Abstract
Background
Hypocretin (Hcrt), an arousal- and feeding-associated peptide is expressed in lateral hypothalamic neurons that project to the ventral tegmental area (VTA). Intra-VTA Hcrt reinstates morphine-conditioned place preferences, and intracerebroventricular and intra-VTA corticotropin-releasing factor (CRF) reinstate cocaine-seeking. Each is presumed to act at least in part through actions local to the VTA. Here we examined the possibility that VTA perfusion of Hcrt reinstates cocaine-seeking and, if so, whether it does so through the VTA mechanism that is implicated in reinstatement by CRF.
Methods
Rats were trained to lever-press for intravenous cocaine (2 weeks) and then underwent extinction training (saline substituted for cocaine: 3 weeks). Reinstatement behavior was tested and VTA dialysates were collected and assayed for glutamate or dopamine following footshock or perfusion of Hcrt or CRF, with or without Hcrt or CRF antagonists, into the VTA.
Results
VTA perfusion of Hcrt-1 or footshock stress reinstated cocaine-seeking and caused release of VTA glutamate and dopamine. The effects of Hcrt-1 were blocked by a selective Hcrt-1 antagonist but not a CRF antagonist, and were not mimicked by Hcrt-2. The Hcrt-1 antagonist did not block CRF-dependent footshock-induced reinstatement or glutamate or dopamine release. The behavioral and neurochemical effects of Hcrt-1 were attenuated but not blocked by kynurenic acid, an ionotropic glutamate antagonist that blocks footshock-induced reinstatement and glutamate release.
Conclusions
While Hcrt and CRF are known to interact in some area of the brain, in the VTA proper they appear to have largely independent actions on the mesolimbic dopamine mechanisms of cocaine-seeking.
Keywords: hypocretin, orexin, addiction, relapse, ventral tegmental area, cocaine
INTRODUCTION
Hypocretin (Hcrt) (1), also known as orexin (2), is a recently identified peptide neurotransmitter implicated in arousal, feeding, and addiction (3, 4, 5). Two Hcrt variants, Hcrt-1 (residues 28–66) and Hcrt-2 (residues 69–97) are expressed from a common precursor in a small group of lateral hypothalamic neurons that project widely in the brain (6). Two receptor subtypes (HcrtR1 and HcrtR2) have been identified (2), and immunohistochemical (7) evidence suggests that these receptors are expressed in ventral tegmental area (VTA) dopamine neurons. While electron microscopic evidence revealed only infrequent Hcrt synapses on VTA dopamine neurons (8), an electrophysiological study has demonstrated that a large proportion of VTA dopamine neurons can be directly activated by Hcrt (9). Hcrt was also found to augment glutamatergic activation of VTA dopamine cell firing (10). Thus, Hcrt may have both direct and indirect effects on dopamine neurons. Hcrt-1 infusion directly into the VTA reinstates morphine-seeking (11) and Hcrt infusion into the cerebral ventricles reinstates cocaine-seeking (3), presumably by activating the mesocorticolimbic dopamine system (11, 12), a system implicated in the rewarding effects of cocaine (13, 14, 15) and in the ability of stress to reinstate cocaine-seeking (16).
A VTA action of corticotropin-releasing factor (CRF) is also known to activate the dopamine system and reinstate cocaine-seeking. Footshock stress causes release of CRF and glutamate in the VTA, and stress-induced reinstatement of cocaine-seeking is blocked by VTA perfusion of CRF and glutamate antagonists (16, 17). The present study was designed to explore the degree to which the reinstatement of cocaine-seeking by Hcrt and CRF share a common mechanism. First, we determined the ability of VTA perfusions of Hcrt-1 or Hcrt-2 to induce VTA glutamate and dopamine release and to reinstate extinguished cocaine-seeking. We then assessed the effects of VTA perfusions of an HcrtR1 antagonist, a glutamate antagonist, and a CRF antagonist on Hcrt-1-induced reinstatement of cocaine-seeking. Finally, we challenged footshock-induced reinstatement of cocaine-seeking by VTA perfusion of an HcrtR1 antagonist.
MATERIALS AND METHODS
Adult male Long-Evans rats were implanted with chronic intravenous catheters and guide cannulae for microdialysis probes and trained to self-administer intravenous cocaine and given two-three weeks of extinction sessions by traditional methods (see Supplemental Information for details). Microdialysis samples were then taken under various conditions as follows:
Experiment 1. VTA perfusion of Hcrt-1 or Hcrt-2 on local neurotransmitter release and reinstatements
One group was tested with VTA perfusion of Hcrt-1 and one with VTA perfusion of Hcrt-2. Hcrt-1 treated rats were tested under 4 experimental conditions: one day with aCSF (vehicle) followed (2 hours later) by Hcrt-1 perfusions and one day with the HcrtR1 antagonist SB-408124 followed (2 hours later) by SB-408124+Hcrt-1 perfusions. The sequence was counterbalanced across days. Hcrt-1 (10 μM) perfusion started after collection of 5 baseline dialysis samples and kept perfused until the end of the test. When SB-408124 (10 μM) was perfused, it was added in the medium 20 min before the collection of the baseline samples until the end of the test. The reinstatement test started 20 min after the initiation of Hcrt-1 perfusion and lasted for 2 hr. The reinstatement testing started with the insertion of the active lever and the illumination of the house light. The testing conditions and the behavioral consequences were the same as during the extinction sessions. Animal responses on the active and inactive levers and the number of saline infusions were recorded.
The Hcrt-2 treated rats were tested under two experimental conditions in one day, first under aCSF perfusion followed by Hcrt-2 (10 μM) perfusion. The two tests were separated by 2 hr.
Experiment 2. VTA perfusion glutamate or CRF antagonist on Hcrt-1-induced local neurotransmitter release and reinstatements
To test the possible involvements of VTA glutamate and CRF in the effects of Hcrt-1, one group of rats was challenged with kynurenic acid (Kyn), an antagonist at ionotropic glutamate receptors and another group was challenged with α-helical CRF, an antagonist at CRF receptors. Each rat was tested under 4 treatment conditions. The 4 treatments for one group were aCSF followed by Hcrt-1 on one day and Kyn followed (2 hours later) by Kyn +Hcrt-1 on the other. The 4 treatments for the other group were aCSF followed by Hcrt-1 on one day and α-helical CRF followed (2 hours later) by α-helical CRF+Hcrt-1 on the other. The sequence was counterbalanced across days. The antagonists were introduced in the perfusion medium 20 min before the collection of the baseline samples. Hcrt-1 was introduced in the perfusion medium 20 min before the reinstatement testing. Drug perfusions were continued until the end of 2 hr behavior tests.
Experiment 3. VTA Hcrt-1 antagonist perfusion on footshock-induced neurotransmitter release and reinstatements
To determine whether VTA Hcrt plays a role in the stress-induced VTA neurotransmitter release and reinstatement, a new group of rats was tested in four conditions, two per day: On one day they were first tested for the effects of SB-408124 (10μM) perfusion in the absence of footshock and then, two hours later, for the effects of footshock during SB-408124 (10μM) perfusion. On the other day control data were first collected with aCSF perfusion in the absence of footshock, and then, two hours later, when footshock was given during aCSF perfusion. Footshock was administered as a 20-minute series of inescapable and unpredictable 0.5 sec shocks (18). The shock intensity was adjusted, for each animal, to a level below the threshold for freezing behaviors (0.4–0.6 mA), the night before reinstatement testing. Footshock was administered at random 40±30 sec intervals. The 2 hr reinstatement testing started immediately after completion of the shock. The no-shock condition was the same as the shock condition except that the wires from the shock generator were not connected to the test box. When SB-408124 was perfused, it was included in the perfusion medium 20 min before the microdialysis baseline collection and maintained until the reinstatement test was completed. Microdialysis samples were collected until the end of the reinstatement testing. Lever-presses and number of saline infusions earned were recorded.
Biochemical analysis of microdialysis samples
Glutamate concentrations were determined by HPLC. We used an ESA pump (ESA 582, ESA, Inc., Chelmsford, MA), a CMA/260 degasser, a CMA/200 refrigerated microsampler, a phase II ODS column (3 μm particle size, 3.2×100 mm, Bioanalytical Systems, Inc., West Lafayette IN), a CMA/280 fluorescence detector, and an ESA model 501 data station (ESA, Inc.). The CMA/280 is a fixed wavelength fluorescence detector operating at a maximal excitation of 330–365 nm and emission of 440–530 nm. We performed precolumn derivation of glutamate with an o-phthalaldehyde/mercaptoethanol reagent (0.4 M borate, 0.04 M phthalaldehyde and 0.4 M 2-mercaptoethanol, pH 10.4). Briefly, 10 μl of the reagent was added to and mixed with the samples by the microsampler. After a 60-sec reaction period at 6° C in the microsampler, 20 μl of the mixture was injected onto the column. The elution of glutamate was achieved with a mobile phase consisting of 0.15 M sodium acetate, 10% methanol and 1.5% tetrahydrofuran at a flow rate of 0.6 ml/min. Following the appearance of the glutamate peak on the chromatogram, an injection of 20 μl of 100% methanol was made by the microsampler before the end of the chromatogram in order to accelerate the elution of the residuals on the column. The detection limit was 0.2-pmol/injection.
Dopamine was measured with an HPLC coupled to an ESA Coulochem II Detector (model 5200) with a dual-electrode microdialysis cell, and an ESA model 501 data station (ESA, Inc., Chelmsford, MA). Samples were manually injected onto the column (3μm particle size, 3 mm×150 mm, Analytical MD-150, ESA, Inc.). The mobile phase for DA separation consisted of 75 mM NaH2PO4, 1.5 mM OSA, 10μM EDTA, and 8% acetonitrile (pH 3.0 adjusted with H3PO4). Dopamine was quantified on both reducing (−250 mV) and oxidizing electrodes (350 mV). The limit of detection for dopamine was approximately 5-fmole/injection.
Drugs
Cocaine hydrochloride and the anesthetics used in the surgery were obtained from the pharmacy department within the Institute. Hcrt-1, Hcrt-2, α-helical CRF, Kyn and SB-408124 were purchased from Sigma (St. Louis, MO). Kyn was dissolved first with a small aliquot of 5 N NaOH and diluted with aCSF to the final concentration. Other drugs were dissolved directly in saline or aCSF when appropriate. Each drug solution was adjusted to pH 7.4 before use.
Histology
After the completion of the microdialysis experiments, the rats were decapitated under anesthesia and their brains were removed and fixed in a 10% formalin solution. After at least 7 days of fixation, the brains were frozen and 50-μm coronal sections were taken. Probe placement was determined under low magnification in wet sections that differentiate fiber bundles from cell body regions and thus identify classic brain landmarks.
Statistical analysis
VTA glutamate and dopamine levels were expressed as the concentrations in the perfusate (means± S.E.M.). Basal values refer to those obtained before the drug was added into the perfusion medium or before footshock was given. When data were expressed as percent of baseline values, the mean concentration of the three samples preceding the drug administration was defined as 100%. Data were analyzed with two-ANOVA with repeated measures over time followed by fisher’s PLSD test. A level of P<0.05 was considered statistical significance.
The total responses on the active or inactive levers were analyzed with two-way ANOVA with repeated measures over treatments followed by Fisher’s PLSD test.
RESULTS
Effects of VTA Hcrt perfusion on local neurotransmitter release and reinstatements of cocaine-seeking
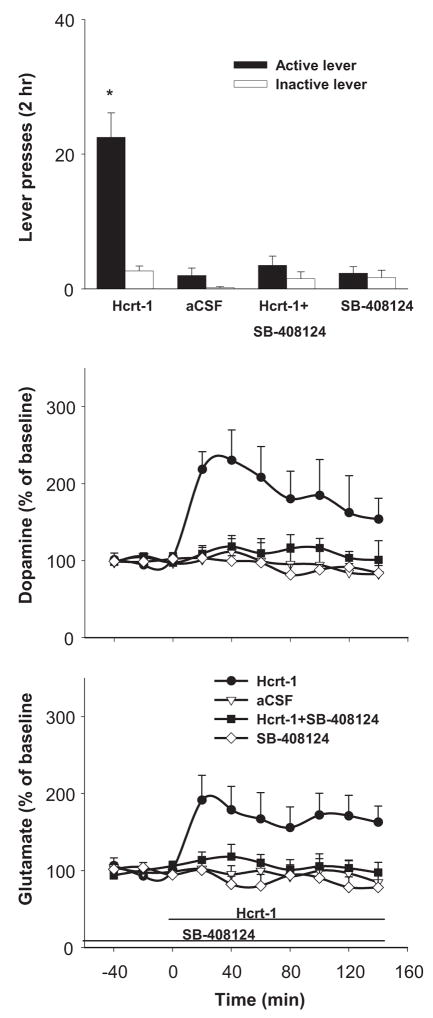
Perfusions of Hcrt-1 (10 μM) through the VTA dialysis probe increased VTA glutamate and dopamine levels and reinstated responding on the lever previously associated with cocaine reinforcement (Fig. 1). Co-perfusion of the Hcrt-1R antagonist SB-408124 blocked both the behavioral (F(3, 30)=24.86, P<0.001) and neurochemical effects of Hcrt-1.(Fig. 1). Two-way ANOVA confirmed a significant Time × treatment interaction for both dopamine (F(27, 180)=2.49, P<0.001) and glutamate (F(27, 180)=2.58, P<0.001). VTA perfusion of SB-408124 alone had no effects ether on dopamine or glutamate levels. Fisher’s PLSD test indicated that VTA dopamine and glutamate levels both were significantly elevated throughout the Hcrt-1 perfusion period.
Figure 1.
VTA perfusion of Hcrt-1 (10 μM) reinstated cocaine-seeking and increased extracellular levels of dopamine and glutamate. VTA perfusion of SB-408124 (10 μM), a selective Hcrt-1 receptor antagonist, while having no effect itself on either the animal’s behavior or basal VTA neurotransmitter levels, blocked the reinstatement and the neurotransmitter increases induced by VTA Hcrt-1. * indicates difference from the other experimental conditions.
Perfusion of Hcrt-2 at the same concentration (10 μM) had no significant effect on lever pressing (F(1, 10)=0.47, P=0.51, see Fig. 1 in Supplemental Information for details), VTA dopamine (F (9, 90)=0.79, P=0.63), or VTA glutamate F(9, 90)=0.11, P=0.98) levels (see Fig. 1 in Supplemental Information for details).
Effects of VTA glutamate or CRF antagonists on Hcrt-1-induced local neurotransmitter release and cocaine-seeking
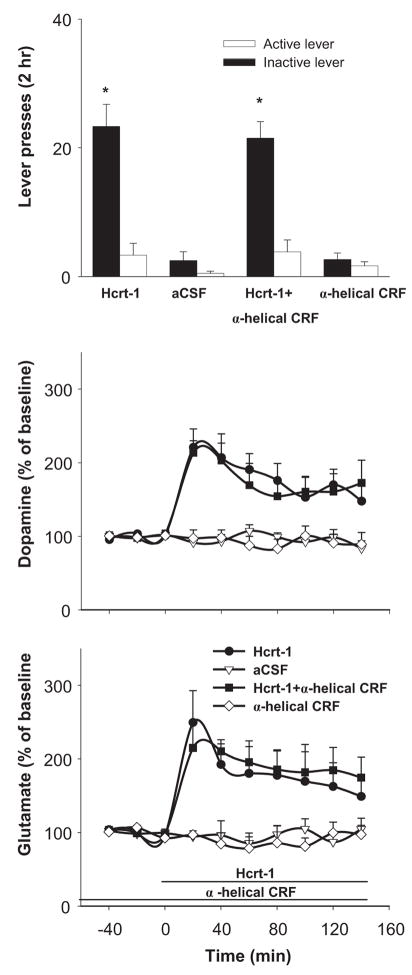
VTA perfusion of Kyn (1 mM) attenuated the VTA dopamine increase induced by VTA Hcrt-1 (treatment X time interaction; F(27, 180)=3.77, P<0.001). Fisher’s PLSD test indicated the elevations of VTA dopamine were significantly lower in the Hcrt-1+Kyn treatment condition than in Hcrt-1 alone condition throughout the initial 80 min. VTA perfusion of Kyn had no effects on Hcrt-1-induced VTA glutamate levels. While ANOVA revealed a significant treatment X time interaction over the 4 treatment conditions (F(27, 180)=3.03, P<0.001), the increases in VTA glutamate were not different from each other between Hcrt-1 and Hcrt-1+kyn treatments. VTA Kyn perfusion also attenuated active lever-pressing induced by VTA Hcrt-1 perfusion (lever × treatment interaction; F(3, 30)=28.78, P<0.001). Fisher’s PLSD test revealed significantly lower active lever-pressing under Hcrt-1+kyn treatment than under Hcrt-1 treatment alone (P<0.05) (Fig. 2).
Figure 2.
VTA perfusion of kynurenic acid (Kyn, 1 mM) attenuated the increase in VTA dopamine levels and the reinstatement of lever-pressing induced by VTA Hcrt-1 (10 μM) perfusion. VTA Kyn perfusion showed no effects on Hcrt-1-induced VTA glutamate levels. * indicates difference from the other experimental conditions. # indicates difference from the Hcrt-1 alone conditions.
VTA perfusion of the CRFR antagonist α-helical CRF (1 μM) had no significant effect on either VTA neurotransmitter levels or lever-pressing induced by VTA Hcrt-1 (Fig. 3).
Figure 3.
VTA perfusion of α-helical CRF (1 μM), an antagonist at CRF receptors, had no effect on VTA Hcrt-1-induced reinstatement of cocaine-seeking or on VTA dopamine or glutamate levels. * indicate difference from the non-Hcrt-1 conditions.
Effects of VTA SB-408124 perfusion on footshock-induced VTA neurotransmitter release and reinstatement of cocaine-seeking
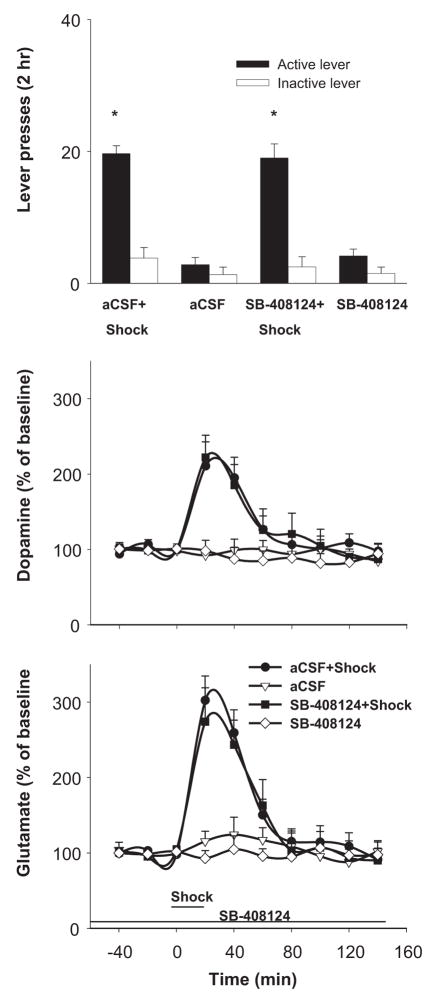
Footshock stress significantly elevated VTA levels of dopamine (F(27, 180)=4.23, P<0.001) and glutamate (F(27, 180)=5.67, P<0.001). The elevations were not different between aCSF and SB-408124 treatments (dopamine, F(9, 90)=0.47, P>0.05; glutamate, F(9, 90)=1.03, P>0.05). SB-408124 or aCSF perfusion alone had no significant effect on VTA dopamine or glutamate levels. Fisher’s PLSD test indicated both dopamine and glutamate levels were significantly elevated (P<0.05) for 40 min following footshock stress. Footshock stress reinstated responding on the active lever (lever × treatment interaction; F(3, 30)=51.15, P<0.001) over the 4 experimental conditions. VTA perfusion of SB-408124 did not significantly affect lever-pressing induced by footshock (P>0.05) (Fig. 4).
Figure 4.
VTA perfusion of the Hcrt-1 antagonist SB-408124, at the concentration (10 μM) that effectively blocks the actions of VTA Hcrt-1, had no effect on VTA dopamine or glutamate levels or on footshock-induced reinstatement of cocaine-seeking. * indicate difference from the no-shock conditions.
Probe placements
The active portions of the microdialysis probes were located primarily in the posterior VTA, usually penetrating the parabrachial pigmented and paranigral nuclei, and occasionally partially intruding the rostral interpeduncular nucleus (see Fig. 2 in Supplemental Information for details). One rat treated with Hcrt-1 in Experiment 1 was excluded because the placement of one probe was dorsal to the VTA. Dopamine levels were not reliably measured in samples from this probe.
DISCUSSION
The present findings add self-administration evidence to conditioned place preference findings that Hcrt acts within the VTA to activate the mesocorticolimbic dopamine system and reinstate drug-seeking; VTA Hcrt-1 perfusion reinstated cocaine-trained responding and elevated VTA dopamine levels, a correlate of increased dopaminergic cell firing (19, 20). Hcrt input to the VTA originates from the lateral hypothalamus, a brain region long implicated in reward and motivation (21). Hcrt administration into VTA induces conditioned place preference (22) and reinstates extinguished place preferences established by morphine (11), presumably by increasing local dopamine cell firing and increasing dopamine release in dopamine terminal fields (7, 23). Thus VTA is a brain region where lateral hypothalamic Hcrt projections interacts with dopaminergic neurons implicated in addiction. While Hcrt axons make few classic synaptic contacts with VTA dopamine neurons, Hcrt is suggested to activate the dopamine system non-synaptically (8) by local “paracrine” diffusion or “volume conduction.”
The Hcrt-1-induced reinstatement of cocaine-seeking appears to mediated primarily by Hcrt-1 receptors. VTA perfusion of selective Hcrt-1 antagonist SB-408124 blocked both the increases in local transmitter release and the reinstatement of responding induced by VTA Hcrt-1. While a non-selective concentration of SB-4008124 was introduced to the dialysate, only a fraction of the dialysis concentration, driven only by a concentration gradient and not by hydraulic pressure, penetrates the membrane and enters the brain. Once outside the probe, the concentration falls off with the square of the distance; thus the concentration in the VTA is more than an order of magnitude lower than the dialysate concentration. Moreover, VTA perfusion of the same concentration of Hcrt-2 that preferentially acts at Hcrt-2 receptors (24, 25) showed no significant effect. The lack of involvement of VTA Hcrt-2 is somewhat surprising considering the similar excitatory actions of the two Hcrt peptides. However, recent studies from various laboratories indicate that activation of Hcrt-2 receptors is significantly less effective in mediating a variety of neurochemical events. Intracranial Hcrt-2 is less potent than Hcrt-1 in elevation of accumbens dopamine levels (7), hypothalamic histamine levels (26), serotonin levels in the dorsal raphe (27) and acetylcholine levels in the pontine reticular formation (28) and cortex (29). Application of both Hcrts to midbrain cultured neurons significantly increases intracellular calcium but Hcrt-1 is an order of magnitude more effective (22). Hcrt-2 immunoreactivity is less abundant than that of Hcrt-1 in most brain regions including the VTA (30). Although it has been well demonstrated that mRNA encoding Hcrt-1 and -2 receptors are equally abundant in the VTA (31), it is not known whether these two subtypes of receptors are equally expressed in this region. Therefore, it is possible that the ineffectiveness of Hcrt-2 in the present study is due to the insufficient activation of the hypocretin 2 receptors. However, the complete blockade by Hcrt-1 antagonists of the effects of Hcrt-1 administered either locally into VTA (present study) or intraventricularly (3) makes it unlikely that Hcrt-2 receptors are significantly involved.
The present study also revealed a partial contribution of VTA glutamate in the effects of Hcrt-1. VTA perfusion of Hcrt-1 induced a two-fold increase in VTA glutamate levels that was blocked by the Hcrt-1 antagonist. Blockade of glutamate action at ionotropic glutamate receptors significantly attenuated, but did not block completely, VTA dopamine increase and reinstatement of cocaine-seeking induced by VTA perfusion of Hcrt-1. In contrast, blockade of glutamate receptors completely blocks the ability of footshock, acting through VTA CRF release, to reinstate cocaine-seeking (16). The fact that reinstatement of cocaine-seeking by VTA CRF is completely glutamate-dependent while reinstatement by VTA Hcrt-1 is not indicates that the mechanism of Hcrt-1-induced activation of the dopamine system is at least partially independent of that involved in CRF-induced activation.
While intracerebroventricular adminstration of an Hcrt-1 antagonist blocks footshock-induced reinstatement of cocaine-seeking (3), VTA administration of the antagonist was not effective in the present study. Thus the VTA is apparently not a locus for interaction of Hcrt with CRF system. VTA perfusion of CRF antagonist α-helical CRF at a dose that blocks footshock-induced VTA neurotransmitter release and reinstatement of cocaine-seeking (16) had no effect on the Hcrt-1-induced neurochemical and behavioral effects, and VTA perfusion of SB-408124 that blocks the effects of VTA Hcrt-1 did not modify footshock-induced VTA glutamate or dopamine levels or footshock-induced drug-seeking. These findings suggest that while VTA Hcrt and CRF each cause local release of both glutamate and dendritic dopamine, they do so through independent inputs to these systems.
Supplementary Material
Acknowledgments
This work was supported by the intramural research program of NIDA, NIH.
FINANCIAL DISCLOSURES
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. Drs. Wang, You, and Wise have no biomedical financial interests and no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Hypocretins and hypocretin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 5.Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- 6.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (hypocretin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, et al. Direct involvement of hypocretinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balcita-Pedicino JJ, Sesack SR. Hypocretin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- 9.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by hypocretins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Hypocretin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic hypocretin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 12.Boutrel B, de Lecea L. Addiction and arousal: the hypocretin connection. Physiol Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise RA, Rompré P-P. Brain dopamine and reward. Ann Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 14.You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;93:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 18.Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- 19.Legault M, Rompré P-P, Wise RA. Chemical stimulation of the ventral hippocmpus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31:241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the hypocretin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur J Neurosci. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- 23.Vittoz NM, Berridge CW. Hypocretin/hypocretin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 24.Smart D, Jerman JC, Brough SJ, Rushton SL, Murdock PR, Jewitt F, et al. Characterization of recombinant human hypocretin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA. The hypocretins are weak agonists at recombinant human hypocretin-1 and hypocretin-2 receptors. Br J Pharmacol. 2000;129:1289–1291. doi: 10.1038/sj.bjp.0703257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishizuka T, Yamamoto Y, Yamatodani A. The effect of hypocretin-A and -B on the histamine release in the anterior hypothalamus in rats. Neurosci Lett. 2002;323:93–96. doi: 10.1016/s0304-3940(01)02552-6. [DOI] [PubMed] [Google Scholar]
- 27.Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. Differential effect of hypocretins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101–1105. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Bernard R, Lydic R, Baghdoyan HA. Hypocretin (hypocretin) receptor subtypes differentially enhance acetylcholine release and activate g protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–171. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- 29.Dong HL, Fukuda S, Murata E, Zhu Z, Higuchi T. Hypocretins increase cortical acetylcholine release and electroencephalographic activation through hypocretin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology. 2006;104:1023–1032. doi: 10.1097/00000542-200605000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, et al. Differential distribution of hypocretin-A and hypocretin-B immunoreactivity in the rat brain and spinal cord. Peptides. 1999;20:1455–1470. doi: 10.1016/s0196-9781(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 31.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of hypocretin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.