Abstract
Monitoring reproductive rates in experiments involving aedine mosquitoes is tedious and time demanding. Here, we demonstrate a protocol for rapid estimation of aedine mosquito egg number. The protocol uses ImageJ, a publicly available image analysis program developed at the US National Institutes of Health. The method relies upon the oviposition behavior of Aedes (i.e., ovipositing on a moist substrate instead of water surface) and upon the contrast between dark-colored aedine eggs and a light, uniformly colored paper that is used as an oviposition substrate. The results for 3 Aedes species show that, following the generation of separate calibration curves for each species, the protocol allows for the accurate and repeatable estimation of samples containing hundreds of aedine eggs. We discuss the use of the protocol for monitoring immature aedine populations in both laboratory and field applications.
Keywords: Egg number, ImageJ, eggs, mosquito, fecundity, digital imagery
INTRODUCTION
Fecundity is an important life-history character in determining mosquito fitness (Briegel and Timmermann 2001). Monitoring reproductive rates in most field and laboratory experiments involving aedine mosquitoes has traditionally involved manually counting eggs using a microscope. Manual calculations are tedious, time consuming, and often subject to human error and bias. Alternative proxies such as measuring pupal mass and wing length have been shown to correlate significantly with fecundity but are often as labor intensive as egg counting (Armbruster and Hutchinson 2002, Blackmore and Lord 2005).
Population biology and vector control research require the counting of multiple samples, each with thousands of aedine eggs. Thus, we have developed a technique to reduce the time and potential error involved in estimating egg number (used here as the quantity of eggs laid) in container-breeding aedine mosquitoes. The procedure is based upon a digital scanner (readily available and relatively inexpensive) and the publicly available ImageJ software, a Java-based image-processing program developed at the US National Institutes of Health (Barboriak et al. 2005).
ImageJ software has become a valuable tool in research involving the analysis of digitized images. It has been used for diverse applications across a broad spatial scale, ranging from molecular experiments involving counting nucleated erythrocytes in blood smears (Gering and Atkinson 2004) to field studies analyzing coral reef expansion (Johnson and Perez 2006). Examples of entomological applications include an examination of ant nest structure by X-ray–computed tomography (Halley et al. 2005) and the early detection of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) pupae in hard red winter wheat (Toews et al. 2006).
Here, we describe a technique that can process large samples in a short time period, compared to traditional manual counts. The technique is repeatable and independent of specialized training required for microscopic methods.
MATERIALS AND METHODS
To determine its robustness and applicability to multiple species, the technique was repeated with 3 aedine species. Mosquitoes used in experiments were the Uju Tet strain of Aedes albopictus Skuse (Dobson and Rattanadechakul 2001, Dobson et al. 2002), the APM strain of Aedes polynesiensis Marks (Dean and Dobson 2004), and the Waco strain of Aedes aegypti (L.) (courtesy of G. Craig, University of Notre Dame). All strains have been in laboratory culture for >50 generations. Maintenance of mosquitoes is as described by Gerberg et al. (1994). A cup lined with brown heavy-weight seed germination paper (Anchor Paper Company, St. Paul, MN) and partially filled with deionized water is provided to adult females. Germination paper is used due to its rough surface, durability, and low cost. However, alternate paper (e.g., filter paper) with a homogeneous, light coloration could be used. The oviposition paper is cut to fit the contours of the cup without overlap and to promote uniform oviposition across the oviposition paper (Fig. 1). Since newly oviposited eggs are white and fragile, eggs are allowed to melanize and develop for 5 days prior to imaging. For egg maturation, Ae. aegypti and Ae. albopictus oviposition papers are held in a vented container over a 7-day period before scanning. Because they tolerate desiccation to a lesser extent compared to Ae. albopictus and Ae. aegypti, Ae. polynesiensis oviposition papers are kept wet until hatching (including scanning while still damp).
Fig. 1.

(A) Example of a scanned oviposition paper with eggs, converted to an 8-bit (black and white) image using ImageJ. (B) Egg paper with background digitally subtracted.
Oviposition papers are placed with eggs toward the imager and held flat using a small stack of white paper. The lid of the scanner is not closed to avoid crushing eggs. Multiple oviposition papers can be placed simultaneously upon the imager, but depending upon the intended downstream use of oviposition papers, care must be taken to prevent transfer of eggs between papers (cross-contamination). If cross-contamination is a concern, the scanner plate must be cleaned and inspected between scans.
To assess the robustness of the approach, 2 scanners from different manufacturers and driven via different computer operating systems (Macintosh and Windows) were tested. The procedure was initially developed using an HP Model 4850 scanner (Hewlett-Packard, Palo Alto, CA) and the Macintosh 10.4 operating system (Apple, Cupertino, CA). Subsequently, the protocol was repeated using a Microtek Scanmaker 6800 (Microtek USA, Carson, CA) and the Windows XP operating system (Microsoft Corporation, Redmond, WA). Other scanner brands with sufficient resolution are appropriate, although their technical definitions will differ. Images were captured using the software supplied with the scanners (HP Precisionscan Pro 3.2 and Microtek Scan Wizard 5 V6.20, respectively) and then analyzed using the ImageJ 1.37 v software (Barboriak et al. 2005). ImageJ versions are available for multiple operating systems (Linux, Macintosh, and Windows), and the source code is freely available online (http://rsb.info.nih.gov/ij/).
For scanning, the PREVIEW function (default settings) is used to generate a histogram for the scanned area (Fig. 2). Both of the employed scanner programs are similar in that they allow for adjustment of HIGHLIGHTS, SHADOWS, and MIDTONES. Only the HIGHLIGHTS setting is adjusted (i.e., SHADOWS and MID-TONES remain at the default settings). The HIGHLIGHTS setting is adjusted to exclude values lighter in color than the eggs and oviposition paper (Fig. 2). Oviposition papers are then scanned in true color at a resolution of ≥600 dots per inch. Scanned images are saved as jpeg files for subsequent ImageJ analysis.
Fig. 2.
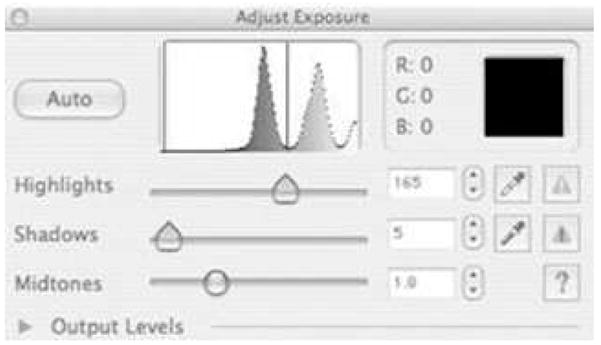
Histogram output from an oviposition paper scanned using an HP Model 4850 scanner (Hewlett-Packard, Palo Alto, CA). The left region (including the leftmost peak) includes the oviposition paper and eggs. The HIGHLIGHTS function is adjusted to deemphasize areas of lighter color (e.g., white background).
In the ImageJ program, images captured by scanners are converted to 8-BIT (black and white) images using the IMAGE: TYPE submenu. Subsequently, the THRESHOLD function (IMAGE: ADJUST submenu) is used (default or AUTO settings) to select only the dark area within the image (i.e., image area containing eggs and not the paper). Following use of the THRESHOLD function, if substantial debris (e.g., dead mosquitoes or other non-egg material) occurs on the paper, this can be digitally removed from the image using the BEZIER tool (select and delete). The TOTAL PARTICLE AREA of eggs is then determined using the ANALYZE: ANALYZE PARTICLES submenu with the following settings: SIZE: 30–INFINITY, CIRCULARITY: 0–1, SUMMARIZE: On, EXCLUDE ON EDGES: On. The minimal size is set to 30 pixel2 to avoid counting small debris. CIRCULARITY is set to include all shapes, and EDGE EXCLUSION is set to exclude objects in contact with the perimeter. The summary information includes a count of objects and TOTAL PARTICLE AREA. These procedures can successfully be combined into a single macro as described on the ImageJ homepage (http://rsb.info.nih.gov/ij/).
Mean digital surface area (mm2) was tested by measuring individual eggs from each of the 3 species used. Digital images (jpeg) of eggs were captured using a microscope- (Leica Microsystems Inc., Bannockburn, IL) mounted camera (Magnafire; Olympus, Melville, NY). Two-dimensional surface area was measured using the MEASURE command of ImageJ. Measurements were calibrated using a slide micrometer with increments of 0.1 mm, which was photographed at the same magnification. All statistical tests were performed using Minitab 15 (Minitab Inc., State College, PA).
RESULTS
Initial efforts were with Ae. albopictus eggs. Eggs on oviposition papers were counted manually using a dissecting microscope and then scanned and analyzed using ImageJ. Oviposition papers of different sizes and with different numbers of eggs were analyzed. Subsequently, calibration curves were generated for 2 additional aedine species: Ae. aegypti and Ae. polynesiensis. As shown in Fig. 3, although different regression equations were obtained for the species, good fits were obtained from calibration plots for all of the species. Overall, more of the experimental variation is explained for Ae. aegypti (R2 = 0.989) and Ae. albopictus (R2 = 0.985) than for Ae. polynesiensis (R2 = 0.957; Fig. 3).
Fig. 3.
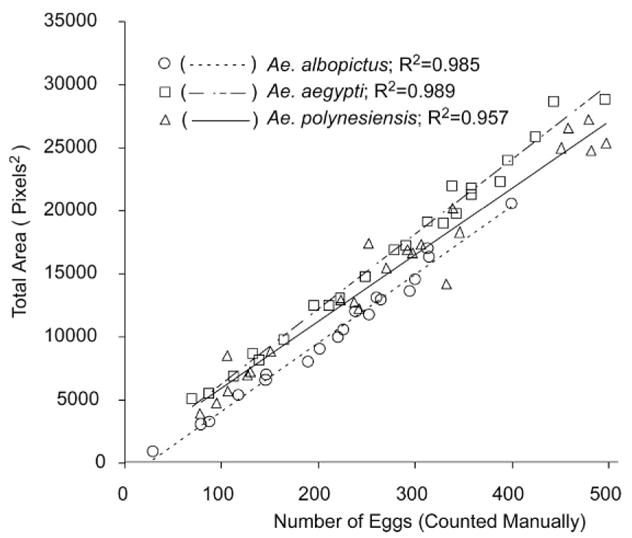
Linear regressions of TOTAL PARTICLE AREA of Aedes albopictus (Y = 54.09X − 1271; N = 19), Ae. aegypti (Y = 59.44X + 337.5; N = 23), and Ae. polynesiensis (Y = 52.80X + 693.2; N = 23) eggs as functions of manual egg counts.
The ImageJ analysis results include 2 measurements that potentially could be used for estimating egg number: PARTICLE COUNT and TOTAL PARTICLE AREA. The PARTICLE COUNT was not as well correlated with the number of eggs (determined by manual counting) as TOTAL PARTICLE AREA (Fig. 4).
Fig. 4.
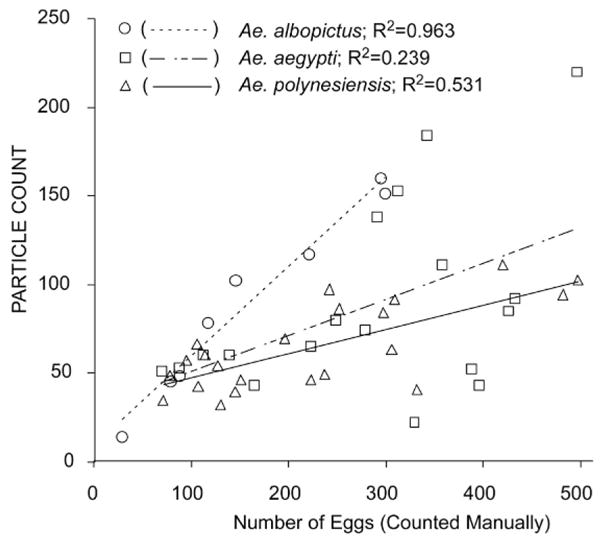
Linear regressions of PARTICLE COUNT of Aedes albopictus (N = 19), Ae. aegypti (N = 23), and Ae. polynesiensis (N = 23) eggs as functions of manual egg counts.
The above procedure was repeated with Ae. albopictus using a second scanner (Scanmaker 6800; Microtek USA) to determine whether the accuracy of the procedure is dependent upon scanner type. The procedure performed equivalently (R2 = 0.992; data not shown).
The scanning process was not observed to affect eggs: hatch rates did not differ between eggs that were scanned (Ae. albopictus, 85% hatch; Ae. aegypti, 92% hatch; Ae. polynesiensis, 78% hatch) or eggs that were not scanned (83%, 94%, and 75%, respectively; t = −0.865; df = 3; P = 0.159; paired t-test).
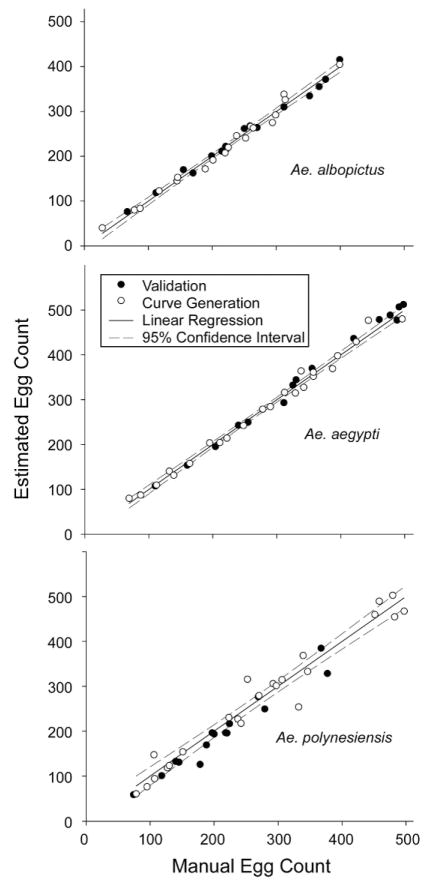
To assess the accuracy of the calibration curves generated for each species, additional samples of varying densities were tested (i.e., different oviposition papers that were not used to generate calibration curve formulas) (Fig. 5). A paired t-test was used to compare egg number estimates generated by the calibration curves and manual counts of the samples. No significant difference was found for Ae. albopictus (t = −0.30; df = 15; P = 0.771) or Ae. aegypti (t = −1.45; df = 14; P = 0.169) in comparing manual counts to estimates. However, there was a significant difference in the comparison of manual counts to estimates for Ae. polynesiensis (t = 3.28; df = 14; P = 0.005).
Fig. 5.
Validation of computer-based egg estimation approach. Egg counts were estimated from oviposition papers with unknown numbers of eggs (“Validation” oviposition papers, solid circles; Aedes albopictus, N = 14; Ae. aegypti, N = 15; and Ae. polynesiensis, N = 15). Subsequently, the numbers of eggs were determined via manual counts. For comparison, the samples used to generate linear regressions (“Curve Generation” oviposition papers, open circles; Aedes albopictus, N = 19; Ae. aegypti, N = 23; and Ae. polynesiensis, N = 23) are plotted along with the linear regressions and 95% confidence intervals. The latter data are the same information shown in Fig. 3.
For egg size, a Box–Cox inverse transformation was performed for digital surface area (mm2) to correct for the non-normal data set and a 1-way ANOVA was used to compare mean egg sizes among the species. A significant difference was found among the 3 species, with Ae. aegypti having significantly larger eggs (F = 33.65; df = 2; P < 0.001) compared to Ae. albopictus and Ae. polynesiensis (Fig. 6). Post hoc comparisons for differences among the means were determined using 95% confidence intervals based upon pooled standard deviations. Similarly, differences were found for the estimated volumes (mm3) of the 3 species calculated from length and width (Sota and Mogi 1992), as measured using ImageJ (data not shown). There was no difference in Ae. albopictus egg size as a function of endosymbiont infection status. In Ae. albopictus, the uninfected Uju Tet strain (7.6 ± 0.5; n = 21) and the naturally superinfected IH strain (8.3 ± 0.2; n = 15) had equal mean egg volumes.
Fig. 6.
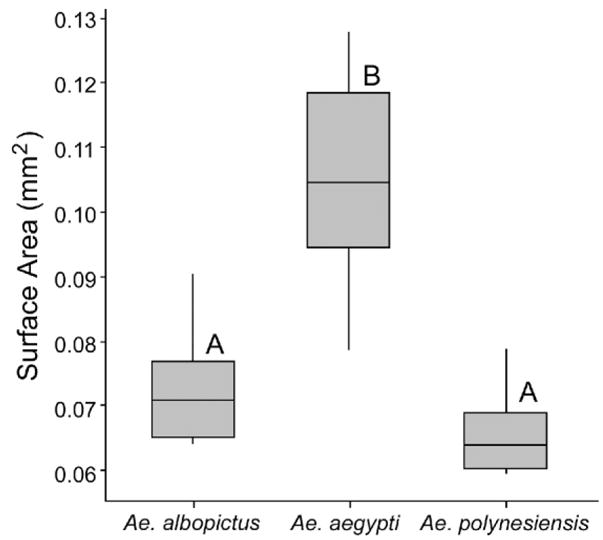
Box plot of egg areas (mm2) measured via ImageJ: Aedes albopictus, N = 12; Ae. aegypti, N = 14; and Ae. polynesiensis, N = 15. Different letters represent statistic groups (P < 0.05).
DISCUSSION
The time required to manually count eggs can be an experimental complication, requiring substantial effort, time, and resources. Advantages of the method described here include reduced time, greater consistency, and reduction of human error and bias. Using this technique, we have processed oviposition papers with thousands of eggs in <5 min, which is a substantial improvement over the ≥30 min per oviposition paper required for manual counting. Furthermore, the scanned images provide permanent records of mosquito reproductive rates, allowing for subsequent manual counting if desired. The described procedure can accurately estimate egg number across a broad range of egg densities and is appropriate for multiple aedine species. The procedure utilizes free software and does not require expensive or specialized hardware.
Only aedine eggs have been examined here due to their oviposition behavior of attaching eggs to a substrate, which simplifies the imaging process. Potentially, an analogous process with species that oviposit on water (e.g., Culex or Anopheles) could be accomplished using a method to concentrate eggs in a monolayer toward the water surface center (e.g., a container with hydrophobic edges) and a device that images from above and at a distance (e.g., a digital camera with a high-resolution macro setting).
Relative to TOTAL PARTICLE AREA–based estimations, egg number estimations based upon PARTICLE COUNT were less predictive for all 3 species. This is likely due to the PARTICLE COUNT algorithm in ImageJ and oviposition tendency in which eggs are deposited in clusters (Judson 1967). Specifically, clusters of adjacent eggs are counted as a single particle when the software fails to recognize individual eggs. The inability to discriminate individual eggs results in poor estimations, particularly at high egg densities. In contrast to the PARTICLE COUNT function, the TOTAL PARTICLE AREA does not rely on the capacity to differentiate single eggs and is less affected by egg clusters. The TOTAL PARTICLE AREA would be affected if eggs were overlaid on top of each other, but this was not observed, even at the highest densities. If necessary, female density or oviposition period can be adjusted.
Although digital estimation is useful for Ae. polynesiensis, lower accuracy was observed relative to Ae. aegypti and Ae. albopictus. The lower accuracy may result from the scanning of wet oviposition paper, which is darker relative to dry paper, reducing the contrast between egg and paper. Residual water around eggs may result in light refraction, blurring the edges of eggs and reducing accuracy of the estimation approach. The eggs of Ae. polynesiensis are less desiccation resistant than those of Ae. albopictus and Ae. aegypti in general (Sota and Mogi 1992). Since maximum egg survivorship was desired for downstream experiments, oviposition papers were kept wet. While the digital estimation procedure was observed to be sufficiently predictive, future accuracy may be improved by using oviposition paper providing greater contrast (e.g., white instead of brown).
The described technique is appropriate for a range of applications and can be potentially extended to additional insects of importance in which fecundity measurements are required. Lab applications that use egg number as an indicator (e.g., cage testing various oviposition attractants for container-breeding Aedes species) would logically benefit from this technique. The approach will also be useful for standardizing mass rearing. Indeed, a similar approach is currently being used in an Ae. albopictus mass rearing effort associated with a Sterile Insect Technique program that is ongoing in Italy to predict potential larval availability (estimated egg number × hatch rate) (Bellini et al. 2007). This digitally based egg estimate procedure can be used with oviposition cup placement (Steinly et al. 1991) for estimating mosquito populations in regions dominated by single species (e.g., Aedes sierrensis (Ludlow) in coastal California, Ae. polynesiensis in rural French Polynesia). However, we note that field applications would be complicated in regions where multiple species lay eggs on the oviposition substrate. Due to interspecific variation within egg morphology (Steinwascher 1984), a single formula may be unreliable in estimating egg number for substrates containing egg of multiple species. Likewise, temporal or spatial differences in developmental conditions may result in intra-specific variation in egg size (Alto and Juliano 2001), requiring calibration. Among the species mentioned in this paper we have found Ae. aegypti to have significantly larger eggs than Ae. albopictus and Ae. polynesiensis among our laboratory strains. However, the egg estimation approach could be effective in laboratory rearing and geographic areas where one species accounts for a majority of eggs collected in ovitraps.
Acknowledgments
We thank Corey Brelsfoard, Michail Mitov, Amanda Skidmore, and M. Saiful Islam for assistance with experiments and data collection. We also thank 2 anonymous reviewers for their comments. This research was supported by National Institutes of Health grant A1-51533. This is publication 08-08-098 of the University of Kentucky Agricultural Experiment Station.
REFERENCES CITED
- Alto BW, Juliano SA. Precipitation and temperature effects on populations of Aedes albopictus: implications for range expansion. J Med Entomol. 2001;38:646–656. doi: 10.1603/0022-2585-38.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster P, Hutchinson RA. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae) J Med Entomol. 2002;39:699–704. doi: 10.1603/0022-2585-39.4.699. [DOI] [PubMed] [Google Scholar]
- Barboriak DP, Padua AO, York GE, MacFall JR. Creation of DICOM: aware applications using ImageJ. J Digit Imaging. 2005;18:91–99. doi: 10.1007/s10278-004-1879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S. Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests. Dordrecht, The Netherlands: Springer; 2007. pp. 505–515. [Google Scholar]
- Blackmore MS, Lord CC. The relationship between size and fecundity in Aedes albopictus. J Vector Ecol. 2005;25:212–217. [PubMed] [Google Scholar]
- Briegel H, Timmermann SE. Aedes albopictus (Diptera: Culicidae): physiological aspects of development and reproduction. J Med Entomol. 2001;38:566–571. doi: 10.1603/0022-2585-38.4.566. [DOI] [PubMed] [Google Scholar]
- Dean J, Dobson S. Characterization of Wolbachia infections and interspecific crosses of Aedes (Stegomyia) polynesiensis and Ae. (Stegomyia) riversi (Diptera: Culicidae) J Med Entomol. 2004;41:894–900. doi: 10.1603/0022-2585-41.5.894. [DOI] [PubMed] [Google Scholar]
- Dobson S, Marsland E, Rattanadechakul W. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002;160:1087–1094. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera:Culicidae) J Med Entomol. 2001;38:844–849. doi: 10.1603/0022-2585-38.6.844. [DOI] [PubMed] [Google Scholar]
- Gerberg EJ, Barnard DR, Ward RA. Manual for mosquito rearing and experimental techniques. Am Mosq Control Assoc Bull. 1994;5:1–98. [Google Scholar]
- Gering E, Atkinson CT. A rapid method for counting nucleated erythrocytes on stained blood smears by digital image analysis. J Parasitol. 2004;90:879–881. doi: 10.1645/GE-222R. [DOI] [PubMed] [Google Scholar]
- Halley JD, Burd M, Wells P. Excavation and architecture of Argentine ant nests. Insect Soc. 2005;52:350–356. [Google Scholar]
- Johnson KG, Perez ME. Skeletal extension rates of cenozoic caribbean reef corals. Palaios. 2006;21:262–271. [Google Scholar]
- Judson CL. Behavior in the mosquito Aedes aegypti: preliminary studies of physiological control mechanisms. Biol Bull. 1967;133:369–377. doi: 10.2307/1539832. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M. Survival time and resistance to desiccation of diapause and nondiapause eggs of temperate Aedes (Stegomyia) mosquitoes. Entomol Exp Appl. 1992;63:155–161. [Google Scholar]
- Steinly B, Novak R, Webb D. A new method for monitoring mosquito oviposition in artificial and natural containers. J Am Mosq Control Assoc. 1991;7:649–650. [PubMed] [Google Scholar]
- Steinwascher K. Egg size variation in Aedes aegypti: relationship to body size and other variables. Am Midl Natur. 1984;112:76–84. [Google Scholar]
- Toews MD, Pearson TC, Campbell JF. Imaging and automated detection of Sitophilus oryzae (Coleoptera: Curculionidae) pupae in hard red winter wheat. J Econ Entomol. 2006;99:583–592. doi: 10.1603/0022-0493-99.2.583. [DOI] [PubMed] [Google Scholar]