS, Campbell CH, Gametchu B. The dynamic and elusive membrane estrogen receptor-alpha. Steroids. 2002;67:429–437. doi: 10.1016/s0039-128x(01)00172-6. [DOI] [PubMed] [Google Scholar]
- 105.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor [beta], but not estrogen receptor [alpha], to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc. Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 107.Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007;12:293–300. doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- 108.Subah Packer C. Estrogen protection, oxidized ldl, endothelial dysfunction and vasorelaxation in cardiovascular disease: New insights into a complex issue. Cardiovasc. Res. 2007;73:6–7. doi: 10.1016/j.cardiores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 109.Joy S, Siow RCM, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, Mann GE. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/hsp90 involving erk1/2 and akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006;281:27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 110.Kiss AL, Turi A, Mullner N, Kovacs E, Botos E, Greger A. Oestrogen-mediated tyrosine phosphorylation of caveolin-1 and its effect on the oestrogen receptor localisation: an in vivo study. Mol. Cell. Endocrinol. 2005;245:128–137. doi: 10.1016/j.mce.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 111.Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through galpha(i) J. Biol. Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 112.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (er46) in human endothelial cells. Proc. Natl. Acad. Sci. USA. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. Spalmitoylation modulates human estrogen receptor-alpha functions. Biochem. Biophys. Res. Commun. 2004;316:878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 114.Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 115.Moriarty K, Kim KH, Bender JR. Estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 116.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with src/erk phosphorylation cascade. Proc. Natl. Acad. Sci. USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Edwards DP, Boonyaratanakornkit V. Rapid extranuclear signaling by the estrogen receptor (er): Mnar couples er and src to the map kinase signaling pathway. Mol. Interv. 2003;3:12–15. doi: 10.1124/mi.3.1.12. [DOI] [PubMed] [Google Scholar]
- 118.Barletta F, Wong CW, McNally C, Komm BS, Katzenellen-bogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and mnar in the activation of csrc. Mol. Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 119.Cheskis BJ. Regulation of cell signalling cascades by steroid hormones. J. Cell Biochem. 2004;93:20–27. doi: 10.1002/jcb.20180. [DOI] [PubMed] [Google Scholar]
- 120.Mishra SK, Balasenthil S, Nguyen D, Vadlamudi RK. Cloning and functional characterization of pelp1/mnar promoter. Gene. 2004;330:115–122. doi: 10.1016/j.gene.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 121.Mishra SK, Talukder AH, Gururaj AE, Yang Z, Singh RR, Mahoney MG, Franci C, Vadlamudi RK, Kumar R. Upstream determinants of estrogen receptor-{alpha} regulation of metastatic tumor antigen 3 pathway. J. Biol. Chem. 2004;279:32709–32715. doi: 10.1074/jbc.M402942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator pelp1 in histone h1 displacement in cancer cells. Cancer Res. 2004;64:6416–6423. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 123.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: Why is the endothelium so special? Sci. STKE. 2005;2005:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 124.Greger JG, Guo Y, Henderson R, Ross JF, Cheskis BJ. Characterization of mnar expression. Steroids. 2006;71:317–322. doi: 10.1016/j.steroids.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 125.Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, San-jay A, Collinge M, Baron R, Sessa WC, Bender JR. Variant estrogen receptor c-src molecular interdependence and c-src structural requirements for endothelial no synthase activation. Proc. Natl. Acad. Sci. 2007;104:16468–16473. doi: 10.1073/pnas.0704315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marquez-Garban DC, Chen H-W, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam H-S, Rutledge S, Mekonnen B, Hauze D, Nagpal S, Freedman LP. Mnar plays an important role in era activation of Src/MAPK and pi3k/Akt signaling pathways. Steroids. 2008;73(9-10):901–905. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 128.Rajhans R, Nair HB, Nair SS, Cortez V, Ikuko K, Kirma NB, Zhou D, Holden AE, Brann DW, Chen S, Tekmal RR, Vadlamudi RK. Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: Potential estrogen receptor autocrine signaling loop in breast cancer cells. Mol. Endocrinol. 2008;22:649–664. doi: 10.1210/me.2007-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–387. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 130.Flototto T, Niederacher D, Hohmann D, Heimerzheim T, Dall P, Djahansouzi S, Bender HG, Hanstein B. Molecular mechanism of estrogen receptor (er)[alpha]-specific, estradiol-dependent expression of the progesterone receptor (pr) b-isoform. J. Steroid Biochem. Mol. Biol. 2004;88:131–142. doi: 10.1016/j.jsbmb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 131.Filardo EJ. Epidermal growth factor receptor (egfr) transactivation by estrogen via the g-protein-coupled receptor, gpr30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 132.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 133.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a g protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 134.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006;346:904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 135.Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. Gpr30 contributes to estrogen-induced thymic atrophy. Mol. Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of gpr30: a transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier K-H. Gpr30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149(10):4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 138.Filardo EJ, Quinn JA, Sabo E. Association of the membrane estrogen receptor, gpr30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids. 2008;73:870–873. doi: 10.1016/j.steroids.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 139.Sakamoto H, Matsuda K-i, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of g protein-coupled receptor-30, a g protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–5850. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- 140.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor gpr30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 141.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of gpr30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin. Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 142.ENCODE, project, consortium. Identification and analysis of functional elements in 1% of the human genome by the encode pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, Bell I, Cheung E, Drenkow J, Dumais E, Patel S, Helt G, Ganesh M, Ghosh S, Piccolboni A, Sementchenko V, Tammana H, Gingeras TR. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 144.Pennisi E. Genomics: DNA study forces rethink of what it means to be a gene. Science. 2007;316:1556–1557. doi: 10.1126/science.316.5831.1556. [DOI] [PubMed] [Google Scholar]
- 145.Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome as an rna machine. Science. 2008;319:1787–1789. doi: 10.1126/science.1155472. [DOI] [PubMed] [Google Scholar]
- 146.Ambros V. The evolution of our thinking about microRNAs. Nat. Med. 2008;14:1036–1040. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 147.Ruvkun G. The perfect storm of tiny rnas. Nat. Med. 2008;14:1041–1045. doi: 10.1038/nm1008-1041. [DOI] [PubMed] [Google Scholar]
- 148.Baulcombe DC. Short silencing RNA: The dark matter of genetics? Cold Spring Harb. Symp. Quant. Biol. 2006;71:13–20. doi: 10.1101/sqb.2006.71.052. [DOI] [PubMed] [Google Scholar]
- 149.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verghese ET, Hanby AM, Speirs V, Hughes TA. Small is beautiful: microRNAs and breast cancer-where are we now? J. Pathol. 2008;215:214–221. doi: 10.1002/path.2359. [DOI] [PubMed] [Google Scholar]
- 151.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hock J, Meister G. The argonaute protein family. Genome Biol. 2008;9:210. doi: 10.1186/gb-2008-9-2-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr. Opin. Genet. Dev. 2005;15:200–205. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Sen GL, Blau HM. A brief history of rnai: the silence of the genes. FASEB J. 2006;20:1293–1299. doi: 10.1096/fj.06-6014rev. [DOI] [PubMed] [Google Scholar]
- 155.Berkhout B, Jeang K-T. Riscy business: MicroRNAs, pathogenesis, and viruses. J. Biol. Chem. 2007;282:26641–26645. doi: 10.1074/jbc.R700023200. [DOI] [PubMed] [Google Scholar]
- 156.Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J. Endocrinol. 2005;187:327–332. doi: 10.1677/joe.1.06426. [DOI] [PubMed] [Google Scholar]
- 157.Chendrimada TP, Finn KJ, Ji X, Baillat D, Gregory RI, Liebhaber SA, Pasquinelli AE, Shiekhattar R. MicroRNA silencing through risc recruitment of eif6. Nature. 2007;447:823–828. doi: 10.1038/nature05841. [DOI] [PubMed] [Google Scholar]
- 158.Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front. Biosci. 2008;13:2537–2547. doi: 10.2741/2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Friedman RC, Farh KK-H, Burge CB, Bartel D. Most mammalian mrnas are conserved targets of microRNAs. Genome Res. 2008 doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 161.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome p450 1b1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 162.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (mirna) mir-206 targets the human estrogen receptor-{alpha} (er{alpha}) and represses er{alpha} messenger rna and protein expression in breast cancer cell lines. Mol. Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 163.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 164.Gaur A, Jewell DA, Liang Y, Ridzon D, Moore JH, Chen C, Ambros VR, Israel MA. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 165.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu C-G, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, Bolondi L, Negrini M. Cyclin g1 is a target of mir-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 166.Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the microRNA hsa-mir-200c leads to reduced expression of transcription factor 8 and increased expression of e-cadherin. Cancer Res. 2007;67:7972–7976. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 167.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 168.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Gitt A, Spiteri I, Das PP, Caldas C, Miska E, Esteller M. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 169.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the pten tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28:2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 171.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates myc and reverts myc-induced growth in burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 172.Sylvestre Y, De Guire V, Querido E, Mukhopadhyay UK, Bourdeau V, Major F, Ferbeyre G, Chartrand P. An e2f/mir-20a autoregulatory feedback loop. J. Biol. Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 173.Thai T-H, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 174.Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-rna cluster by e2f transcription factors. J. Biol. Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- 175.Zhang W, Dahlberg JE, Tam W. MicroRNAs in tumorigenesis: a primer. Am. J. Pathol. 2007;171:728–738. doi: 10.2353/ajpath.2007.070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou Y, Yau C, Gray JW, Chew K, Dairkee SH, Moore DH, Eppenberger U, Eppenberger-Castori S, Benz CC. Enhanced nf kappa b and ap-1 transcriptional activity associated with antiestrogen resistant breast cancer. BMC Cancer. 2007;7:59. doi: 10.1186/1471-2407-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'Brien-Jenkins A, Katsaros D, Hat-zigeorgiou A, Gimotty PA, Weber BL, Coukos G. MicroRNAs exhibit high frequency genomic alterations in human cancer. Proc. Natl. Acad. Sci. USA. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 180.Russo J, Tahin Q, Lareef MH, Hu YF, Russo IH. Neoplastic transformation of human breast epithelial cells by estrogens and chemical carcinogens. Environ. Mol. Mutagen. 2002;39:254–263. doi: 10.1002/em.10052. [DOI] [PubMed] [Google Scholar]
- 181.Russo J, Lareef MH, Tahin Q, Hu YF, Slater C, Ao X, Russo IH. 17beta-estradiol is carcinogenic in human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2002;80:149–162. doi: 10.1016/s0960-0760(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 182.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 183.Wiseman RA. Breast cancer: critical data analysis concludes that estrogens are not the cause, however lifestyle changes can alter risk rapidly. J. Clin. Epidemiol. 2004;57:766–772. doi: 10.1016/j.jclinepi.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 184.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 185.Hammond SM. MicroRNAs as oncogenes. Curr. Opin. Genet. Dev. 2005 doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 186.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, mir-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 187.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. Ras is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 188.Diederichs S, Haber DA. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res. 2006;66:6097–6104. doi: 10.1158/0008-5472.CAN-06-0537. [DOI] [PubMed] [Google Scholar]
- 189.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66:1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 190.Giannakakis A, Coukos G, Hatzigeorgiou A, Sandaltzopoulos R, Zhang L. miRNA genetic alterations in human cancers. Expert Opin. Biol. Ther. 2007;7:1375–1386. doi: 10.1517/14712598.7.9.1375. [DOI] [PubMed] [Google Scholar]
- 191.Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, Wells W, Kauppinen S, Cole CN. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 192.Gusev Y, Schmittgen TD, Lerner M, Postier R, Brackett D. Computational analysis of biological functions and pathways collectively targeted by co-expressed microRNAs in cancer. BMC Bioinformatics. 2007;8(Suppl 7):S16. doi: 10.1186/1471-2105-8-S7-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27:4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of aib1 mRNA. Mol. Cell. Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. Coordinate suppression of erbb2 and erbb3 by enforced expression of micro-RNA mir-125a or mir-125b. J. Biol. Chem. 2007;282:1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- 196.Creighton CJ, Nagaraja AK, Hanash SM, Matzuk MM, Gunaratne PH. A bioinformatics tool for linking gene expression profiling results with public databases of microRNA target predictions. RNA. 2008;14:2290–2296. doi: 10.1261/rna.1188208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cohen A, Shmoish M, Levi L, Cheruti U, Levavi-Sivan B, Lubzens E. Alterations in micro-ribonucleic acid expression profiles reveal a novel pathway for estrogen regulation. Endocrinology. 2008;149:1687–1696. doi: 10.1210/en.2007-0969. [DOI] [PubMed] [Google Scholar]
- 198.Kovalchuk O, Tryndyak VP, Montgomery B, Boyko A, Kutanzi K, Zemp F, Warbritton AR, Latendresse JR, Kovalchuk I, Beland FA, Pogribny IP. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle. 2007;6:2010–2018. doi: 10.4161/cc.6.16.4549. [DOI] [PubMed] [Google Scholar]
- 199.Dai R, Phillips RA, Zhang Y, Khan D, Crasta O, Ahmed SA. Suppression of lps-induced ifn{gamma} and nitric oxide in splenic lymphocytes by select estrogen-regulated mirna: a novel mechanism of immune modulation. Blood. 2008;112(12):4591–4597. doi: 10.1182/blood-2008-04-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christen-son LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008;149(12):6207–6212. doi: 10.1210/en.2008-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carletti MZ, Christenson LK. MicroRNA in the ovary and female reproductive tract. J. Anim Sci. 2008 doi: 10.2527/jas.2008-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu S-J, Ren G, Liu J-L, Zhao Z-A, Yu Y-S, Su R-W, Ma X-H, Ni H, Lei W, Yang Z-M. MicroRNA expression and regulation in mouse uterus during embryo implantation. J. Biol. Chem. 2008;283:23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- 203.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, eralpha and erbeta, in estrogen target tissues in vivo through the use of an eralpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- 204.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 205.Ferguson AT, Lapidus RG, Baylin SB, Davidson NE. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995;55:2279–2283. [PubMed] [Google Scholar]
- 206.Yang X, Ferguson AT, Nass SJ, Phillips DL, Butash KA, Wang SM, Herman JG, Davidson NE. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–6894. [PubMed] [Google Scholar]
- 207.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (er)-alpha by DNA methyltransferase and histone deacetylase inhibition in human er-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 208.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl cpg binding proteins and histone deacetylase 1 from the estrogen receptor {alpha} (er) promoter upon reactivation in er-negative human breast cancer cells. Mol. Endocrinol. 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 209.Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated er recruits distinctive corepressor complexes. Cancer Res. 2006;66:6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J. Cell. Mol. Med. 2008;12:227–240. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. Mir-206 expression is down-regulated in estrogen receptor {alpha}-positive human breast cancer. Cancer Res. 2008;68:5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- 212.Zhao J-J, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates eralpha and associates with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 213.Sun M, Estrov Z, Ji Y, Coombes KR, Harris DH, Kurzrock R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008;7:464–473. doi: 10.1158/1535-7163.MCT-07-2272. [DOI] [PubMed] [Google Scholar]
- 214.Buchan JR, Parker R. Molecular biology: the two faces of mirna. Science. 2007;318:1877–1878. doi: 10.1126/science.1152623. [DOI] [PubMed] [Google Scholar]
- 215.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor {alpha}-src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 216.Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of eralpha domains with known nuclear functions. Mol. Endocrinol. 2005;19:277–289. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- 217.Klinge CM. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008;105:1342–1351. doi: 10.1002/jcb.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chen J-Q, Russo PA, Cooke C, Russo IH, Russo J. Er[beta] shifts from mitochondria to nucleus during estrogen-induced neoplastic transformation of human breast epithelial cells and is involved in estrogen-induced synthesis of mitochondrial respiratory chain proteins. Biochimica et Biophysica Acta (BBA) - Mol. Cell Res. 2007;1773:1732–1746. doi: 10.1016/j.bbamcr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 219.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the g2-m checkpoint in mda-mb-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 220.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 221.Tsang WP, Kwok TT. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13(10):1215–22. doi: 10.1007/s10495-008-0256-z. [DOI] [PubMed] [Google Scholar]
- 222.Ovcharenko D, Kelnar K, Johnson C, Leng N, Brown D. Genome-scale microRNA and small interfering rna screens identify small RNA modulators of trail-induced apoptosis pathway. Cancer Res. 2007;67:10782–10788. doi: 10.1158/0008-5472.CAN-07-1484. [DOI] [PubMed] [Google Scholar]
- 223.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, Calin GA, Liu CG, Andreeff M, Croce CM. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 224.Ivanovska I, Ball AS, Diaz RL, Magnus JF, Kibukawa M, Schelter JM, Kobayashi SV, Lim L, Burchard J, Jackson AL, Linsley PS, Cleary MA. MicroRNAs in the mir-106b family regulate p21/cdkn1a and promote cell cycle progression. Mol. Cell. Biol. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 226.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu C-g, Kipps TJ, Negrini M, Croce CM. Mir-15 and mir-16 induce apoptosis by targeting bcl2. Proc. Natl. Acad. Sci. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, Johnson JM, Cummins JM, Raymond CK, Dai H, Chau N, Cleary M, Jackson AL, Carleton M, Lim L. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol. Cell. Biol. 2007;27:2240–2252. doi: 10.1128/MCB.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Mir-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu C-G, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol. Cell. Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The mir-200 family and mir-205 regulate epithelial to mesenchymal transition by targeting zeb1 and sip1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 231.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol. Hum. Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 232.Cloonan N, Brown MK, Steptoe AL, Wani S, Chan WL, Forrest AR, Kolle G, Gabrielli B, Grimmond SM. The mir-17-5p microRNA is a key regulator of the g1/s phase cell cycle transition. Genome Biol. 2008;9:R127. doi: 10.1186/gb-2008-9-8-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yan L-X, Huang X-F, Shao Q, Huang M-Y, Deng L, Wu Q-L, Zeng Y-X, Shao J-Y. MicroRNA mir-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang CH, Lee DY, Deng Z, Jeyapalan Z, Lee SC, Kahai S, Lu WY, Zhang Y, Yang BB. MicroRNA mir-328 regulates zonation morphogenesis by targeting cd44 expression. PLoS ONE. 2008;3:e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]