Abstract
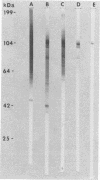
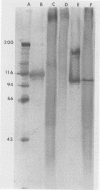
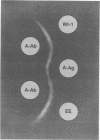
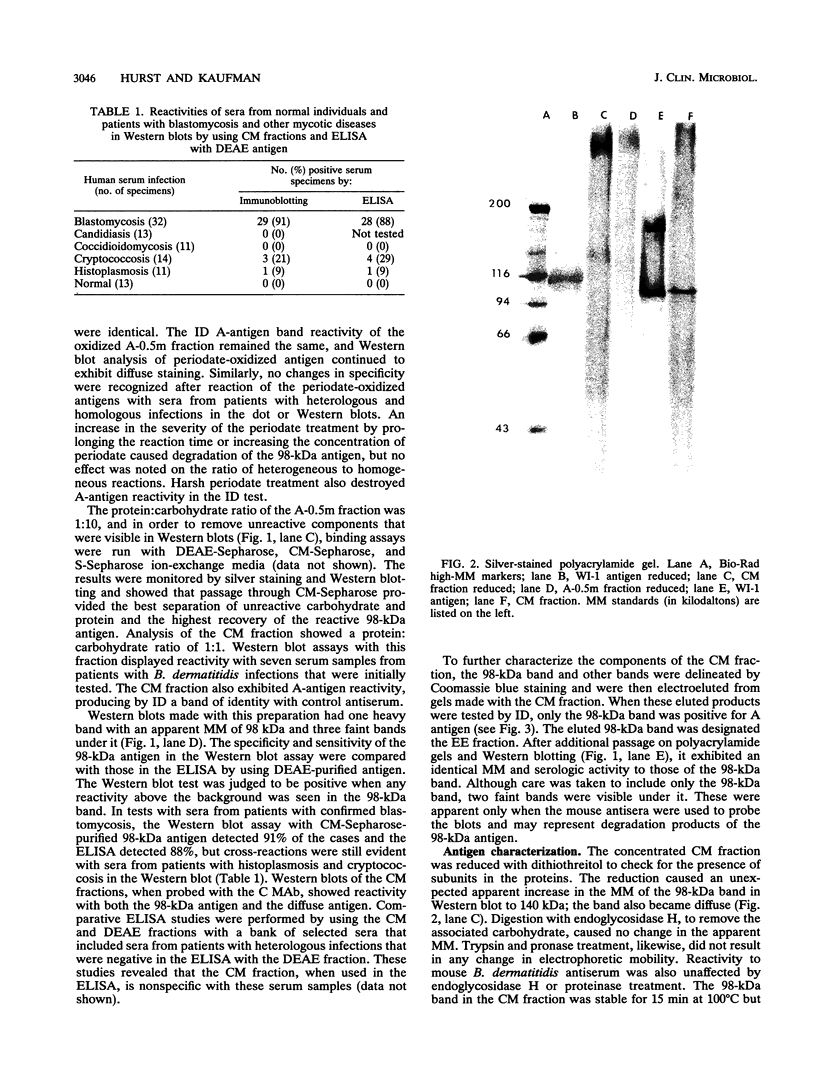
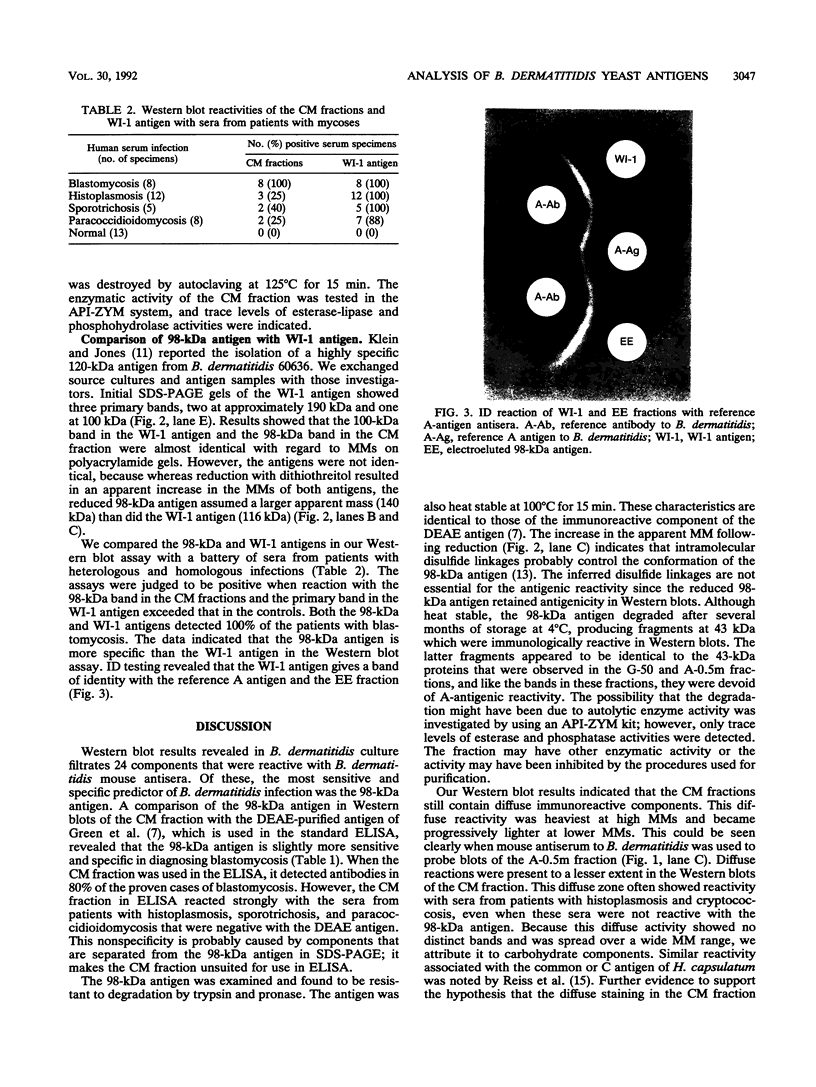
A major 98-kDa extracellular protein antigen of Blastomyces dermatitidis was shown in Western blot (immunoblot) analysis to be highly reactive with serum antibodies from patients with blastomycosis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of yeast form B. dermatitidis culture filtrates and cell extracts demonstrated over 50 proteins, only 24 of which were immunoreactive. Of these, a 98-kDa protein was found to be the most specific and was isolated. This protein was found in both broth culture filtrates and extracts of yeast forms. Western blot tests with this antigen detected serum antibodies in 91% of 32 patients with clinically proven blastomycosis, in contrast to an enzyme-linked immunosorbent assay (ELISA) with DEAE-purified antigen, which detected 88% of the cases. The Western blot test also exhibited lower reactivity with a panel of sera from patients with heterologous fungal infections that were cross-reactive in the ELISA with DEAE-purified B. dermatitidis antigen. The 98-kDa protein electroeluted from polyacrylamide gels was identical to the diagnostic A antigen used in the blastomycosis immunodiffusion test. Comparison of the 98-kDa antigen with a previously described 120-kDa yeast form cell wall protein antigen of B. dermatitidis showed that these two antigens are almost identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Fransson L. A. Periodate oxidation of L-iduronic acid residues in dermatan sulphate. Carbohydr Res. 1974 Sep;36(2):339–348. doi: 10.1016/s0008-6215(00)83055-4. [DOI] [PubMed] [Google Scholar]
- Green J. H., Harrell W. K., Johnson J. E., Benson R. Preparation of reference antisera for laboratory diagnosis of blastomycosis. J Clin Microbiol. 1979 Jul;10(1):1–7. doi: 10.1128/jcm.10.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- Kane J. Conversion of Blastomyces dermatitidis to the yeast form at 37 degrees C and 26 degrees C. J Clin Microbiol. 1984 Sep;20(3):594–596. doi: 10.1128/jcm.20.3.594-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., McLaughlin D. W., Clark M. J., Blumer S. Specific immunodiffusion test for blastomycosis. Appl Microbiol. 1973 Sep;26(3):244–247. doi: 10.1128/am.26.3.244-247.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Jones J. M. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J Clin Invest. 1990 Jan;85(1):152–161. doi: 10.1172/JCI114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., Davis J. P. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986 Mar;1(1):29–39. [PubMed] [Google Scholar]
- Owen M. J., Barber B. H., Faulkes R. A., Crumpton M. J. Albumin associated with purified pig lymphocyte plasma membrane. Biochem J. 1980 Oct 15;192(1):49–57. doi: 10.1042/bj1920049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht L. D., Philips J. R., Eckman M. R., Sarosi G. A. Self-limited blastomycosis: a report of thirteen cases. Am Rev Respir Dis. 1979 Nov;120(5):1109–1112. doi: 10.1164/arrd.1979.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Reiss E., Knowles J. B., Bragg S. L., Kaufman L. Monoclonal antibodies against the M-protein and carbohydrate antigens of histoplasmin characterized by the enzyme-linked immunoelectrotransfer blot method. Infect Immun. 1986 Sep;53(3):540–546. doi: 10.1128/iai.53.3.540-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosi G. A., Hammerman K. J., Tosh F. E., Kronenberg R. S. Clinical features of acute pulmonary blastomycosis. N Engl J Med. 1974 Mar 7;290(10):540–543. doi: 10.1056/NEJM197403072901004. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Maddison S. E., Beatty A. L., Moss D. M. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J Immunol. 1984 May;132(5):2607–2613. [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Turner S., Kaufman L., Jalbert M. Diagnostic assessment of an enzyme-linked immunosorbent assay for human and canine blastomycosis. J Clin Microbiol. 1986 Feb;23(2):294–297. doi: 10.1128/jcm.23.2.294-297.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. D., Larsh H. W. Identification of the active precipitin components in a purified preparation of the A antigen of Blastomyces dermatitidis. Infect Immun. 1981 Jul;33(1):171–177. doi: 10.1128/iai.33.1.171-177.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]