Abstract
OBJECTIVE
The objective of this study was to determine the minimum threshold level at which maximum anatomic prolapse predicts bothersome pelvic floor symptoms.
STUDY DESIGN
We performed a cross-sectional study of women older than 40 years undergoing gynecologic and urogynecologic examinations using Pelvic Organ Prolapse Quantification (POP-Q) examinations to assess support and Pelvic Floor Distress Inventory questionnaires to assess symptoms. Across the spectrum of prolapse severity, we calculated receiver operating characteristic (ROC) curves and areas under the curves (AUCs) for each symptom.
RESULTS
Of 296 participants, age was 56.3 ± 11.2 years, and 233 (79%) were white. POP-Q stage was 0 in 39 (13%), 1 in 136 (46%), 2 in 89 (30%), and 3 in 33 (11%). ROC analysis for each symptom revealed an AUC of 0.89 for bulging/protrusion; 0.81 for splinting to void; 0.55–0.62 for other prolapse and urinary symptoms; and 0.48–0.56 for bowel symptoms. Using a threshold of 0.5 cm distal to the hymen, the sensitivity (69%) and specificity (97%) were high for protrusion symptoms but poor for most other symptoms considered.
CONCLUSION
Vaginal descensus 0.5 cm distal to the hymen accurately predicts bulging/protrusion symptoms; however, we could not identify a threshold of prolapse severity that predicted other pelvic floor symptoms.
Keywords: bulging and protrusion symptoms, Pelvic Floor Distress Inventory, Pelvic Organ Prolapse Quantification System, prolapse, receiver operating characteristic curve, threshold
Based on the current Pelvic Organ Prolapse Quantification (POP-Q) staging system,1 pelvic organ support is classified into 5 stages, with stage 0 representing no descent of the pelvic organs and stage 4 representing complete prolapse. Historically, studies have used variable reference points to identify anatomic failure or the threshold for abnormal support, and the POP-Q system does not specify a threshold (stage or individual point) for the definition of abnormal support. Moreover, stages were assigned based on expert opinion and were not derived from scientific data. Using a definition of stage 2 or greater, pelvic organ prolapse has been noted among 37% of women presenting for annual gynecologic examinations.2 However, many women with stage 2 support are asymptomatic, so it is not clear that a clinical finding of stage 2 support should be equated with a disease state.
Swift et al2 attempted to define a threshold beyond which prolapse symptoms reliably identify maximum vaginal wall descensus. This large study of 1004 total subjects included 7% with prolapse at or distal to the hymen and 2% with stage 3–4 support. They compared the maximum prolapse descent with the presence or absence of 7 symptoms. The only symptom predicted by anatomic prolapse severity was feeling or seeing something bulging. Specifically, the authors found that prolapse greater than −0.5 cm (0.5 cm proximal to the hymen) was the best threshold for discriminating women with this symptom. No other symptom was predicted by the severity of prolapse.
Several studies have shown associations between anatomic measurements of prolapse and specific symptoms, most notably the symptom of a visible and palpable protrusion3–5 or obstructive voiding symptoms.4 Yet correlations between symptoms and anatomic prolapse severity have been weak.5,6 Few studies have attempted to define the relationship between anatomic prolapse and symptoms using validated quality-of-life measures. The purpose of this study was to determine the minimum threshold level at which maximum anatomic prolapse predicts bothersome pelvic floor symptoms.
Materials and Methods
This was a secondary analysis of a cross-sectional study of women with and without pelvic floor dysfunction designed to assess the impact of pelvic floor disorders on sexual function.7 The study was funded by the National Institutes of Health and approved by the Johns Hopkins Institutional Review Board.
Women over 40 years of age who were scheduled for ambulatory gynecologic or urogynecologic care were offered participation. We included women seeking care at 1 of 5 outpatient sites affiliated with Johns Hopkins Medical Institutions in metropolitan Baltimore, MD. Women under 40 years of age were excluded because the prevalence of pelvic floor disorders would be too low among younger women to draw meaningful conclusions. We also excluded pregnant women and those who could not complete questionnaires in English. Women who had never been sexually active were also excluded because the primary study evaluated the relationship between sexual function and pelvic floor dysfunction.
After obtaining informed consent, subjects completed a research questionnaire and underwent a gynecologic examination. The questionnaire included the Pelvic Floor Distress Inventory 20 (PFDI) and Pelvic Floor Impact Questionnaire 7 (PFIQ)8 as validated measures of pelvic floor dysfunction symptom severity and its impact on quality of life. The PFDI is a 20-item symptom inventory that can be used to generate scores for 3 subscales: the urinary distress inventory (UDI), colorectalanal distress inventory (CRADI), and the pelvic organ prolapse distress inventory (POPDI). Each scale is scored from 0 to 100, with higher scores indicating greater symptom burden. The PFDI summary score is the sum of these 3 scale scores.
The PFIQ also has subscale scores, one of which is the Pelvic Organ Prolapse Impact Questionnaire (POPIQ). Epidemiologic Studies Depression Scale (CES-D) and Short-Form Health Survey (SF-12)9 questionnaires were also administered as part of the original study protocol. Subjects were asked “the reason for your visit with the doctor or nurse.”
A POP-Q examination was performed on each woman in the supine position. Investigators were trained using an instructional video, and POP-Q examinations were observed by the primary investigator, a urogynecologist. Twelve physician investigators demonstrated competency in performing this examination before the study, and individual competency was reconfirmed throughout the study. For each subject, we defined the most dependent site of prolapse. This was measured in centimeters from the hymen. Distances proximal to the hymen were assigned negative values and distances distal to the hymen were assigned positive values. Given the range of prolapse observed in this population (−3.0 to 6.0), we considered anatomic measurements in 0.5-cm increments as defined by the POP-Q system.
We examined the relationship between prolapse severity and 20 symptom outcomes, consisting of the 20 items that comprise the PFDI. For each PFDI item, a positive or bothersome symptom was defined as the presence of the symptom plus a bother greater than or equal to “somewhat.”
For each symptom outcome, we calculated a receiver operating characteristic (ROC) curve. For each curve, the disease state was determined by the presence or absence of the symptom outcome. The ROC curves were generated by plotting sensitivity vs 1 minus the specificity for all relevant values of the anatomic measurement for the most dependent site of prolapse from −3.0 to 6.0. We used the ROC curves to estimate the minimum threshold level for the measured anatomic prolapse that would best predict women who had bothersome pelvic floor symptoms.
For each ROC curve, we calculated the area under the curve, which is a measure of the probability that the test (in this case, severity of prolapse) will allow correct classification for the presence of disease (in this case, presence of a bothersome PFDI symptom). By definition, a test with no ability to predict individuals with the condition will have an area under the curve of 0.5. A test with perfect ability to predict individuals with the condition will have an area under the curve of 1.0. If the 95% confidence interval for the area under the curve includes 0.5, the test is not likely to accurately predict between those with and without the relevant condition.
We performed similar ROC analysis for maximum anterior (Ba) and posterior (Bp) vaginal wall descensus. In an additional stratified analysis, patients were subdivided according to pain and depression scores. Subjects were considered to have pain if their response was greater than 1 (not at all) to the SF-12 question, “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” Subjects were considered to have depression if their score on the CES-D was 16 or greater. ROC analysis was performed for those with and without pain as well as those with and without depression to look for confounding effects. In a final stratified analysis, ROC analysis was also performed on the subsets of subjects presenting to the urogynecology and gynecology clinics to evaluate for spectrum bias.
For each ROC curve with an area under the curve statistically greater than 0.5, likelihood ratios were generated for each measured value of maximum anatomic prolapse along the curve. We used the likelihood ratio to select a minimum threshold level of maximum prolapse that best predicts women with the symptom. A likelihood ratio of greater than 10 was chosen because a likelihood ratio in this range provides strong evidence of a condition.
Using this criterion, the minimum threshold level of maximum anatomic prolapse defined clinically significant prolapse, the point at which anatomic prolapse and bothersome pelvic floor symptoms coexist. We applied this threshold to the population to identify women who met this definition for clinically significant prolapse. Mean PFDI, POPDI, PFIQ, and POPIQ scale scores were compared between women with and without clinically significant prolapse using the Student t test. P <.05 was considered statistically significant.
Results
Of the 305 study participants, POP-Q examinations were completed on 296 (97%). Of the 296 with complete POP-Q data, 188 subjects were seen in the gynecology clinics and 108 subjects in the urogynecology clinics. Among the gynecology clinic population, 139 subjects(74%) were seen for annual gynecologic care, 6 (3%) sought treatment for pelvic floor conditions (including “problems with bladder function” and “problems with bowel function”) and 43 (23%) came for treatment of other gynecologic complaints (including menstrual complaints, pelvic pain, menopause, abnormal cytology, and vaginal discharge). Among the urogynecology clinic population, 87 subjects (81%) sought treatment for pelvic floor conditions of prolapse, bowel, or bladder dysfunction, 11 (10%) had gynecologic complaints, 7 (6%) were for annual gynecologic care, and 3 did not respond to the question.
The mean age of our population was 56.3 ±11.2 years, mean parity was 2.7 ± 1.8, and mean body mass index was 28.7 ± 6.6 (kg/m2). Two hundred thirty-three (79%) were white, 46 (16%) were African American, 10 (3%) were Asian, and 7 (2%) listed other or had missing data. One hundred ninety-one (65%) were menopausal and 73 (25%) had a previous hysterectomy. One hundred thirty-six (46%) had pain that interfered with normal work, and 83 (28%) had depressive symptoms in the range associated with major depression. POP-Q support was stage 0 in 39 (13%), stage 1 in 136 (46%), stage 2 in 88 (30%), and stage 3 in 33 (11%).
Table 1 summarizes the results of the ROC analysis. As shown, the ROC curves with the greatest areas under the curve were those for the symptoms of a visible or palpable bulge and splinting to void. For these 2 items, prolapse severity had at least moderate ability to predict symptomatic women, with areas under the curve of 0.89 for visible or palpable bulge (95% confidence interval [CI], 0.83–0.95) and 0.81 (95% CI, 0.63–0.99) for splinting to void.
TABLE 1.
Receiver operator characteristic curve analysis for prolapse symptoms and maximum vaginal descensus
| Pelvic floor distress inventory symptoms | Area under the curve | Standard error | 95% confidence interval |
|---|---|---|---|
| Pelvic Organ Prolapse Distress Inventory | |||
| Low abdominal pressure | 0.62 | 0.037 | 0.55–0.69 |
| Heaviness or dullness | 0.61 | 0.040 | 0.53–0.68 |
| Bulging or protrusion | 0.89 | 0.031 | 0.83–0.95 |
| Splinting to defecate | 0.55 | 0.042 | 0.47–0.63 |
| Incomplete voiding | 0.62 | 0.038 | 0.55–0.70 |
| Splinting to void | 0.81 | 0.092 | 0.63–0.99 |
| Urinary Distress Inventory | |||
| Urinary frequency | 0.59 | 0.033 | 0.52–0.65 |
| Urge incontinence | 0.62 | 0.033 | 0.56–0.68 |
| Stress incontinence | 0.59 | 0.033 | 0.52–.65 |
| Leaks small amounts | 0.61 | 0.033 | 0.54–0.67 |
| Difficulty emptying | 0.58 | 0.047 | 0.49–0.67 |
| Pain or discomfort | 0.55 | 0.040 | 0.47–0.63 |
| Colorectal-Anal Distress Inventory | |||
| Straining to defecate | 0.56 | 0.035 | 0.49–0.63 |
| Incomplete emptying | 0.48 | 0.035 | 0.41–0.55 |
| Fecal incontinence, flatus | 0.56 | 0.034 | 0.49–0.63 |
| Fecal incontinence, liquid | 0.52 | 0.041 | 0.44–0.60 |
| Fecal incontinence, solid | 0.56 | 0.078 | 0.41–0.71 |
| Pain with defecation | 0.53 | 0.051 | 0.43–0.63 |
| Fecal urgency | 0.53 | 0.038 | 0.45–0.60 |
| Rectal prolapse | 0.53 | 0.052 | 0.43–0.63 |
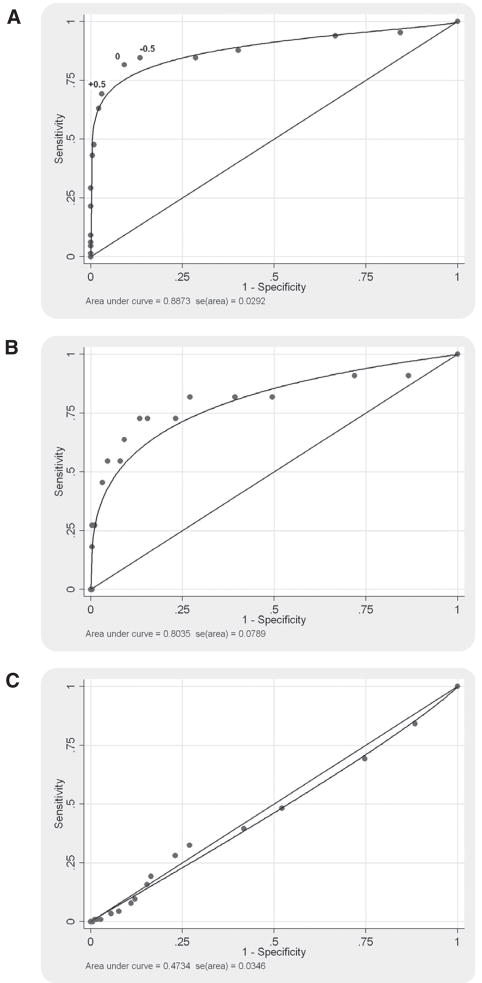
For 8 other symptoms (including 3 items from the POPDI scale and 4 items from the UDI scale), the area under the ROC curve was significantly greater than 0.5 but less than 0.7, suggesting limited ability to predict symptomatic women. These include low abdominal pressure, heaviness, incomplete voiding, urinary frequency, urge and stress incontinence, and leakage of small amounts. For the remaining 10 PFDI-20 items, including all 8 items of the CRADI scale, the area under the ROC curve was not significantly different from 0.5, suggesting no ability for prolapse severity to predict symptomatic women. Representative ROC curves are shown in the Figure.
FIGURE.
Representative ROC curves for individual PFDI symptoms
A, Visible and palpable bulging or protrusion with threshold points for −0.5, 0, and +0.5 marked on the plot; B, splinting to void; C, incomplete bowel emptying.
We used the ROC analyses to identify a best threshold for predicting prolapse symptoms (Table 2). The likelihood ratios (LRs) identified a threshold between 0 (LR 8.9) and 0.5 (LR 22.8) for the symptom of a visible or palpable bulging or protrusion. The threshold level was unchanged when subdivided into gynecology and urogynecology clinic populations. The best threshold for symptoms of splinting to void was observed between 2 (LR 6.7) and 2.5 (LR 11.9). With a threshold of 0.5 or greater, the sensitivity and specificity of physical examination for the symptom of vaginal bulging or protrusion were 69% and 97%. At this threshold, the highest percentage of participants (91%) was correctly classified.
TABLE 2.
Sensitivity, specificity, and likelihood ratios for various discriminatory thresholds for most dependent site of prolapse and symptoms of visible/palpable bulge and splinting to void
| Visible/palpable bulge |
Splinting to void |
||||||
|---|---|---|---|---|---|---|---|
| Threshold POP-Q (equal or greater than) | Na | Sensitivity | Specificity | LR | Sensitivity | Specificity | LR |
| −3 | 296 | 1.00 | 0.00 | 1.00 | 1.00 | 0.00 | 1.00 |
|
| |||||||
| −2.5 | 257 | 0.95 | 0.16 | 1.13 | 0.91 | 0.13 | 1.05 |
|
| |||||||
| −2 | 215 | 0.94 | 0.33 | 1.41 | 0.91 | 0.28 | 1.26 |
|
| |||||||
| −1.5 | 150 | 0.88 | 0.60 | 2.18 | 0.82 | 0.51 | 1.65 |
|
| |||||||
| −1 | 121 | 0.85 | 0.71 | 2.96 | 0.82 | 0.61 | 2.08 |
|
| |||||||
| −0.5 | 86 | 0.85 | 0.87 | 6.31 | 0.82 | 0.73 | 3.03 |
|
| |||||||
| 0 | 74 | 0.82 | 0.91 | 8.97 | 0.73 | 0.77 | 3.14 |
|
| |||||||
| 0.5 | 52 | 0.69 | 0.97 | 22.85 | 0.73 | 0.85 | 4.71 |
|
| |||||||
| 1 | 46 | 0.63 | 0.98 | 29.14 | 0.73 | 0.87 | 5.45 |
|
| |||||||
| 1.5 | 33 | 0.48 | 0.99 | 55.08 | 0.64 | 0.91 | 6.98 |
|
| |||||||
| 2 | 29 | 0.43 | 1.00 | 99.51 | 0.55 | 0.92 | 6.76 |
|
| |||||||
| 2.5 | 19 | 0.29 | 1.00 | 0.55 | 0.95 | 11.96 | |
|
| |||||||
| 3 | 14 | 0.22 | 1.00 | 0.45 | 0.97 | 14.39 | |
|
| |||||||
| 3.5 | 6 | 0.09 | 1.00 | 0.27 | 0.99 | 25.91 | |
|
| |||||||
| 4 | 4 | 0.06 | 1.00 | 0.27 | 1.00 | 77.73 | |
|
| |||||||
| 5 | 3 | 0.05 | 1.00 | 0.18 | 1.00 | 51.82 | |
|
| |||||||
| 6 | 1 | 0.02 | 1.00 | 0.00 | 1.00 | ||
LR, likelihood ratio.
Cumulative number above threshold.
The sensitivity and specificity of the symptom of splinting to void were 73% and 85%. Using a threshold of 0.5, we classified 52 women with “clinically significant” prolapse (18%) and 244 women who did not meet this criterion. We compared PFDI, POPDI, PFIQ, and POPIQ scores between women in these 2 groups using a threshold of 0.5 (Table 3). When comparing the quality-of-life measures, we found significant differences in the PFDI, POPDI, PFIQ, and POPIQ scores between groups.
TABLE 3.
Comparison of mean scores for 4 quality-of-life measures between women with and without “clinically significant prolapse,” defined using the threshold of 0.5 cm for clinically relevant prolapse
| Quality-of-life measures | Mean score for those with prolapse ≥ 0.5 cm (95% CI) (n = 52) | Mean score for those without prolapse (95% CI) (n = 244) | P |
|---|---|---|---|
| Pelvic Floor Distress Inventory | 81.9 (65.2–98.6) | 47.4 (41.3–53.4) | <.0001 |
| Pelvic Organ Prolapse Distress Inventory | 32.0 (26.0–37.9) | 10.0 (8.1–11.9) | <.0001 |
| Pelvic Floor Impact Questionnaire | 47.8 (30.4–65.2) | 25.8 (20.3–31.3) | <.0027 |
| Pelvic Organ Prolapse Impact Questionnaire | 17.6 (10.0–25.2) | 5.3 (3.3–7.3) | <.0001 |
We repeated ROC analysis for points Ba and Bp to determine whether anterior or posterior compartment measurements were better able to predict PFDI symptoms. We found no differences in predictive ability to measure prolapse, urinary, or bowel symptoms based on either compartment (data not shown). We found that anterior and posterior prolapse at least 0.5 cm distal to the hymen was specific but not sensitive for bulging/protrusion symptoms and did not predict women with other pelvic floor symptoms.
We also repeated ROC analysis to look for potential effect modification from depressive and pain symptoms. Restricting the analysis to women without depressive symptoms, we found slightly improved diagnostic accuracy for symptoms of bulging/protrusion (area under the curve 0.91) and splinting to void (area under the curve 0.94) symptoms. Areas under the curve for other symptoms only marginally increased. Restricting the analysis to women without pain also increased the area under the curve for bulging/protrusion (0.98), but we found no difference for splinting to void or other urinary and bowel symptoms.
When we repeated the ROC analysis for symptoms of bulging/protrusion subdividing patients by clinic category, we discovered that anatomic prolapse may have less predictive ability to identify these symptoms in the gynecology clinic population, compared with the urogynecology clinic population (area under the curve 0.73 vs 0.92, respectively). However, the overall pattern of observations was similar. Specifically, prolapse 0.5 cm distal to the hymen was sensitive and specific for bulging/protrusion symptoms; however, we could not identify a threshold of prolapse severity that predicted women with other pelvic floor symptoms.
Comment
Maximum vaginal descensus 0.5 cm or more distal to the hymenal remnant is the threshold for bulging/protrusion symptoms, but we found that prolapse severity is a weak predictor of other PFDI prolapse (POPDI) and urinary (UDI) symptoms and offers little or no predictive value for bowel (CRADI) symptoms. In this population, the threshold for splinting to void was more distal to the hymenal remnant (2–2.5 cm), indicating that more severe prolapse is necessary before this symptom develops.
We selected these thresholds based on a positive likelihood ratio greater than 10. With a likelihood ratio of 10, the odds of the symptom are increased 10-fold. In other words, the odds of a symptom are 10 times higher among women with prolapse beyond a given threshold than among women with less advanced prolapse. However, in selecting a specific threshold, the relative balance between sensitivity and specificity must be considered.
Based on our ROC results (Table 2 and Figure, A), a threshold of 0 increased sensitivity to 82% and decrease specificity by 91%, whereas a threshold of −0.5 increased sensitivity to 85% and decreased specificity to 87%. One could argue that the 0 or −0.5 thresholds better optimize sensitivity and specificity. Using these other thresholds, 25% or 29% of participants would be classified with “clinically significant” prolapse. We chose a likelihood ratio of 10 to establish our threshold, and this proved to be the best for correctly classifying the highest percentage of participants (91%). If we were selecting our criterion to measure successful surgical treatment of prolapse, a high negative predictive value might be preferred to maximize diagnostic sensitivity.
We are aware of only 1 other study using ROC analysis to identify diagnostic thresholds for pelvic floor symptoms.2 Swift et al2 found that prolapse greater than −0.5 cm (0.5 cm proximal to the hymen) was the best threshold for predicting the symptoms of “feeling or seeing something bulging.” In their study, no other symptom was predicted by the severity of prolapse. The differences in subject enrollment, questionnaires, and analysis technique may explain the subtle discrepancy in the selected threshold for clinically significant prolapse, compared with our study.
Other investigators have evaluated the association between symptoms and the prolapse severity. These correlations are generally weak to moderate.2–5 The exception is the symptom of visible or palpable bulging or protrusion with a minimum threshold level at or near the hymenal remnant.2–5 When we repeated ROC analysis for Ba and Bp vaginal wall descensus, we also found no differences in predictive ability to measure prolapse, urinary, or bowel symptoms. This is consistent with other research suggesting that bowel and bladder symptoms do not correlate with the location of prolapse (anterior or posterior vaginal wall).5,6,10,11
We looked at the possible impact of depressive and pain symptoms (as measured with the CES-D and SF-12, respectively) on our analysis. Patients without depression or pain had a higher area under the curve for bulging/protrusion and splinting to void, suggesting that the relationship between prolapse and symptoms is qualitatively stronger among women without depression or chronic pain. The diagnostic accuracy of other symptoms was not affected. Thus, although chronic pain and depression may blunt the strength of the relationship between prolapse severity and pelvic floor symptoms because of more complex perception and reporting of symptoms, the diagnostic accuracy of anatomic prolapse severity for predicting pelvic floor symptoms is not substantially affected by these conditions.
Because we recruited women seeking clinical care, a potential limitation of our analysis is the possibility that our results could be affected by spectrum bias. Indeed, our analyses suggest that the diagnostic accuracy of prolapse severity was somewhat lower in the gynecologic population, compared with the women seeking urogynecologic care. However, our overall conclusion in both populations was similar, especially with respect to the poor diagnostic accuracy for the majority of pelvic floor symptoms considered. The optimal threshold for the clinical diagnosis of prolapse was similar in these 2 groups.
The results of this study are strengthened by the systematic collection of symptom and anatomic data using validated quality-of-life instruments. The choice of ROC analysis enhanced our ability to evaluate strength of associations and define an anatomic threshold for the most predictive symptom. Another advantage of our study design is the selection of a population that included normal gynecology and urogynecology patients. This provided us with a spectrum of prolapse severity and a relatively high proportion of women with pelvic floor disorders. However, a potential limitation of our research may be the generalizability of our findings. Johns Hopkins is a tertiary institution. As such, women seeking care at our clinical sites, even in an outpatient suburban setting, may not be representative of the general population.
Our results suggest that it is difficult to identify clinically significant prolapse based on a combined definition of anatomic support defects and pelvic floor symptoms other than vaginal bulging/protrusion. The specific threshold for bulging and protrusion symptoms appears to be near the hymen, but the definition of clinically significant prolapse remains controversial. If prolapse is defined as stage 2 or greater, the prevalence would be 37% among women presenting for gynecologic examinations2 and 65% among older women in the Women’s Health Initiative.12 If anatomic prolapse is defined using a threshold at or distal to the hymenal remnant, the prevalence decreases to 7% and 26%, respectively, among these 2 populations studied. However, neither of these definitions considers the presence or absence of bothersome symptoms.
Future studies calculating postoperative anatomic failure using ROC analysis for global impression of improvement scores may help to improve our understanding of the relationship between prolapse severity and symptoms. Consideration should be given to reporting anatomic prolapse and symptom burden in clinical research.
Acknowledgments
This study was supported in part by Grant K23HD045806 from the National Institute of Child Health and Human Development.
Footnotes
Presented at the 34th Annual Scientific Meeting of the Society of Gynecologic Surgeons, Savanna, GA, April 14-16, 2008.
Reprints not available from the authors.
References
- 1.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 2.Swift S, Woodman P, O’Boyle A, et al. Pelvic organ support study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 3.Bradley CS, Nygaard IE. Vaginal wall descensus and pelvic floor symptoms in older women. Obstet Gynecol. 2005;106:759–66. doi: 10.1097/01.AOG.0000180183.03897.72. [DOI] [PubMed] [Google Scholar]
- 4.Tan JS, Lukacz ES, Menefee SA, Powell CR, Nager CW San Diego Pelvic Floor Consortium. Predictive value of prolapse symptoms: a large database study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:203–9. doi: 10.1007/s00192-004-1243-8. [DOI] [PubMed] [Google Scholar]
- 5.Ghetti C, Gregory WT, Edwards SR, Otto LN, Clark AL. Pelvic organ descent and symptoms of pelvic floor disorders. Am J Obstet Gynecol. 2005;193:53–7. doi: 10.1016/j.ajog.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332–7. doi: 10.1067/mob.2001.119078. [DOI] [PubMed] [Google Scholar]
- 7.Handa VL, Cundiff G, Chang HH, Helzlsouer KJ. Female sexual function and pelvic floor disorders. Obstet Gynecol. 2008;111:1045–52. doi: 10.1097/AOG.0b013e31816bbe85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193:103–13. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Weber AM, Walters MD, Ballard LA, Booher DL, Piedmonte MR. Posterior vaginal prolapse and bowel function. Am J Obstet Gynecol. 1998;179:1446–9. doi: 10.1016/s0002-9378(98)70008-0. [DOI] [PubMed] [Google Scholar]
- 11.Burrows LJ, Meyn LA, Walters MD, Weber AM. Pelvic symptoms in women with pelvic organ prolapse. Obstet Gynecol. 2004;104:982–8. doi: 10.1097/01.AOG.0000142708.61298.be. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard I, Bradley C, Brandt D Women’s Health Initiative. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104:489–97. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]