Abstract
Clinical and experimental data support a link between endothelial dysfunction and inflammation. Inflammatory cytokines are important protagonists in formation of atherosclerotic plaque, eliciting effects throughout the atherosclerotic vessel. Importantly, the development of atherosclerotic lesions, regardless of the risk factor, e.g., diabetes, hypertension, obesity, is characterized by disruption in normal function of the endothelial cells. Endothelial cells, which line the internal lumen of the vasculature, are part of a complex system that regulates vasodilation and vasoconstriction, growth of vascular smooth muscle cells, inflammation, and hemostasis, maintaining a proper blood supply to tissues and regulating inflammation and coagulation. Current concepts suggest that the earliest event in atherogenesis is endothelial dysfunction, manifested by deficiencies in the production of nitric oxide (NO) and prostacyclin. The focus of this review is to summarize recent evidence showing the effects of inflammation on vascular dysfunction in ischemic-heart disease, which may prompt new directions for targeting inflammation in future therapies.
Keywords: inflammation, vascular dysfunction, cytokines, ischemic-heart disease, nitric oxide, vasodilation
Introduction
Ischemic heart disease (IHD) is the leading health-related cause of mortality in the Western world. Although recent trends show decreases in mortality of patients with IHD, well over 50% of patients exhibit myocardial infarction or sudden death. A hallmark of IHD is the development of coronary vascular lesions, which are linked to well known risk factors such as diabetes and obesity—conditions associated with inflammation. The purpose of this review is to highlight the importance of, and some causes of inflammation in endothelial dysfunction that inevitably leads to vascular disease.
Role of inflammation in endothelial dysfunction
Inflammation is a protective response of tissue that eliminates causative agents and debris and is closely tied to repair. Endothelial dysfunction is a broad term that implies diminished production or availability of nitric oxide (NO) and/or an imbalance in the relative contribution of endothelium-derived relaxing and contracting factors, such as endothelin-1 (ET-1) [48, 70], angiotensin, and oxidants. NO, generated by the conversion of the amino acid L-arginine to NO and L-citrulline by the enzyme NO synthase, is the key endothelium-derived relaxing factor that plays a pivotal role in the regulation of vascular tone and vasomotor function [49]. Small amounts NO produced by endothelial cells result in smooth muscle relaxation and vasodilation and are anti-thrombogenic to platelets. Impaired endothelium-dependent vasodilation in coronary arteries with established atherosclerosis results in paradoxical vasoconstriction, which may result in reduced myocardial perfusion and myocardial ischemia. However, endothelial dysfunction, as assessed in terms of vasomotor dysfunction, can occur well before the structural manifestation of atherosclerosis and thus can serve as an independent predictor of future cardiovascular events [6, 49].
Constituents of inflammation are circulating cells, connective tissue cells, extracellular matrix (ECM) and basement membrane (BM). An acute inflammation can be determined in minutes to days by the presence of neutrophils and fluid protein exudates. Inflammation is terminated when the injurious stimulus is removed and all the mediators are dissipated or inhibited. The three stages of acute inflammation in a vessel are vasodilation, increased permeability of microvasculature, and vascular stasis. The delayed sustained response is observed as endothelial cells undergo cytoskeletal changes that disrupt junctions in venules and capillaries, which takes 4–6 h after the mediator stimulus and lasts for days. The factors involved in the delayed sustained response are: interleukin (IL)-1, tumor necrosis factor-alpha (TNF-α, interferon-gamma (INF-γ, hypoxia and sublethal injury. Cytokines are produced mainly by lymphocytes and macrophages after stimulation by toxins, injury or inflammatory mediators. Lymphokines, monokines, chemokines, colony stimulating factors, interleukins, and growth factors, etc., are cytokines. The general properties of cytokines are: (1) short half-life, (2) modulation of the immune response, (3) production/modulation by many types of cells, (4) redundancy, (5) interactive with other cytokines and, (6) recognition by specific receptors.
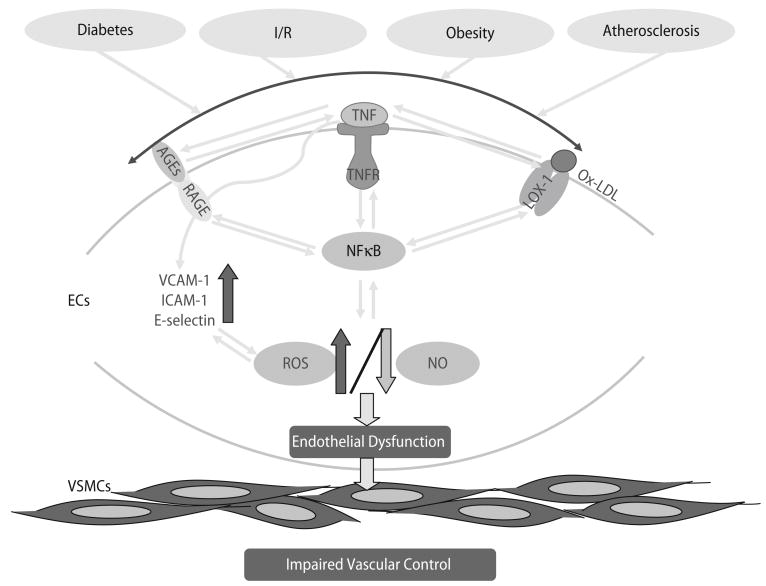
Various infections cause endothelial dysfunction (Fig. 1). The cause of inflammation may be multi-factorial, but it is probable that various chronic infectious processes contribute [47]. When endothelial cells (ECs) undergo inflammatory activation, the increased expression of selections, vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) promotes the adherence of monocytes [10]. Adhesion molecule expression is induced by proinflammatory cytokines such as IL-1β and TNF-α, by the acute-phase protein, C-reactive protein (CRP), that is produced by the liver in response to IL-6, by protease-activated receptor signaling, by ox-LDL uptake via ox-LDL receptor-1 (LOX-1), and by CD40/CD40 ligand (CD40L and CD154) interactions [10, 49, 54].
Fig. 1.
The indicators of endothelial dysfunction in cardiovascular diseases (CVD) are portrayed. Normally, endothelial cells regulate the homeostasis of the vessel wall. The healthy endothelium is not leaky, anti-adhesive and is able to relax vascular smooth muscle cells. However, when risk factors (diabetes, ischemia/reperfusion, obesity and atherosclerosis etc.) disturb endothelial cells, which induce endothelial dysfunction and vascular remodelling. We have an impaired vascular control when the damaged endothelium is leaky, sticky and unable to relax vascular smooth muscle cells. In brief, central to the endothelial dysfunction is oxidative stress. The oxidative stress is induced by the production of reactive oxidative species (ROS) and this induces NF-κB. Key to the production of ROS is AGE/RAGE and TNF-α signaling, but ox-LDL/LOX-1 signaling is also involved in ROS production. The interactions among oxidative stress, AGE/RAGE, TNF-α/TNFR and ox-LDL/LOX-1 are because oxidative stress induces NF-κB, and this transcription factor can induce AGE, TNF-α and ox-LDL expression; and TNF-α can induce RAGE and LOX-1 expression. Thus, the oxidative stress of diabetes, begets more oxidative stress, eventually inducing endothelial dysfunction, because of decreased bioavailability of nitric oxide (NO, due to the reaction between NO and O2 · −)
One chronic consequence of endothelial dysfunction is the initiation of the pathological sequalae leading to vascular disease. In this process, the first evolution of a fatty streak toward a complex lesion is typified by the proliferation of smooth muscle cells (SMCs), their migration toward the intima, and their synthesis of collagen. Continued release of cytokines, such as MCP-1, by activated ECs, T-cells, and foam cells not only perpetuates inflammation and lipid accumulation within the atheroma but also influences SMC activity. Disruption of the vulnerable atherosclerotic plaque, on exposure to hemodynamic stresses, can trigger thrombosis, culminating in acute myocardial infarction [49]. Usually, the outcomes of acute inflammation are resolution, scarring, and abscess formation, which progress to chronic inflammation. Vascular endothelial cells play an important role in the control of vascular tone. The reasons for coronary endothelial dysfunction are complex and may involve ischemia/reperfusion injury. Smoking, obesity, hypertension, diabetes, physical inactivity and hypercholesterolemia are established atherogenic risk factors. Influenced by these risk factors, atherosclerosis is considered a chronic inflammatory disease, associated with endothelial dysfunction and other profound changes in the vascular system [26]. Chronic inflammation is a major contributing factor to atherosclerosis and various markers of inflammation, fibrinolysis and coagulation are upregulated in patients with established atherosclerotic disease [26]. While myocardial function has already recovered, endothelial cells are more severely impaired than smooth muscle cells, and this injury persists beyond myocardial stunning [20]. Thus, endothelial-dependent dysfunction can still impair vasodilation while ventricular dysfunction has already resolved, which confirms the concept that myocardial and vascular stunning represent relatively independent phenomena [20].
For vascular homeostasis, endothelial cells are of utmost importance and they produce a variety of mediators, surface proteins, and autocoids involved in vasomotion, coagulation, and inflammation [31]. TNF-α plays an important role in the corruption of vascular homeostasis in certain pathologies (Table 1). The involvement of TNF-α in the pathogenesis of shock-induced cardiac dysfunction and in acute myocardial infarction (AMI), chronic heart failure, atherosclerosis, viral myocarditis, and cardiac allograft rejection [33] is well accepted. TNFα and other cytokines (CRP, IL-6) are elevated in, and thought to contribute to, insulin resistance [16, 17, 66]. Elevated levels of TNF-α are also associated with coronary artery disease. Moreover, TNF-α inhibits endothelium-dependent NO-mediated dilation of coronary arterioles by ceramide-induced activation of JNK and subsequent production of superoxide via xanthine oxidase [10, 68]. Because myocardial ischemia elevates TNF-α levels, production of this cytokine could contribute to inadequate regulation of coronary blood flow during the development of ischemic heart disease [68]. Such a proposal has been suggested by Heusch’s group in which the production of TNF-α during microembolization has contributed significantly to the evolution of ischemic damage [53]. Furthermore, Rask-Madsen et al. [39] demonstrated that TNF-α inhibited both insulin-stimulated glucose and endothelium-dependent vasodilation in humans. TNF-α inhibits NO-mediated endothelium-dependent vaso- relaxation in small coronary arteries via sphingomyelinase activation and consequent O2 · − production in ECs [11, 14, 28, 32, 69].
Table 1.
Effects of TNFα and potential mechanisms for affecting endothelial function including the respective references (Refs)
| Effects of TNF-α | Potential mechanisms | References |
|---|---|---|
| Induce RAGE expression | NF-κB activation | [25] |
| Induce ICAM-1 expression Induce VCAM-1 expression Induce E-Selectin expression | NF-κB activation | [24] |
| Induce LOX-1 expression | NF-κB activation | [43] |
| Activation of eNOS | S1P receptors, NSMase2 and SK1 activation | [11] |
| Induce ROS formation decrease NO production increase superoxide production | Activation of CAPK; activation of NAD(P)H oxidase; activation of XO | [5, 14, 16, 21, 38] |
TNF-α is expressed at low concentrations in the healthy heart, and such TNF-α is mainly located to the vascular endothelium. Left ventricular function as well as prognosis of heart failure patients is not improved following administration of TNF-α antibodies [41]. The ischemia-induced increase in the myocardial TNF-α concentration, however, contributes to irreversible tissue injury, since administration of TNF-α antibodies reduced infarct size following coronary artery occlusion/reperfusion [41]. TNF-α is the mediator responsible for the profound contractile dysfunction following coronary micro-embolization [13, 14]. The diverse signaling events that regulate varied TNF-α responses are all initiated by the binding of heterotrimeric ligand to one of two cell surface receptors (TNFR1 and TNFR2) [58]. Gilmont et al. [21] have shown a direct effect of TNF-α on ECs, whereby the cells are rendered more susceptible to oxidant injury accompanying reperfusion in rat pulmonary artery endothelial cells. Usually, TNFR1 can be released (shed) from the cellular membrane, becoming a soluble receptor and modulating the activity of circulating TNF, decreasing the amount of TNF that can be bound to the cell receptor initiating the signaling pathway. Several mutations on TNFR1 have been described and most of them generate a bound receptor that cannot be released from the cell membrane and cannot elicit the modulation function on TNF-α related inflammatory processes; moreover, the cellular receptor TNFR2 remains functionally intact, therefore TNF-α related processes are enhanced, inducing the inflammation cascade and cell apoptosis. TNF-α is one of the key inflammatory mediators that is expressed during a variety of inflammatory conditions and initiates the expression of an entire spectrum of inflammatory cytokines ranging from many interleukins to interferons. Current evidence suggests a strong relation between inflammation and endothelial dysfunction.
Roles of AGE/RAGE signaling in inflammation-induced endothelial dysfunction
The basis for advanced glycation end products (AGEs) being involved in the pathophysiological sequelae of vascular dysfunction in type II diabetes is found in the hyperglycemia that occurs in this disease, and the fact that hyperglycemia leads to the production of AGEs. Hyperglycemia and oxidant stress promote nonenzymatic glycoxidation of proteins and lipids [15]. The final products of these reactions, termed AGEs, have both direct and indirect effects [15]. Direct activities of AGEs include the capacity to alter vessel wall architecture through formation of intermolecular crosslinks and trapping of plasma components, reducing elasticity. AGE-mediated generation of low levels of reactive oxygen species can result in quenching of the endogenous vasorelaxant, nitric oxide [15]. The most characterized AGE binding protein is the receptor for AGEs (RAGE). RAGE, a 45-kDa protein belonging to the immunoglobulin superfamily, is present on the cell surface of a variety of cells, including endothelial cells, mononuclear phagocytes, and hepatocytes [56]. RAGE is a multiligand receptor that also binds to several proteins in the S100 family including S100A12 (EN-RAGE) and S100b. S100b and S100A12 are calcium binding proteins with inflammatory properties [56]. Activation of RAGE by its various ligands reportedly induces a variety of proinflammatory and procoagulant cellular responses, resulting from the activation of NF-κB, including the expression of VCAM-1, TNF-α, IL-6, and tissue factor (TF) [56]. By interacting with the receptor for AGE (RAGE), AGEs magnify their biologic effects considerably by recruiting cellular elements. RAGE is a member of the immunoglobulin superfamily of cell surface molecules with a ligand repertoire, including AGEs, S100/cal-granulins, amyloid-β peptide, and amphoterin [15]. Two general outcomes of RAGE-mediated cellular activation include an elicitation of a proinflammatory phenotype, resulting from expression of mediators (cytokines) and effectors (metalloproteinases and tissue factor), and upregulation of the receptor itself. The latter has the potential to create a positive feedback loop in which ligand-induced expression of RAGE places more receptors on the cell surface to potentiate subsequent rounds of RAGE-induced cellular activation [15].
In humans with diabetes, the increase in circulating AGE has been found to parallel the severity of diabetic kidney disease. AGEs accumulate more quickly than normal in the blood and arteries of patients with diabetes [50, 65]. Diabetic rats treated with pimagedine (aminoguanidine, prevents AGE formation) showed a reversal of inadequate blood flow to the nerves and gradual improvement of the nerves’ ability to transmit signals. This suggests that pimagedine may have potential for treating diabetic neuropathy [27]. Once AGEs begin forming they become self-perpetuating and irreversible in many tissues. Some glycosylated proteins undergo complex chemical interactions with other proteins, even in the absence of continued high blood sugar. Theoretically, as AGEs become self-perpetuating and well-established in certain tissues, and even if the diabetes were cured and blood sugar returned to normal, the AGEs might continue to increase, thereby leading to diabetic complications [27, 50, 65]. The interaction of AGE with its receptor (RAGE) induces the production of reactive oxygen species (ROS), which can stimulate the cascade leading to NF-κB-induced transcriptional events. NF-κB will induce expression of TNF-α [9, 51, 62], which can lead to further production of ROS, and in turn induce the formation of AGE products. This possible amplification is shown in Fig. 1, where we present an heuristic model for interactions between AGE/RAGE and TNF-α signaling.
Role of oxidative stress in endothelial dysfunction
The production of ROS by TNF-α and AGE/RAGE signaling would compromise the bioavailability of NO. NO protects vessels from atherogenic insult via several mechanisms, including alteration of LDL modification, platelet and leukocyte function, SMC modification, and maintenance of vascular homeostasis [7]. Enzymes involved in the metabolism of NO and ROS may play a role in the decreased availability of NO in atherosclerosis [7]. NO inhibits thrombus formation, vascular contraction, and smooth muscle cell proliferation [52]. Treatment with NO donors or gene therapy with nitric oxidase synthase (NOS) reduced lesion formation after vascular injury in both animals and humans [5, 7, 42, 63]. Many experimental studies suggest that increased O2 · − production accounts for a significant proportion of the NO deficit in diabetic vessels. Potential sources of vascular O2 · − production include NAD(P)H-dependent oxidases [38, 55], xanthine oxidase [61], lipoxygenase, mitochondrial oxidase and NO synthase [57]; NAD(P)H oxidases [29, 30] appear to be the principal source of superoxide production in several animal models of vascular disease [35–37], including diabetes [6, 46, 59]. Furthermore, NAD(P)H oxidase proteins and activity are present in human blood vessels, including atherosclerotic coronary arteries [1], and in saphenous veins and mammary arteries from patients with coronary artery disease [25], which suggests that this oxidase system plays an important role in vascular disease states [22]. Within the context of diabetic vascular disease, Guzik et al. [24] have recently described the mechanisms of increased O2 · − production in human diabetes mellitus. They [24] found that basal O2 · − release was significantly elevated in vessels from patients with diabetes: Western immunoblot analysis showed increased levels of the p22phox membrane-bound subunit and the p67phox and p47phox cytosolic subunits in both veins and arteries from diabetic patients. Moreover, engagement of RAGE triggers signaling cascades in which activation of NAD(P)H oxidase recruits multiple downstream pathways, including p21ras, the mitogen-activated protein kinases, the Jak/stat pathway, phosphatidylinositol three-kinase, cdc42/rac, and nuclear translocation of NF-κB [15]. TNF-α activates the transcription of proinflammatory gene products including E-selectin, ICAM-1 and VCAM-1 via a translocation of NF-κB from the cytoplasm into the cell nucleus and subsequent induction of the cell adhesion molecules promotor reporter genes [40].
As mentioned previously, NF-κB links TNF-α and AGE/RAGE signaling because the expression of TNF-α is induced by NF-κB [11, 44, 51]. However, there is no report elucidating the role of TNF-α in endothelial dysfunction in type II diabetes and the mechanisms by which TNF-α induces this injury. Therefore, investigations were conducted to determine how type II diabetic-induced coronary endothelial dysfunction is mediated by TNF-α to decipher the causal mechanisms [18]. AGE/RAGE signaling stimulates the production of O2 · −, which could further both the oxidative stress, and the impaired bioavailability of NO. O2 · − is the chemical precursor to many ROS, such as H2O2, and ONOO−. Thus, the impaired NO function may be the direct result of the overproduction of O2 · − induced by TNFα and AGE/RAGE.
Over the last two decades, scientists world-wide have focused on the mechanisms and manifestations of diabetes. The basis for the manifestations of type II diabetes on endothelial dysfunction and vascular disease are incompletely understood. Diabetes impairs NO-mediated dilation in coronary arterioles. Short-term treatment of type II diabetes mellitus mice with the PPAR-γ activator rosiglitazone augments NO-mediated, flow-dependent dilation of coronary arterioles, despite the presence of hyperglycemia and hyperinsulinemia. These changes are associated with a reduction in vascular NAD(P)H oxidase activity and enhancement of vascular catalase activity demonstrating a functionally important antioxidant activity of the anti-inflammatory PPAR-γ ligand [2]. The inflammatory cytokine, TNF-α, affects intracellular insulin signaling in fat, skeletal muscle and other insulin sensitive tissues by inhibiting kinase activity in the proximal part of the insulin signaling pathway [39]. Rask-Madsen [39] showed that the forearm blood flow response to ACh was inhibited by TNF-α and the inhibitory effect of TNF-α was larger during co-infusion of insulin: their results demonstrate that TNF-α plays a pivotal role in insulin mediated endothelial function. AGE/RAGE signaling also stimulates the production of O2 · −, which could further both the oxidative stress, and the impaired bioavailability of NO.
Current concepts in endothelial dysfunction and inflammation
eNOS transduction in atherosclerotic human carotid artery results in high expression without any measurable activity of the recombinant protein. The defect in the atherosclerotic vessels is neither caused by co-factor deficiency nor enhanced NO breakdown [52]. Circulating and cardiac TNF-α concentrations increase in response to myocardial ischemia/reperfusion within minutes, most likely by release from macrophages, monocytes, and mast cells [3]. Classic ischemic preconditioning decreases cardiac and circulating TNF-α concentrations during sustained ischemia and reduces myocardial infarct size in rabbits [3]. TNF-α is responsible for progressive contractile dysfunction and delayed protection against infarction in coronary microembolization [45]. Belosjorow S et al.’s study, while insightful, neither proves nor disproves the causal involvement of TNF-α in the signal cascade from classic ischemic preconditioning [3].
Recently, investigations into the pathophysiological manifestations of ischemic heart disease focus on assessing a potential role of the inflammatory cytokine, TNF-α, in ischemia/reperfusion injury using a murine genetic model, TNF++/++ mice (transgenic mice that overexpress TNF-α) [19, 67]. The TNF++/++ transgenic model offers a unique approach to assess the role played by TNFα in many cardiovascular diseases. This approach also examines the mechanisms underlying the endothelial dysfunction of coronary artery in pathological conditions such as coronary artery disease and other cardiovascular related health problems of particular importance in the United States. The authors also utilize genetic models for obesity and type II diabetes (Leprdb mouse), the heterozygote lean controls (m Leprdb), and Leprdb mice null for TNFα (dbTNF-/dbTNF-). TNF-α is a key inflammatory mediator expressed during a variety of inflammatory conditions. Furthermore, TNF-α initiates the expression of an entire spectrum of inflammatory cytokines ranging from many interleukins to interferon. The hypothesis regarding diabetes diverges when considering the enzyme system responsible for this pathophysiological disease. Diabetes is one of the leading risk factors for the development of coronary artery and peripheral vascular diseases. Before vascular disease develops in diabetics, endothelial dysfunction occurs. In fact, endothelial dysfunction appears to be a hallmark underlying vascular disease of many etiologies. Understanding endothelial dysfunction is critical because the progression of vascular disease may be halted if endothelial dysfunction is remediated. To delineate a potential cause of endothelial dysfunction is to test the hypothesis that TNF-α induces inflammation responsible for endothelial dysfunction in type II diabetes. Current evidence shows that endothelial function was normal in diabetic mice that were lacking TNF-α (TNF knockout in the Leprdb diabetic mouse) [18]. Different inflammatory intracellular pathways are known to be involved in the development of diabetic cardiomyopathy [60]. Type I and type II diabetes mellitus has experimentally been characterized by intramyocardial inflammation, including increased TNF-α expression, oxidative stress and myocardial fibrosis [60]. TNF-α is a pro-inflammatory cytokine with various biological effects, involving the activation of mitogen-activated protein kinase (MAPK), e.g., the extracellular signal-regulated kinase (ERK) [60]. TNF-α-antagonism attenuates the development of experimental diabetic cardiomyopathy associated with a reduction of intramyocardial inflammation and cardiac fibrosis [60]. Diabetic mice have elevated expression of TNF-α, suggesting that this inflammatory cytokine produces, or at least contributes to, endothelial dysfunction in diabetes. The endothelial dysfunction associated with TNF-α in diabetes was related to excess production of the free radical, superoxide. Finally, advanced glycosylation end product and the receptor for these products seemed to amplify TNF-α expression in diabetes; thus, TNF-α and AGE/RAGE signaling play pivotal roles in endothelial dysfunction in type II diabetes [18].
This work may provide new approaches for the treatment of vasculopathy in type II diabetes vis-à-vis the use of antibodies against TNF-α or its receptors, or decoy receptors for this inflammatory agent. This poses a novel and exciting possibility for therapeutic amelioration of this disease. It is critical to attempt anti-TNF-α therapy in models of pathology, because this new kind of therapy has great potential to become the basis for such intervention in the future. This work illustrates the current views on how basic theoretical research can point the way to understanding the mechanism of type II diabetes and how directed searches for new therapeutic drugs can be discovered and conducted. There is also interest in applying these models, as well as other murine genetic models, to the study of cardiovascular diseases like hypertension, atherosclerosis and diabetes at the molecular, cellular, and intact tissue levels. The possibility of using soluble TNF receptor therapy that is being used in the treatment of rheumatoid arthritis, has been also discussed [12, 23, 34].
In native coronary vessels, TNF-α contributes to the progression from stable to unstable plaques by augmenting the local inflammatory response via multiple effects [4]. TNF-α-induced effects include the release of cell surface adhesion molecules, the increase in thrombotic activity, and induction of endothelial and smooth muscle cell apoptosis by increased formation of ROS within the plaque [4]. The periprocedural release of plaque-derived TNF-α possibly represents the amount and activity of the atherosclerotic process and might be a predictor for restenosis [4]. The importance of a lipid lowering and anti-inflammatory statin therapy in established atherosclerosis is well described and several studies have established an additional role for statin therapy besides its lipid lowering properties due to direct anti-inflammatory effects in atherosclerosis [26]. The results from Kalsch et al. [26] may illuminate possible anti-inflammatory and anti-atherogenic implications of a statin therapy in early subclinical atherosclerotic disease since atorvastatin directly reduces platelet expression of CD ligand and simvastatin attenuates endotoxin-induced tissue factor formation. The current studies on the role of LOX-1 in atherosclerosis [64] documents the direct evidence that endothelial dysfunction in atherosclerosis is mediated, at least in part, via the interaction of ox-LDL with its receptor, LOX-1, which in turn stimulates endothelial generation of superoxide radicals by activation of NAD(P)H oxidase. These results may contribute to the development of novel adjunctive therapies using anti-ox-LDL and/or anti-LOX-1 antibodies or soluble receptors to prevent endothelial dysfunction following atherosclerosis [43, 64].
Conclusion
Mounting evidence shows that disturbed endothelial function may be an early marker of an ongoing atherosclerotic process. Thus, endothelial dysfunction has increasingly been recognized to play an important role in a number of conditions associated with a high prevalence of atherosclerotic CVDs, including obesity and diabetes. The identification of elevated CRP as a transient independent risk factor for endothelial dysfunction might provide an important clue to link a systemic marker of inflammation to progression of atherosclerotic disease. Available evidence suggests that low-grade inflammation is accompanied by a decreased bioavailability of endogenous NO and that TNF-α may play a key role in these events. Thus, randomized longitudinal studies are now needed to investigate whether or not various anti-inflammatory treatment strategies (such as anti-TNF-α treatment) improve endothelial function in ischemic-heart disease patients and, more importantly, also decrease the unacceptable high cardiovascular mortality rate in this patient group.
New animal models, diagnostic techniques and therapeutic agents are expected to be developed from this work. The early appearance of TNF-α in the disease process and its association with subsequent inflammation indicates that the role of TNF-α warrants further study. Understanding endothelial dysfunction is critical, because this condition precedes the development of coronary disease; thus, if endothelial dysfunction can be rectified, the progression of vascular disease may be halted.
Acknowledgments
The Figure design was created by Dr. Xiuping Chen. Funding Sources: Support from American Heart Association Scientist Development Grant (110350047A), Pfizer Atorvastatin Research Award (2004-37) and NIH grants (RO1-HL077566 and RO1-HL085119) to Dr. Cuihua Zhang.
Footnotes
Conflicts of Interest: None.
References
- 1.Azumi H, Inoue N, Takeshita SEA. Expression of NADH/NAD(P)H oxidase p22phox in human coronary arteries. Circulation. 1999;100:1494–1498. doi: 10.1161/01.cir.100.14.1494. [DOI] [PubMed] [Google Scholar]
- 2.Bagi Z, Koller A, Kaley G. PPAR-g activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2004;286:H742–H748. doi: 10.1152/ajpheart.00718.2003. [DOI] [PubMed] [Google Scholar]
- 3.Belosjorow S, Bolle I, Duschin A, Heusch G, Schulz R. TNF-alpha antibodies are as effective as ischemic preconditioning in reducing infarct size in rabbits. Am J Physiol Heart Circ Physiol. 2003;284:H927–H930. doi: 10.1152/ajpheart.00374.2002. [DOI] [PubMed] [Google Scholar]
- 4.Bose D, Leineweber K, Konorza T, Zahn A, Brocker-Preuss M, Mann K, Haude M, Erbel R, Heusch G. Release of TNF-alpha during stent implantation into saphenous vein aortocoronary bypass grafts and its relation to plaque extrusion and restenosis. Am J Physiol Heart Circ Physiol. 2007;292:H2295–H2299. doi: 10.1152/ajpheart.01116.2006. [DOI] [PubMed] [Google Scholar]
- 5.Busk M, Mertz H, Espersen GT, Rasmussen K, Maeng M. Effects of pentoxifylline on the vascular response to injury after angioplasty in rabbit iliac arteries. Basic Res Cardiol. 2007;17:17. doi: 10.1007/s00395-007-0694-8. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Niroomand F, Liu Z, Zankl A, Katus HA, Jahn L, Tiefenbacher CP. Expression of nitric oxide related enzymes in coronary heart disease. Basic Res Cardiol. 2006;101:346–353. doi: 10.1007/s00395-006-0592-5. [DOI] [PubMed] [Google Scholar]
- 8.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 9.De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–E88. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 10.Deisher TA, Haddix TL, Montgomery KF, Pohlman TH, Kaushansky K, Harlan JM. The role of protein kinase C in the induction of VCAM-1 expression on human umbilical vein endothelial cells. FEBS Lett. 1993;331:285–290. doi: 10.1016/0014-5793(93)80354-w. [DOI] [PubMed] [Google Scholar]
- 11.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor alpha through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 12.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure-an analysis of the cytokine database from the vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 13.Dorge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 14.Downey JM, Omar B, Ooiwa H, McCord J. Superoxide dismutase therapy for myocardial ischemia. Free Rad Res Comms. 1991;12/13:703–720. doi: 10.3109/10715769109145850. [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Matsumoto C, Schwartz A, Schmidt AM, Stern DM, Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am J Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- 17.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol. 2007;102:393–411. doi: 10.1007/s00395-007-0674-z. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor alpha-induces endothelial dysfunction in leprdb mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, Chilian WM, Zhang C. TNF-alpha contributes to endothelial dysfunction by up-regulation arginase in I/R injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–1275. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- 20.Garcia SC, Pomblum V, Gams E, Langenbach MR, Schipke JD. Independency of myocardial stunning of endothelial stunning? Basic Res Cardiol. 2007;102:359–367. doi: 10.1007/s00395-007-0657-0. [DOI] [PubMed] [Google Scholar]
- 21.Gilmont RR, Dardano A, Engle JS, Adamson BS, Welsh MJ, Li T, Remick DG, Smith DJJ, Rees RS. TNF-alpha potentiates oxidant and reperfusion-induced endothelial cell injury. J Surg Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 23.Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T, Lien E, Frøland SS, Aukrust P. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation. 2001;103:220. doi: 10.1161/01.cir.103.2.220. [DOI] [PubMed] [Google Scholar]
- 24.Guzick TJ, Mussa S, Gastaldi DEA. Mechanisms of increased vascular superoxide production in human diabetes mellitus. Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 25.Guzik TJ, West NEJ, Black EEA. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:e85–e90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 26.Kalsch T, Elmas E, Nguyen XD, Suvajac N, Kluter H, Borggrefe M, Dempfle CE. Endotoxin-induced effects on platelets and monocytes in an in vivo model of inflammation. Basic Res Cardiol. 2007;102:460–466. doi: 10.1007/s00395-007-0667-y. [DOI] [PubMed] [Google Scholar]
- 27.Kihara M, Schmelzer JD, Poduslo JF, Curran GL, Nickander KK, Low PA. Aminoguanidine effects on nerve blood flow, vascular permeability, electrophysiology, and oxygen free radicals. Proc Natl Acad Sci USA. 1991;88:6107–6111. doi: 10.1073/pnas.88.14.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47(phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002;90:143–150. doi: 10.1161/hh0202.103615. [DOI] [PubMed] [Google Scholar]
- 31.Luscher TM, Steffel J. Sweet and sour: unraveling diabetic vascular disease. Circ Res. 2007;102:9–11. doi: 10.1161/01.RES.0000303937.73170.31. [DOI] [PubMed] [Google Scholar]
- 32.Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meldrum DM, Cleveland JC, Cain BS, Meng X, Harken AH. Increased myocardial tumor necrosis factor-α in a crystalloid-perfused model of cardiac ischemia-reperfusion injury. Ann Thorac Surg. 1998;65:439–443. doi: 10.1016/s0003-4975(97)01297-6. [DOI] [PubMed] [Google Scholar]
- 34.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 35.Mukherjee TK, Mukhopadhyay S, Hoidal JR. The role of reactive oxygen species in TNF-alpha-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells. Biochim Biophys Acta. 2005;1744:213–223. doi: 10.1016/j.bbamcr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Muzaffar S, Shukla N, Angelini G, Jeremy JY. Nitroaspirins and morpholinosydnonimine but not aspirin inhibit the formation of superoxide and the expression of gp91phox induced by endotoxin and cytokines in pig pulmonary artery vascular smooth muscle cells and endothelial cells. Circulation. 2004;110:1140–1147. doi: 10.1161/01.CIR.0000139851.50067.E4. [DOI] [PubMed] [Google Scholar]
- 37.Pritchard KA, Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 38.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rask-Madsen C, Dominguez H, Ihlemann NEA. Tumor-necrosis factor-a inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circ Res. 2003;203:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt A, Geigenmuller S, Volker W, Buddecke E. The antiatherogenic and antiinflammatory effect of HDL-associated lysosphingolipids operates via Akt → NF-kappaB signalling pathways in human vascular endothelial cells. Basic Res Cardiol. 2006;101:109–116. doi: 10.1007/s00395-005-0582-z. [DOI] [PubMed] [Google Scholar]
- 41.Schulz R, Aker S, Belosjorow S, Heusch G. TNF in ischemia/reperfusion injury and heart failure. Basic Res Cardiol. 2004;99:8–11. doi: 10.1007/s00395-003-0431-x. [DOI] [PubMed] [Google Scholar]
- 42.Seidel M, Billert H, Kurpisz M. Regulation of eNOS expression in HCAEC cell line treated with opioids and proinflammatory cytokines. Kardiol Pol. 2006;64:153–158. discussion 159–160. [PubMed] [Google Scholar]
- 43.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Shu HB, Agranoff AB, Nabel EG, Leung K, Duckett CS, Neish AS, Collins T, Nabel GJ. Differential regulation of vascular cell adhesion molecule 1 gene expression by specific NF-kappa B subunits in endothelial and epithelial cells. Mol Cell Biol. 1993;13:6283–6289. doi: 10.1128/mcb.13.10.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-{alpha} in coronary microembolization: progressive contractile dysfunction vs. delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 46.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidase in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 47.Stenvinkel P. Endothelial dysfunction and inflammation—is there a link? Nephrol Dial Transplant. 2001;16:1968–1971. doi: 10.1093/ndt/16.10.1968. [DOI] [PubMed] [Google Scholar]
- 48.Sury MD, Frese-Schaper M, Muhlemann MK, Schulthess FT, Blasig IE, Tauber MG, Shaw SG, Christen S. Evidence that N-acetylcysteine inhibits TNF-alpha-induced cerebrovascular endothelin-1 upregulation via inhibition of mitogen- and stress-activated protein kinase. Free Radic Biol Med. 2006;41:1372–1383. doi: 10.1016/j.freeradbiomed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 50.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25:1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781–25790. doi: 10.1074/jbc.M001235200. [DOI] [PubMed] [Google Scholar]
- 52.Tanner FC, van der Loo B, Shaw S, Greutert H, Bachschmid MM, Berrozpe M, Rozenberg I, Blau N, Siebenmann R, Schmidli J, Meyer P, Luscher TF. Inactivity of nitric oxide synthase gene in the atherosclerotic human carotid artery. Basic Res Cardiol. 2007;102:308–317. doi: 10.1007/s00395-007-0650-7. [DOI] [PubMed] [Google Scholar]
- 53.Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Buchert A, Kruger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 54.True AL, Rahman A, Malik AB. Activation of NF-kappaB induced by H(2)O(2) and TNF-alpha and its effects on ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L302–L311. doi: 10.1152/ajplung.2000.279.2.L302. [DOI] [PubMed] [Google Scholar]
- 55.Ushio-Fukai M, Zafari AM, Fukui TEA. p22phox is a critical component of the superoxide-generating NADH/NAD(P)H oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Bio Chem. 1996;277:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 56.Valencia JV, Mone M, Zhang J, Weetall M, Buxton FP, Hughes TE. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes. 2004;23:743–751. doi: 10.2337/diabetes.53.3.743. [DOI] [PubMed] [Google Scholar]
- 57.Vasquez-Vivar J, Kalyanaraman B, Martasek PEA. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 59.Warnholtz A, Nickenig G, Schulz EEA. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis:evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 60.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschope C. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 61.White CR, Darley-Usmar V, Berrington WREA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercolesterolemic rabbits. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf G. Free radical production and angitensin. Curr Hypertens Rep. 2000;2:167–173. doi: 10.1007/s11906-000-0078-z. [DOI] [PubMed] [Google Scholar]
- 63.Xia Z, Liu M, Wu Y, Sharma V, Luo T, Ouyang J, McNeill JH. N-acetyl-cysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol. 2006;550:134–142. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, Gao X, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic apo-E knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 65.Yagihashi S, Kamijo M, Baba M, Yagihashi N, Nagai K. Effect of aminoguanidine on functional and structural abnormalities in peripheral nerve of STZ-induced diabetic rats. Diabetes. 1992;41:47–52. doi: 10.2337/diab.41.1.47. [DOI] [PubMed] [Google Scholar]
- 66.Yudkin GS, Stehouwer CD, Emeis JJEA. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 68.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-alpha impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-α-induced impairment of endothelium-dependent vasorelation in coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H1758–H1794. doi: 10.1152/ajpheart.00318.2002. [DOI] [PubMed] [Google Scholar]
- 70.Zhao RZ, Chen X, Yao Q, Chen C. TNF-alpha induces interleukin-8 and endothelin-1 expression in human endothelial cells with different redox pathways. Biochem Biophys Res Commun. 2005;327:985–992. doi: 10.1016/j.bbrc.2004.12.109. [DOI] [PubMed] [Google Scholar]