Abstract
Background
Anergia (lack of energy) is a newly delineated, criterion-based geriatric syndrome. Since heart failure (HF) is a common chronic condition among older adults and a since a cardinal symptom of HF is reduced energy, we characterized the degree of anergia in subjects with HF and evaluated its relevance to disease severity, functional performance and quality of life.
Methods
Prospective 3-month cohort study among a convenience sample of 61 subjects (61±15 years, 48% women, EF 41±16%) with NYHA Class I-III HF were studied. The criterion for anergia was based upon the major criterion “sits around for lack of energy” and any two of six minor criteria. Principal measures in addition to demographic and clinical characteristics included functional performance (NYHA class, 6 minute walk, cardiopulmonary exercise testing), plasma B-type natriuretic peptide and quality of life (SF-12 and Minnesota Living with Heart Failure Questionnaire). To evaluate the relevance of anergia to daily function, each subject wore an actigraph, a watch-like wrist device that continuously and automatically monitors patient activity levels and energy expenditure, for 3 months.
Results
Anergia was prevalent in 39% of this population. Anergia was associated with decrements in functional capacity (higher NYHA class and lower six minute walk distance) as well as reduction in quality of life but was not associated with ejection fraction. Actigraphy data demonstrated that HF subjects with anergia spent significantly less time performing moderate physical activity and the peak activity counts per day were significantly lower than HF subjects without anergia. Additionally, the amplitude of circadian rhythm was lower, suggesting altered sleep and activity patterns in HF subjects with anergia compared to those without anergia. Over the 3 months of follow-up, there was a significant association between anergia and inter-current hospitalization.
Conclusions
Anergia is significantly associated with several of the cardinal domains of heart failure. Its presence is associated with demonstrable differences in both physical activity and circadian rhythm as measured by actigraphy and an increased risk of hospitalizations. Accordingly, anergia may be a target for intervention among heart failure subjects.
Introduction
Physical symptoms are common and distressing problems for patients with chronic disease, including heart failure, and are among the strongest predictors of health-related quality of life.(1-3) Fatigue is a cardinal feature of the syndrome of heart failure(4) and has been reported as the one of the most common symptoms experienced by older adults with heart failure affecting from 20-75%.(5,6) Fatigue has been shown to have independent long-term prognostic implications in patients with heart failure suggesting that fatigue needs to be effectively evaluated not only because symptom alleviation is a target for treatment, but also because of the potential for the treatment of fatigue to influence the prognosis in patients with HF.(4;6) Previous investigations have shown that fatigue in patients with HF is associated with both clinical and psychosocial variables, including anemia, hypothyroidism, sleep disorders, depression, social isolation and disengagement, offering a wide range of targets for intervention.(7-9)
Anergia (lack of energy), a newly delineated criterion based geriatric syndrome that is analogous conceptually to fatigue but is a more persistent state, which has been shown to affect a majority of community dwelling older adults and be of a severe nature in 18%. Additionally, anergia was very distressing among older adults, and older adults with anergia were more often hospitalized and had more office visits, ER visits, and home care services; and had higher mortality rates. Anergia is a concern that is encountered by all medical providers of care to older persons and was strongly associated with cardiovascular syndromes.(10) Since heart failure (HF) is a common chronic condition among older adults and since a principal feature of HF is reduced energy, we sought to characterize the degree of anergia in subjects with heart failure and evaluate its relevance to other standard measures of disease severity, functional performance and quality of life. Additionally, in order to explore the multifaceted nature of anergia and its impact on activities in daily life, we employed continuous monitoring of activity levels using actigraphs, devices that are worn on the wrist, like a watch and automatically monitor activity levels and energy expenditure. The hypotheses to be tested were that anergia: (1) would be a prevalent concern among subjects with heart failure and of sufficient magnitude to warrant clinical evaluation and treatment, (2) would be strongly associated with measures of functional performance, B-type natriuretic peptide levels, quality of life and hospitalizations, and (3) be associated with significant impact on day to day activities including exercise and sleep patterns.
Methods
Study Subjects
Consecutive subjects from the Advanced Cardiac Care Center of Columbia University Medical Center, who had a clinical diagnosis of heart failure, were approached regarding participation in this observational study if they met the inclusion and exclusion criteria. Inclusion criteria included: outpatients, New York Heart Association Class I-III, ambulatory, including with a walker and living in a traditional residence, apartment, and non-communal adult home where they move about freely and frequently and are primarily responsible for scheduling their sleep and daily activities. The diagnosis of heart failure based on the criteria developed by Rich et al(11) which requires a history of acute pulmonary edema or the occurrence of 2 of the following that improves with diuretic therapy without another identifiable cause: dyspnea on exertion, paroxysmal nocturnal dyspnea, orthopnea, bilateral lower extremity edema, or exertional fatigue. Additionally, for those subjects with an EF >50% the European Society of Cardiology criteria(12), were employed to further adjudicate the diagnosis. Subjects were not eligible if they reported problems with skin or extremities that would limit their ability to wear an actigraph 24hrs/day for nine months or were on continuous intravenous inotropes or mechanical circulatory support or could not provide informed consent. The Columbia University Medical Center Institutional Review Board approved the protocol and informed consent was obtained in all subjects.
Study Design
This is a 9-month prospective cohort study, in which heart failure patients were provided an Actical actigraph device (Minimitter Inc, Respironics, Bend, Oregon) and instructed to wear it continuously on their non-dominant wrist. At baseline and at every 3 months for a total of 4 visits each subject undergoes a targeted physical exam including review of concomitant medications, co-morbid diagnoses, BNP, measures of functional capacity and inter-current events (e.g. hospitalizations). The study coordinator on a monthly basis downloaded data from the actigraph during a personal home visit. The current data focuses on the baseline clinical data, data generated at the first 3-month visit, and intervening actigraph data.
Characterization of Anergia, Sleep and Quality of Life
Anergia was defined as a criterion based syndrome based on seven questions, as described previously (Table 1).(10) Sleepiness was assessed by the Epworth Sleepiness Scale.(13;14) To evaluate health related quality of life, we employed a disease specific measure, the Minnesota Living with Heart Failure Questionnaire (MLWHFQ),(15) and a more general scale, the Short Form-12 (16).
Table 1. Prevalence of components used to define anergia.
| Anergia components | N of Subjects (%) (n=61) |
|---|---|
| Recently not enough energy | 38 (62%) |
| Felt slowed physically in month | 31 (51%) |
| Doing less than usual in month | 13 (21%) |
| Any slowness is worse in morning | 16 (26%) |
| Sits around a lot for lack of energy | 26 (43%) |
| Wakes up feeling tired | 37 (61%) |
| Naps during the day (more than 2 hours) | 15 (25%) |
Functional Performance
This was quantified by clinical assessment of New York Heart Association functional class along with a six minute walk test(17). In a subset of patients, cardiopulmonary exercise tests were reviewed for data delineating functional capacity as determined by peak VO2 in ml/kg/min.
Actigraphy
The Actical® (MiniMitter, Respironics, Inc, Bend, Oregon) is a small, wrist watch-like omni-directional accelerometer that provides real-time ambulatory monitoring and can quantify patient activity levels and energy expenditure. Patients wore the device on the non-dominant wrist on a continuous basis, including during sleep. Data was recorded using a 1-minute epoch, resulting in an activity count for each minute of the day. All measures were computed as the average of the first twenty-eight days of valid data for each patient. A four hour block of total inactivity recorded by the device was interpreted as lack of compliance, and therefore rendered a day invalid. Further, patients were excluded due to non-compliance if they did not achieve 28 days of valid data within the first 56 days. Several standard measures were computed from the activity count using the Actical software, including Energy Expenditure and time spent at each of four activity levels (sedentary, light, moderate, and vigorous). Additionally, the following measures were derived from the activity counts. M10, a measure of the patient's daytime activity, is the average total activity count of the most active 10 hours in the day (18). L5, a measure of the patient's nocturnal restlessness, is the average total activity count of the least active 5 hours in the day (18). For both M10 and L5, two variations were computed, representing a daily and weekly value for each. Two new variations of these metrics were used to measure the most active periods of the day: M0.5 and M0.1 are the total activity counts for the most active 30 and 6 minutes of the day, respectively.
Several non-parametric circadian measurements were then calculated. Relative Amplitude (RA) measures the amplitude of the patient's circadian rhythm, and is calculated by the formula (M10-L5)/(M10+L5), using the weekly M10 and L5 values (19). Ideally, patient daytime activity (M10) is high and sleep activity (L5) is low, resulting in a RA value near 1. Inter-daily Stability (IS) measures the stability of activity pattern from day to day, and is high if the patient exhibits a daily routine, with periods of high and low activity occurring at roughly the same time every day. Intra-daily Variability (IV) is an index of fragmentation of rest-activity rhythms in a single day, and is low if the patient is able to maintain high and low activity levels for a sustained period of time.(18)
Statistical Analysis
Data are expressed as mean ± SD, unless otherwise noted. Since data on BNP was not normally distributed, it was log transformed for further analyses. The differences in demographic and clinical measures were compared between the groups with and without anergia. A chi-square analysis with Fisher's exact test was used for dichotomous variables and a student's t-test for un-paired comparisons was employed for continuous variables. Associations between anergia and cardinal features of heart failure (ejection fraction, exercise performance, and quality of life) were determined by use of Pearson's correlation coefficient. In order to evaluate associations of clinical and demographic features with subsequent hospitalizations, we performed a multivariate logistic regression analysis using a forward selection model. The following parameters were entered into the multivariate model: age, NYHA Class, 6-minute walk distance, B-type natriuretic peptide levels, depression and anergia status. A p value <0.05 was considered to be statistically significant. SAS for Windows (Version 8.0, SAS Institute Inc., Cary, North Carolina) was used for all analyses.
Results
The demographic and clinical characteristics of the study population are delineated in Table 2. Subjects ranged in age from 25 to 93 years of age and were multi-ethnic, with nearly equal men and women. Subjects had, on average, NYHA class II heart failure, which was demonstrated in reduced six-minute walk distances and reduced peak oxygen consumption. The average EF was 41±16, with 36% of the sample having an EF>50%. Concordant with other heart failure populations, numerous other co-morbid conditions were present with an average of 2 co-morbidities per subject. Subjects had reductions in quality of life on the physical domain of the SF-12 and on both physical and mental domains of the MLWHFQ.
Table 2. Demographic and Clinical Characteristics.
| Parameter | Overall (n=61) | No-Anergia (n=37) | Anergia (n=24) |
|---|---|---|---|
| Age (years) | 61±15 | 58±15 | 65±15 |
| Gender (% female) | 48% | 49% | 46% |
| Ethnicity (%) | |||
| -Non-Hispanic | 85 | 84 | 87 |
| -Hispanic | 15 | 16 | 13 |
| Race | |||
| -White | 62 | 62 | 63 |
| -Black | 20 | 16 | 25 |
| -Asian/Pacific Islander | 15 | 16 | 13 |
| -Other | 3 | 5 | 0 |
| BMI | 28±6 | 28±6 | 29±6 |
| Regular Exercise* | 28% | 38% | 13% |
| VO2 (ml/kg/min) | 17±3 | 18±3 | 15±3 |
| BNP (pg/ml) | 379±544 | 280±451 | 537±647 |
| Log BNP | 5.0±1.6 | 4.7±1.6 | 5.5±1.5 |
| Six minute walk (meters)* | 386±116 | 418±112 | 337±108 |
| Ejection Fraction (%) | 41±16 | 39±15 | 44±17 |
| NYHA Class* | 2.0±0.6 | 1.9±0.5 | 2.3±0.6 |
| SF-12 | |||
| -Physical* | 39±11 | 42±10 | 36±12 |
| -Mental | 50±11 | 53±9 | 44±11 |
| Minnesota Living with HF | |||
| -Overall* | 37±24 | 28±21 | 53±24 |
| -Physical* | 17±10 | 13±9 | 23±9 |
| -Emotional* | 8±7 | 6±7 | 11±6 |
| Co-Morbidities (%) | |||
| -Hypertension | 54 | 51 | 58 |
| -Diabetes | 23 | 22 | 25 |
| -Ischemic HD | 16 | 19 | 13 |
| -Sleep Disorder | 13 | 8 | 21 |
| -Depression* | 13 | 5 | 25 |
| -Hypothyroidism | 15 | 22 | 4 |
| -Anemia | 26 | 19 | 38 |
| -Chronic Pain | 16 | 11 | 25 |
| -COPD | 15 | 11 | 21 |
p <0.05 for comparison of anergia and non-anergic subjects
Anergia, of significant magnitude to be associated with increased morbidity and mortality(10), was prevalent in 39% of this population. HF subjects with anergia did not differ from those without anergia in age, gender, race, ethnicity, and prevalence of obesity nor ejection fraction but were more often diagnosed with depression. However, heart failure subjects with anergia demonstrated decrements in functional capacity (higher NYHA class and lower six minute walk distance) and reported significantly less regular exercise than subjects without anergia. Additionally, significant differences in the physical domain of quality of life as determined by the SF-12 and both physical and emotional domains as assessed by the MLWHFQ were present. Plasma B-type natriuretic peptide tended to be higher and peak VO2 tended to be lower (p<0.1) among individuals with heart failure and anergia as compared to the heart failure subjects without anergia. Hypothyroidism, chronic pain and anemia tended to be more common in the heart failure subjects with anergia as compared to their non-anergia counterparts.
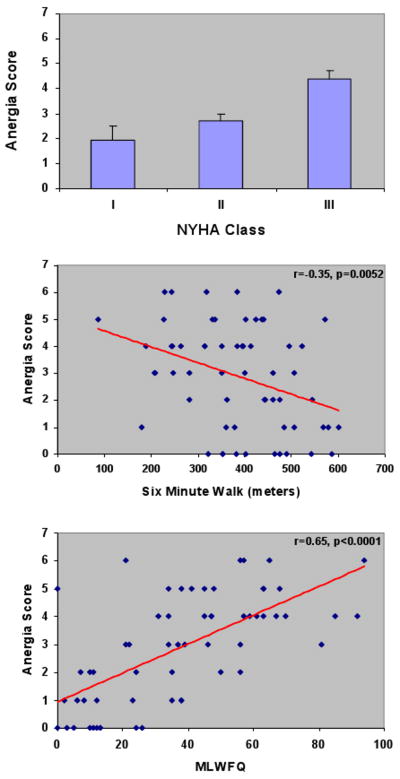
As demonstrated in figure 1, there were significant positive associations of anergia when graded on a linear scale (range 0 to 7) with NYHA class (p< 0.01 by ANOVA) as well as positive associations with overall quality of life as assessed by the MLWHFQ and inverse associations with six-minute walk distance. There was no significant association between anergia and levels of plasma B-type natriuretic peptide (r=0.106, p = 0.43). Evaluation of actigraphy data during the initial 28 days demonstrated that heart failure subjects with anergia spent less time performing moderate physical activity per day, and the peak activity counts for a six minute and 30 minute period per day were significantly lower than heart failure subjects without anergia. Analysis of the circadian rhythm measures IS and IV did not differ between heart failure subjects with and without anergia, however RA was lower, indicating lower circadian rhythm amplitude in heart failure subjects with anergia compared to those without anergia (Table 3). Significant inverse correlations between anergia grade (0 to 7) and the RA were present (r=-0.379, p=0.0048).
Figure 1.
Table 3. Actigraphy Data.
| Parameter | Overall (n=56) | No-Anergia (n=35) | Anergia (n=21) |
|---|---|---|---|
| Energy Expenditure (kcal) | |||
| Total EE | 922±365 | 946±360 | 884±380 |
| Activity Time (minutes) | |||
| Sedentary | 665±148 | 660±122 | 674±187 |
| Light | 693±119 | 689±90 | 701±159 |
| Moderate | 81±50 | 91+53* | 65±41 |
| Vigorous | 0.2±0.4 | 0.2±0.4 | 0.1±0.4 |
| Activity (counts/day) | |||
| Activity Counts | 283608±103337 | 298631±105132 | 258571±97587 |
| M0.1 –Day | 9778±3655 | 10647±3738* | 8328±3077 |
| M0.5 – Day | 24188±8203 | 25880±8285* | 21369±7418 |
| M10 – Day | 21571±7623 | 22829±7752 | 19475±7088 |
| L5 – Day | 1016±976 | 886±515 | 1241±1143 |
| M10 Consecutive week | 17839±6564 | 18710±6695 | 16470±6264 |
| L5 Consecutive week | 2177±1525 | 1954±1209 | 2528±1901 |
| Circadian Rhythm | |||
| IS | 0.47±0.09 | 0.48±0.09 | 0.45±0.09 |
| IV | 0.98±0.21 | 0.99±0.21 | 0.96±0.21 |
| RA | 0.78±0.11 | 0.81±0.08* | 0.74±0.12 |
p <0.05 for comparison of anergic and non-anergic subjects
During the initial 3 months of follow-up, among the heart failure subjects with anergia at baseline, 16 (67%) had persistent anergia at 3-months, and of those without anergia at baseline, 5 (14%) developed anergia over time. Of note, subjects without anergia at baseline who subsequently developed anergia had similar decrements in daily energy expenditures as subjects who reported anergia (780±290 vs. 649±206 kcal/day) which was significantly lower than subjects without anergia and those that improved (946±372 vs. 953±455 kcal/day, respectively). During the same follow-up period, seven of the 24 heart failure subjects with anergia at baseline (29%) had an inter-current hospitalization, while only 2 of the 37 heart failure subjects without anergia (5%) had an inter-current hospitalization (p=0.02, Odds ratio (95% CI) of 7.2 (1.35-38.47). In a multivariate logistic regression analysis to evaluate for independent associations with inter-current hospitalizations that included age, NYHA Class, 6-minute walk distance, B-type natriuretic peptide levels, depression and anergia status, anergia emerged as only predictor of hospitalization (Odds Ratio of 7.7 with a 95% CI of 1.43-41.56, p=0.02).
Discussion
The principal findings of this prospective cohort study are that anergia, a syndrome of lack of energy, is: (1) common in subjects with heart failure; (2) significantly associated with several of the cardinal domains of heart failure including subjective and objective measures of functional capacity, reduced quality of life (but not ejection fraction); (3) associated with several co-morbid conditions that could be causal or contributing factors; (4) is a potentially modifiable condition, as demonstrated by the ways in which the presence and degree of anergia change over a 3 month period; and (5) associated with significant differences in circadian rhythm patterns and activity as assessed by continuous monitoring using actigraphy. Collectively, these data suggest that further evaluation and understanding of the factors that contribute to the development of anergia warrant additional attention and that anergia may be a target for intervention among heart failure subjects, which could potentially be assessed using actigraphy.
Based on data from a multi-ethnic population based sample, anergia was associated with a range of clinical symptoms and diseases, extensive use of health services and increased mortality, and was therefore proposed as a central feature for identifying, evaluating and treating older adults with health related problems in quality of life.(10) The current data extend these observations to a population of predominately older adults, who have a specific disorder, namely heart failure. The relationship between anergia or lack of energy and functional status has important implications for subjects with heart failure. First, lack of energy is associated with increased morbidity and mortality. In the COMET trial, a randomized prospective study comparing the efficacy of the beta-blockers metoprolol versus carvedilol in patients with systolic heart failure, fatigue, as assessed on a five point scale, was significantly related to increased mortality and development of worsening heart failure. In a multivariate Cox regression analysis including 16 baseline covariates, fatigue remained a significant predictor for developing worsening heart failure (RR 1.09 per unit; 95% CI 1.02-1.18; P = .02).(4) Additionally, fatigue limits daily activities, has negative consequences on health and independence and has been noted as the main reason for not participating in outpatient HF treatment programs.(20)
Although clinicians believe that fatigue is a symptom amenable to treatment, there has been little focused attention on interventions aimed directly at reducing HF-related fatigue. First, the ability to validate and reliably identify HF subjects with lack of energy has contributed to the clinical inertia. Second, the broad differential diagnosis and the lack of a standardized and systematic approach to the evaluation and management of fatigue in subjects with heart failure undoubtedly contribute to the inactivity on the part of providers. Third, the lack of effective treatments for fatigue and/or the lack of health care systems and appropriate reimbursement to institute specific therapies, such as cardiac rehabilitation, may contribute to a discrepancy between the level of patient's suffering and degree of clinical intervention. Finally, the inability to objectively monitor the impact of therapeutic interventions on energy levels and the reliance on cardiovascular measures that have not been shown to be associated with the severity of fatigue nor the change in energy in response to treatment,(21) has also contributed to therapeutic inertia in this arena. This is reflected in heart failure disease management guidelines (22), which emphasize the symptomatic nature of the heart failure syndrome but do not address the management of symptoms such as fatigue.
Our data suggests that a simple self administered criterion based approach may facilitate the identification and monitoring of subjects with heart failure who have degrees of lack of energy that warrant clinical intervention, as they are associated with reduced functional capacity, quality of life and subsequent morbid events such as hospitalizations. Additionally, the current data suggest that physical activity levels are reduced in HF subjects with anergia, and also that circadian rhythm is altered in these same subjects. Accordingly, efforts to address these two causal or contributing factors for anergia in HF subjects seem like fruitful areas for initial intervention that could be monitored by actigraphy.
Actigraphy is a methodology for recording and analyzing activity from small, computerized devices worn on the body.(23) A majority of the published reports have focused on the utility of these devices to quantify sleep, estimated by scoring algorithms that are relatively consistent with polysomnography scored sleep.(24;25) Additionally, the technology has been applied to estimate physical activity in population based studies, mainly focused on children(26), and in certain chronic conditions for which it is a useful methodology to investigate group differences,(27;28) sleep-pattern variations over time, and the effects of interventions.(29) Discrepancies between self report and actigraphy measures have been documented(30) suggesting that they provide complimentary and important information on relevant parameters. However, we are aware of only two other studies that have been performed in heart failure subjects that have employed actigraphy.(31;32) Both of these studies focused predominately on HF subjects with systolic heart failure and were cross sectional observational cohort studies that employed actigraphy data for a duration of 3 to 14 days. These studies reaffirmed the independent associations of sleep continuity as assessed by actigraphy with functional performance and mental health in stable heart failure patients(31) and demonstrated that in the presence of sleep disorder breathing, subjects with HF demonstrated less daytime activity, greater movements during sleep and greater fragmentation index with actigraphy monitoring than those without sleep disordered breathing.(32) Collectively, these data suggest a mechanistic link between heart failure, sleep disorders and impairments in health related quality of life that may be operative through increases in anergia.
Accordingly, a focus of the current work is to develop strategies that improve patient outcomes and enhance heart failure disease management programs with real time data proven to correlate with disease state and self reported quality of life. The current data coupled with previous investigations suggests that actigraphy could be useful to evaluate the effectiveness of the approaches aimed at targeting anergia or its contributing or causal factors, with parameters from actigraphy serving as a principal measure of efficacy.
This is a small, but prospective study among a predominately older, multi-ethnic cohort of subjects with HF from an urban community. Additionally, subjects were recruited from the Advanced Cardiac Care Center (formerly Heart Failure Clinic) of our institution and thus the generalizability of these data to patients with less advanced disease is not clear. Confirmation of these findings and exploration of additional questions raised by these data will require larger datasets. Additionally, several of the clinical examinations (e.g. echocardiograms and cardiopulmonary exercise tests) were performed as part of routine clinical care in close proximity but not on the same day as the assessments of anergia, and thus the effect of intervening time between assessments is a potential confounder. The criterion employed to define anergia and quantify its severity has good psychometric properties that were derived from latent class analysis with high content, clinical and face validities, good internal and inter-rater consistent reliabilities, as well as well as concurrent and predictive validity for future morbidity and mortality.(10) However, these criteria should be viewed as preliminary, with formalized criteria for anergia awaiting prospective investigation and future study to define consensus. The energy expenditure prediction equations employed by actigraphy have been shown to be valid(33;34) but small differences have been detected in comparison with calorimetry, raising concerns regarding the ability of these devices to accurately characterize whether specific physical activity goals are being achieved.(35) Finally, questions about the need to control for artifacts and the duration of actigraphy recording have been raised, which we addressed by employing a recording period that was relatively long for this technology (e.g. 28 days), thereby providing reliable measures that can capture important variations across time.
In conclusion, this small, prospective cohort 3 month study of subjects with HF demonstrated that anergia was common and was significantly associated with several of the cardinal domains of heart failure. Longitudinal analysis of real world physical activity and circadian rhythm using actigraphy revealed demonstrable differences in those HF subjects with and without anergia. Additionally, during 3 months of follow-up, anergia was strongly associated with inter-current hospitalizations. Collectively, these data suggest that anergia may be a target for intervention among heart failure subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Walke LM, Gallo WT, Tinetti ME, Fried TR. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164(21):2321–2324. doi: 10.1001/archinte.164.21.2321. [DOI] [PubMed] [Google Scholar]
- 2.Walke LM, Byers AL, Gallo WT, Endrass J, Fried TR. The association of symptoms with health outcomes in chronically ill adults. J Pain Symptom Manage. 2007;33(1):58–66. doi: 10.1016/j.jpainsymman.2006.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo S, Doering LV, Widener J, Moser DK. Predictors and effect of physical symptom status on health-related quality of life in patients with heart failure. Am J Crit Care. 2008;17(2):124–132. [PubMed] [Google Scholar]
- 4.Ekman I, Cleland JG, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11(4):288–292. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR. Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med. 2007;167(22):2503–2508. doi: 10.1001/archinte.167.22.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes S, Gott M, Payne S, Parker C, Seamark D, Gariballa S, et al. Prevalence of symptoms in a community-based sample of heart failure patients. J Pain Symptom Manage. 2006;32(3):208–216. doi: 10.1016/j.jpainsymman.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista LS, Moser DK, Westlake C, Pike N, Ter-Galstanyan A, Dracup K. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs. 2008;23(1):12–17. doi: 10.1111/j.1751-7117.2008.07275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falk K, Granger BB, Swedberg K, Ekman I. Breaking the vicious circle of fatigue in patients with chronic heart failure. Qual Health Res. 2007;17(8):1020–1027. doi: 10.1177/1049732307306914. [DOI] [PubMed] [Google Scholar]
- 9.Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue and anaemia in patients with chronic heart failure. Eur J Heart Fail. 2006;8(7):744–749. doi: 10.1016/j.ejheart.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Gurland B, Maurer MS. Self-Reported Lack of Energy (Anergia) among Elders in a Multiethnic Community. J Gerontol A Biol Sci Med Sci. 2008;63(7):707–14. doi: 10.1093/gerona/63.7.707. [DOI] [PubMed] [Google Scholar]
- 11.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 13.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21(1):27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Choi JB, Nelesen R, Loredo JS, Mills PJ, ncoli-Israel S, Ziegler MG, et al. Sleepiness in obstructive sleep apnea: a harbinger of impaired cardiac function? Sleep. 2006;29(12):1531–1536. doi: 10.1093/sleep/29.12.1531. [DOI] [PubMed] [Google Scholar]
- 15.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing the psychometric properties of the Minnesota Living with Heart Failure questionnaire. Nurs Res. 2005;54(4):265–272. doi: 10.1097/00006199-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Ni H, Toy W, Burgess D, Wise K, Nauman DJ, Crispell K, et al. Comparative responsiveness of Short-Form 12 and Minnesota Living With Heart Failure Questionnaire in patients with heart failure. J Card Fail. 2000;6(2):83–91. doi: 10.1054/jcaf.2000.7869. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 18.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the Circadian Rest-Activity Rhythm in Aging and Alzheimer's Disease. Biol Psychiatry. 1990;(27):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 19.Van Someren EJW, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright Light Therapy: Improved Sensitivity to Its Effects on Rest-Activity Rhythms in Alzheimer Patients By Application of Nonparametric Methods. Chronobiology International. 1999;16(4):505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 20.Ekman I, Fagerberg B, Skoog I. The clinical implications of cognitive impairment in elderly patients with chronic heart failure. J Cardiovasc Nurs. 2001;16(1):47–55. doi: 10.1097/00005082-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Shah MR, Hasselblad V, Stinnett SS, Kramer JM, Grossman S, Gheorghiade M, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4(3):297–304. doi: 10.1016/s1388-9842(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 22.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12(1):23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6(2):113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 26.de Vries SI, Bakker I, Hopman-Rock M, Hirasing RA, van M W. Clinimetric review of motion sensors in children and adolescents. J Clin Epidemiol. 2006;59(7):670–680. doi: 10.1016/j.jclinepi.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Landis CA, Frey CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JL. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res. 2003;52(3):140–147. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Friedman L, Benson K, Noda A, Zarcone V, Wicks DA, O'Connell K, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 29.Redeker NS, Wykpisz E. Effects of age on activity patterns after coronary artery bypass surgery. Heart Lung. 1999;28(1):5–14. doi: 10.1016/s0147-9563(99)70038-5. [DOI] [PubMed] [Google Scholar]
- 30.Harris TJ, Owen CG, Victor CR, Adams R, Cook DG. What factors are associated with physical activity in older people, assessed objectively by accelerometry? Br J Sports Med. 2008 doi: 10.1136/bjsm.2008.048033. [DOI] [PubMed] [Google Scholar]
- 31.Redeker NS, Hilkert R. Sleep and quality of life in stable heart failure. J Card Fail. 2005;11(9):700–704. doi: 10.1016/j.cardfail.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Hastings PC, Vazir A, O'Driscoll DM, Morrell MJ, Simonds AK. Symptom burden of sleep-disordered breathing in mild-to-moderate congestive heart failure patients. Eur Respir J. 2006;27(4):748–755. doi: 10.1183/09031936.06.00063005. [DOI] [PubMed] [Google Scholar]
- 33.Slootmaker SM, Chin APM, Schuit AJ, van MW, Koppes LL. Concurrent validity of the PAM accelerometer relative to the MTI Actigraph using oxygen consumption as a reference. Scand J Med Sci Sports. 2008 doi: 10.1111/j.1600-0838.2007.00740.x. [DOI] [PubMed] [Google Scholar]
- 34.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 2007;15(10):2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- 35.Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of Physical Activity Intensity Predictions by ActiGraph, Actical, and RT3 Accelerometers. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]