Abstract
Background
Inadequate control for potential methodological biases has been suggested as an explanation for the conflicting findings concerning the relationship between body mass index (BMI) and mortality.
Methods
Using data from the Shanghai Women’s Health Study, a prospective cohort study conducted in a relatively lean population, we examined the associations of BMI at various ages and average adult BMI with mortality, and evaluated the impact of potential biases related to preexisting illness and cigarette smoking on the associations. Included in the analysis were 74,896 women aged 40 to 70 years with anthropometrics taken by trained interviewers at baseline (1996–2000). Recalled body sizes at ages 20 and 50 were also obtained for older women. Participants were followed through April 2007 by biennial home visits and linkage with the vital statistics registry.
Results
During a mean follow-up of 7.4 years, 2,389 deaths occurred. In initial analyses, both low and high levels of baseline BMI were associated with an increase in mortality, whereas mortality increased monotonically with increasing levels of average adult BMI, BMI at age 50, and, to a lesser extent, BMI at age 20. A direct monotonic relationship between baseline BMI and mortality emerged after accounting for potential biases. Controlling for potential biases also strengthened the positive associations of average adult BMI and BMI at ages 20 and 50 with mortality, with a hazard ratio comparing the highest vs. the lowest quartiles of average adult BMI reaching 2.19 (95% CI, 1.67–2.88).
Conclusion
High BMI during adulthood consistently predicts mortality risk after accounting for potential biases.
Keywords: Body mass index, Epidemiology, Mortality, Methodological bias
INTRODUCTION
It has been argued that conflicting findings regarding the relationship between body weight and mortality can be reconciled by carefully considering potential sources of bias.1–4 The most serious concern in studies of body weight and mortality is that low body weight may be the result rather than the cause of a preexisting illness that leads ultimately to death, a phenomenon referred to as reverse causation.1–4 This phenomenon could underlie, at least in part, the elevated risk of death associated with leanness observed in many epidemiologic studies. Another possible concern is related to confounding by cigarette smoking.1–4 Smoking is known to be associated with reduced body weight and is a major cause of premature death. As with preexisting disease, smoking could also exaggerate the risk of death among individuals with low body weight. Given their potential threats to the validity of studies, it has been recommended that biases due to reverse causation and confounding by smoking need to be addressed carefully in data analyses by limiting analyses to individuals who have no preexisting chronic disease at study baseline, have no substantial weight loss prior to baseline, and have never smoked.1–4
Weight loss due to clinical or subclinical illness and preferential loss of lean body mass often occur in old age, posing a particular methodological challenge for studies of older people. 1–3 Therefore, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) assessed in old age may not capture the true long-term adverse health effect of excess adiposity.1 BMI assessed at a younger age or average adult BMI may be more informative than BMI assessed in old age for elucidating the contribution of adiposity to mortality.1,5,6
We evaluated the impact of potential methodological biases on the relationship between BMI and mortality in the Shanghai Women’ Health Study (SWHS). In addition to BMI at study baseline, we examined BMI at 20 and 50 years of age and average adult BMI in relation to mortality.
METHODS
The SWHS is a population-based, prospective cohort study of adult Chinese women. Details of the study methods have been described elsewhere.7 Briefly, all women 40 to 70 years of age living in seven typical urban communities of Shanghai were invited to participate in the study. Between December 1996 and May 2000, a total of 74,942 women were recruited, with a participation rate of 92.7%. Baseline surveys and anthropometric measurements were carried out at participants’ homes by trained interviewers. Structured questionnaires were used during the surveys to obtain information on demographics, diet and other lifestyle habits, weight history (including weights at ages 20 and 50 years for older women), medical history, and other characteristics. The food-frequency and physical-activity questionnaires used in the SWHS have been validated.8,9
Anthropometry
Participants were asked to wear light indoor clothing when they were measured for weight, height, and circumferences of the waist and hips by trained interviewers at baseline. Measurements were conducted uniformly according to a standard protocol. Weight was measured to the nearest 0.1 kg using a digital weight scale that was calibrated every 6 months. Height and circumferences were measured to the nearest 0.1 cm. All measurements were taken twice, and the average of the weight and height measurements was used to calculate baseline BMI.
BMI at age 20 years was derived from recalled weight and height at age 20, and BMI at age 50 was derived from recalled weight at age 50 and measured height at baseline for women over 50 years of age. Finally, average adult BMI was calculated as a measure of usual adult BMI based on BMI at baseline and at ages 20 and 50 years.
Outcome Ascertainment
Participants were followed up by means of biennial in-person contact and record linkage to the Shanghai Cancer Registry and the Shanghai Vital Statistics Registry. The primary end point for the present analysis was death from all causes that occurred after the baseline survey, with follow-up through April 30, 2007. As of this date, follow-up for the vital status of participants was more than 99% complete. The underlying cause of death was determined on the basis of death certificates, which were coded uniformly according to the codes of the International Classification of Diseases, Ninth Revision (ICD-9) by trained health professionals at the Shanghai Vital Statistics Unit. In particular, we examined deaths from cancer (ICD-9 codes 140 through 208) and cardiovascular disease (CVD, codes 390 through 459).
Statistical Analysis
We first conducted analyses among all participants with BMI measured at baseline (n=74,896). We then focused the analysis on women over 50 years of age (n=36,035) for whom BMI at various ages was assessed. Of these older women, 72.3% (n=26,055) had complete data on BMI at ages 20 and 50, BMI at baseline (51–70 years), and average adult BMI. Women were categorized according to quartiles of BMI at various ages and average adult BMI, with the lowest quartiles serving as the reference categories. Cox proportional hazards models were used, with age as the time scale, to estimate hazard ratios (HRs) of death associated with each BMI category and their 95% confidence intervals (CIs) and to adjust for potential confounders.10 Entry time was defined as age at enrollment, and exit time was defined as age at death or censoring. Tests for linear trend in risk across BMI categories were performed by using the median value for each BMI category and modeling them as continuous variables. Covariates considered for analyses of BMI at baseline, BMI at age 50, and average adult BMI included age (continuous); education level (4 categories); occupation (3 categories); family income (4 categories); menopausal status (premenopausal or postmenopausal); use of hormone therapy (yes or no); amount of regular exercise (hours/week, 4 categories) over the past 5 years; cigarette smoking (yes or no); alcohol consumption (yes or no); and intake of saturated fat, vegetables, and fruits (continuous). For analysis of BMI at age 20, the multivariate model included age and exercise during adolescence. In addition, we used restricted cubic spline regression, a flexible statistical technique, to evaluate the association between BMI and mortality.11 The median value of BMI was treated as the reference point, with knots placed at the 5th, 50th, and 95th percentiles of the BMI distribution. To make the graph more stable and meaningful, subjects with extreme BMI (below the 1st percentile or above the 99th percentile) were excluded from the data set used to fit the spline model.
Of the total participants, 2,111 were ever smokers, including current and former smokers, 7,451 reported previous coronary heart disease, stroke, or cancer at baseline, 745 died within the first 3 years of follow-up, and 1,713 had lost more than 10% of their weight since the age of 50. Because these women had characteristics that might contribute to biases, analyses were conducted both with and without including them to demonstrate the influence of potential methodological factors. We evaluated the proportional hazards assumption by including interaction terms between exposure variables and follow-up time and found no evidence of violating the assumption. Statistical analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC). All statistical tests were based on 2-sided probability.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. The study was approved by the relevant Institutional Review Boards for human research in China and in the United States.
RESULTS
Table 1 presents the characteristics of the study population. The mean age (standard deviation [SD]) of the participants was 52 years (9.1) at baseline. The mean values (SD) for BMI at baseline, at ages 50 and 20 years, and for average adult BMI were 24.0 (3.4), 23.5 (3.3), 19.6 (2.5), and 22.6 (2.5), respectively.
Table 1.
Selected characteristics of the study population
| Variable | |
|---|---|
| Age, mean (SD), y | 52.0 (9.1) |
| Body mass index at baseline, kg/m2 | 24.0 (3.4) |
| Body mass index at age 50, kg/m2 | 23.5 (3.3) |
| Body mass index at age 20, kg/m2 | 19.6 (2.5) |
| Average adult body mass index, kg/m2 | 22.6 (2.5) |
| Education level, % | |
| ≤ Elementary school | 21.6 |
| Middle school | 36.9 |
| High school | 27.9 |
| ≥ College | 13.6 |
| Occupation, % | |
| Professionals, technicians, administrators | 28.7 |
| Clerical and service workers | 20.8 |
| Manufacturing and agricultural workers | 50.6 |
| Annual family income, yuan, % | |
| < 10,000 | 16.2 |
| 10,000 to < 20,000 | 38.3 |
| 20,000 to < 30,000 | 28.1 |
| ≥ 30,000 | 17.5 |
| Postmenopausal, % | 49.6 |
| Hormone therapy, % | 2.1 |
| Ever smoked cigarettes, % | 2.8 |
| Ever drank alcohol, % | 2.2 |
| Regular exercise, mean, hr/wk | 2.0 (3.7) |
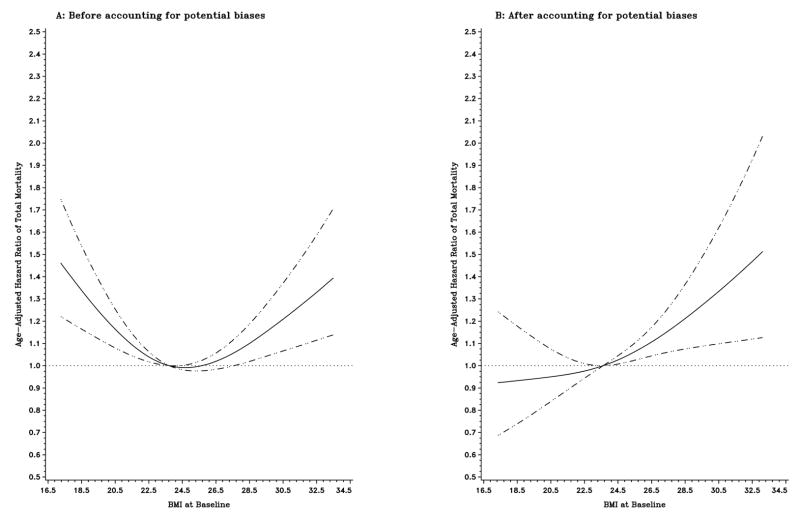
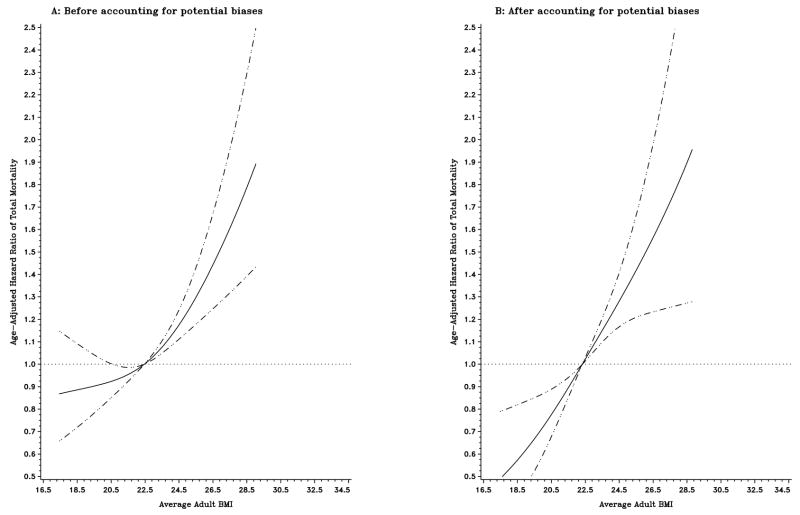
During a mean follow-up of 7.4 years, 2,389 deaths were identified among the entire cohort, with 1,325 occurring in individuals who had conditions potentially contributing to biases. Figure 1 illustrates the influence of control for potential methodological biases on the shape of the curve for age-adjusted HRs of total mortality in relation to baseline BMI among the entire cohort. In an initial analysis including all subjects, both low and high levels of baseline BMI were associated with an increased risk of death (Figure 1A). However, when exclusionary strategies were applied to account for potential biases, including preexisting diseases, early death, weight loss, and smoking, the increase in mortality associated with low levels of baseline BMI was reversed, and a direct monotonic relationship between baseline BMI and mortality emerged (Figure 1B). Figure 2 compares the shapes of the curves for age-adjusted HRs of total mortality associated with average adult BMI in older women before (Figure 2A) and after (Figure 2B) accounting for potential biases, respectively. Unlike the U-shaped relation with baseline BMI in the initial analyses, the risk of death increased monotonically with increasing levels of average adult BMI (Figure 2A). Controlling for potential biases further strengthened the positive association (Figure 2B). Similar patterns were observed for BMI at ages 50 and 20.
Figure 1.
Title: Age-adjusted hazard ratios of total mortality according to baseline BMI before (A; n=73,403) and after (B; n=62,729) accounting for potential biases. Hazard ratios were estimated from a restricted cubic spline Cox regression model. Point estimates are indicated by a solid line and 95% confidence intervals by dashed lines.
Figure 2.
Title: Age-adjusted hazard ratios of total mortality according to average adult BMI before (A; n=25,533) and after (B; n=19,485) accounting for potential biases. Hazard ratios were estimated from a restricted cubic spline Cox regression model. Point estimates are indicated by a solid line and 95% confidence intervals by dashed lines.
Table 2 summarizes the HRs and 95% CIs of total mortality according to quartiles of BMI at various ages and average adult BMI in older women before and after applying exclusionary strategies. After accounting for potential biases, the HRs of death increased consistently with increasing quartiles of BMI. The age-adjusted HRs (95% CIs) of death for the highest vs. the lowest BMI quartiles were 1.74 (1.34–2.25), 2.06 (1.58–2.67), 1.34 (1.05–1.71), and 2.19 (1.67–2.88) for BMI at baseline, BMI at age 50, BMI at age 20, and average adult BMI, respectively. Further adjustment for a range of potential confounders did not alter the results significantly.
Table 2.
Hazard ratios (HRs) of Total Mortality According to Quartiles of BMI at Various Ages and Average Adult BMI
| All Subjects Aged 51–70 y at Baseline (n=26055) |
Excluding Subjects Potentially Contributing to Bias* (n=19883) |
||||
|---|---|---|---|---|---|
| Quartile of BMI, kg/m2 | No. of Deaths | Age-Adjusted HR (95% CI) |
No. of Deaths | Age-Adjusted HR (95% CI) |
Multivariate HR† (95% CI) |
| BMI at study baseline | |||||
| <22.2 | 291 | 1 (Reference) | 87 | 1 (Reference) | 1 (Reference) |
| 22.2–24.3 | 244 | 0.84 (0.71–0.99) | 105 | 1.15 (0.86–1.52) | 1.13 (0.85–1.50) |
| 24.4–26.6 | 299 | 1.01 (0.86–1.18) | 133 | 1.47 (1.12–1.92) | 1.40 (1.07–1.84) |
| ≥26.7 | 353 | 1.11 (0.95–1.30) | 161 | 1.74 (1.34–2.25) | 1.61 (1.23–2.10) |
| P value for trend | 0.04 | <.0001 | 0.0001 | ||
| BMI at age 50 y | |||||
| <21.1 | 245 | 1 (Reference) | 91 | 1 (Reference) | 1 (Reference) |
| 21.1–23.0 | 238 | 0.99 (0.82–1.18) | 116 | 1.34 (1.02–1.76) | 1.32 (1.00–1.74) |
| 23.1–25.3 | 292 | 1.29 (1.08–1.52) | 132 | 1.74 (1.33–2.28) | 1.67 (1.28–2.19) |
| ≥25.4 | 412 | 1.73 (1.47–2.02) | 147 | 2.06 (1.58–2.67) | 1.91 (1.46–2.50) |
| P value for trend | <.0001 | <.0001 | <.0001 | ||
| BMI at age 20 y | |||||
| <18.0 | 297 | 1 (Reference) | 116 | 1 (Reference) | 1 (Reference) |
| 18.0–19.4 | 276 | 0.92 (0.78–1.09) | 117 | 1.03 (0.80–1.34) | 1.02 (0.79–1.32) |
| 19.5–21.3 | 247 | 0.91 (0.77–1.08) | 106 | 1.03 (0.79–1.33) | 1.01 (0.77–1.31) |
| ≥21.4 | 367 | 1.19 (1.02–1.39) | 147 | 1.34 (1.05–1.71) | 1.29 (1.01–1.64) |
| P value for trend | 0.01 | 0.02 | 0.04 | ||
| Average Adult BMI | |||||
| <20.9 | 250 | 1 (Reference) | 78 | 1 (Reference) | 1 (Reference) |
| 20.9–22.4 | 267 | 1.06 (0.89–1.26) | 127 | 1.66 (1.25–2.21) | 1.63 (1.23–2.17) |
| 22.5–24.0 | 292 | 1.14 (0.96–1.35) | 124 | 1.70 (1.28–2.25) | 1.61 (1.21–2.15) |
| ≥24.1 | 378 | 1.44 (1.23–1.69) | 157 | 2.19 (1.67–2.88) | 2.02 (1.53–2.66) |
| P value for trend | <.0001 | <.0001 | <.0001 | ||
Abbreviations: BMI, body mass index; CI, confidence interval.
Excluding subjects who ever smoked, reported previous cardiovascular disease or cancer at baseline or substantial weight loss since age 50, or died within the first 3 years of follow-up.
Analyses of BMI at baseline, age 50, and average BMI adjusted for age; education; occupation; annual family income; menopausal status; hormone therapy; amount of exercise; alcohol consumption; intakes of saturated fat, vegetables, and fruits. For BMI at age 20, adjusted for age and amount of exercise during adolescence.
Table 3 shows the HRs of death from cardiovascular disease and cancer according to quartiles of BMI at various ages and average adult BMI after accounting for potential biases. BMI at baseline, BMI at age 50, and average adult BMI all showed strong positive associations with CVD and cancer mortality, whereas BMI at age 20 showed positive but non-significant associations.
Table 3.
Hazard ratios (HRs) of Cardiovascular or Cancer Mortality by Quartiles of BMI After Excluding Subjects Potentially Contributing to Bias* (n=19883)
| Cardiovascular Mortality | Cancer Mortality | |||||
|---|---|---|---|---|---|---|
| Quartile of BMI, kg/m2 | No. of Deaths | Age-Adjusted HR (95% CI) |
Multivariate HR (95% CI) |
No. of Deaths | Age-Adjusted HR (95% CI) |
Multivariate HR† (95% CI) |
| BMI at study baseline | ||||||
| <22.2 | 13 | 1 (Reference) | 1 (Reference) | 48 | 1 (Reference) | 1 (Reference) |
| 22.2–24.3 | 16 | 1.17 (0.56–2.43) | 1.13 (0.54–2.35) | 61 | 1.21 (0.83–1.77) | 1.19 (0.82–1.74) |
| 24.4–26.6 | 31 | 2.30 (1.20–4.39) | 2.14 (1.11–4.10) | 77 | 1.54 (1.07–2.21) | 1.49 (1.03–2.14) |
| ≥26.7 | 36 | 2.59 (1.37–4.89) | 2.27 (1.19–4.33) | 89 | 1.76 (1.24–2.50) | 1.68 (1.18–2.41) |
| P value for trend | 0.0004 | 0.003 | 0.0006 | 0.002 | ||
| BMI at age 50 y | ||||||
| <21.1 | 17 | 1 (Reference) | 1 (Reference) | 50 | 1 (Reference) | 1 (Reference) |
| 21.1–23.0 | 17 | 1.04 (0.53–2.04) | 1.03 (0.52–2.01) | 72 | 1.52 (1.06–2.19) | 1.52 (1.06–2.18) |
| 23.1–25.3 | 29 | 2.03 (1.12–3.70) | 1.90 (1.04–3.47) | 72 | 1.74 (1.21–2.50) | 1.70 (1.18–2.45) |
| ≥25.4 | 33 | 2.45 (1.37–4.41) | 2.15 (1.18–3.89) | 81 | 2.09 (1.46–2.97) | 2.00 (1.39–2.86) |
| P value for trend | 0.0003 | 0.003 | <.0001 | 0.0002 | ||
| BMI at age 20 y | ||||||
| <18.0 | 23 | 1 (Reference) | 1 (Reference) | 61 | 1 (Reference) | 1 (Reference) |
| 18.0–19.4 | 17 | 0.75 (0.40–1.40) | 0.75 (0.40–1.40) | 78 | 1.33 (0.95–1.86) | 1.31 (0.94–1.84) |
| 19.5–21.3 | 23 | 1.11 (0.62–1.98) | 1.10 (0.62–1.96) | 59 | 1.10 (0.77–1.57) | 1.08 (0.75–1.54) |
| ≥21.4 | 33 | 1.48 (0.87–2.53) | 1.45 (0.85–2.48) | 77 | 1.35 (0.96–1.90) | 1.32 (0.94–1.85) |
| P value for trend | 0.06 | 0.07 | 0.2 | 0.2 | ||
| Average Adult BMI | ||||||
| <20.9 | 12 | 1 (Reference) | 1 (Reference) | 43 | 1 (Reference) | 1 (Reference) |
| 20.9–22.4 | 25 | 2.14 (1.08–4.27) | 2.03 (1.02–4.05) | 76 | 1.80 (1.24–2.62) | 1.78 (1.22–2.60) |
| 22.5–24.0 | 23 | 2.04 (1.01–4.10) | 1.86 (0.92–3.75) | 71 | 1.77 (1.21–2.59) | 1.72 (1.17–2.52) |
| ≥24.1 | 36 | 3.26 (1.69–6.26) | 2.75 (1.42–5.36) | 85 | 2.17 (1.51–3.14) | 2.07 (1.42–3.01) |
| P value for trend | 0.0005 | 0.004 | 0.0001 | 0.0005 | ||
Abbreviations: BMI, body mass index; CI, confidence interval.
Excluding subjects who ever smoked, reported previous cardiovascular disease or cancer at baseline or substantial weight loss since age 50, or died within the first 3 years of follow-up.
Analyses of BMI at baseline, age 50, and average BMI adjusted for age; education; occupation; annual family income; menopausal status; hormone therapy; amount of exercise during the past 5 years; alcohol consumption; intakes of saturated fat, vegetables, and fruits. For BMI at age 20, adjusted for age and amount of exercise during adolescence.
COMMENT
In this cohort study of Chinese women, we found that a U-shaped relationship between baseline BMI and mortality became a positive linear one after accounting for potential methodological biases. Average adult BMI and BMI in midlife showed more robust positive associations with mortality. BMI at age 20 also appeared to predict total mortality, despite its relatively small degree of variation. It is worth noting that unlike previous studies, most of which were conducted in Western populations, our study was conducted in a generally lean population of Chinese women, with the mean values of BMI from age 20 to study baseline all below 25.0, the cut point for overweight. Nevertheless, a consistent, monotonic dose-response relationship was observed between BMI at various ages and subsequent mortality. Given these findings, the importance of achieving and maintaining a healthy weight throughout adulthood cannot be overemphasized.
Our study illustrates clearly the influence of potential methodological biases on the relationship between BMI and mortality. As smoking was uncommon in our study population, the observed significant change in the shape of the curve for BMI at baseline and mortality reflected primarily the effect of controlling for reverse causation bias. Several previous studies have also demonstrated varying associations between BMI and mortality depending on the analytic approaches used.5,6,12–17 For example, in the Nurses’ Health Study, a J-shaped association was observed in an initial analysis of the entire cohort; however, as seen in our study, a clear linear association emerged in a separate analysis that excluded ever smokers, early deaths, and those with significant weight change.12 In the National Institutes of Health-AARP Diet and Health Study, similar to our finding, BMI at age 50 showed a much stronger positive association with mortality than BMI at study baseline when participants were 50 to 71 years old.5 Also consistent with our results, the association of excess adiposity with mortality was found to be markedly strengthened by using average BMI in an analysis of data from the First National Health and Nutrition Examination Survey Epidemiologic Follow-Up Study.6 It remains unclear, however, why a J- or U-shaped relation between BMI and mortality persisted after accounting for baseline disease and smoking status in some other studies.18,19 Although there has been some debate over this issue, the possibility cannot be dismissed that inadequate control for potential biases, particularly the reverse causation bias, may have contributed to the conflicting findings.1,2
Given the increased prevalence of chronic diseases and the reduced validity of BMI as a measure of adiposity in the elderly, simplistic analyses using current BMI could be particularly misleading in this group.1–3 In addition to BMI assessed at earlier ages, other measures of adiposity also need to be considered.20 We and others have shown previously that waist-to-hip ratio was a strong and robust predictor of mortality in women.21–23 Taken together, these findings underscore the importance of taking into account methodological factors in studying the effect of excess adiposity on mortality.
There is compelling evidence supporting the biological plausibility of a positive relation between adiposity and mortality. It is well known that obesity is a major risk factor for numerous chronic diseases such as type 2 diabetes, hypertension, cardiovascular disease, and certain types of cancer.24 Adipose tissue has been recognized as an active endocrine and paracrine organ capable of releasing a large number of cytokines and bioactive mediators, including leptin, adiponectin, interleukin-6 and tumor necrosis factor-α, that play important roles in the pathogenesis of many obesity related diseases.25 Among the key pathways involved are insulin resistance and low-grade systemic inflammation induced by excess adiposity.25,26
Our study is limited mainly by a lack of data to validate the accuracy of recalled body sizes at ages 20 and 50, although recall of past weights is generally known to be fairly accurate.27,28 Another limitation of the study is the possibility of misclassification of the cause of death based solely on death certificates. However, a study evaluating the validity of cause-of-death statistics in urban China, including Shanghai, has shown that information on death certificates was reasonably accurate with regard to major causes of death such as CVD and cancer, suggesting that our results on cause-specific mortality were unlikely to be seriously biased.29 Finally, we cannot rule out completely the possibility of residual confounding due to crudely measured or unmeasured covariates, despite having adjusted for a range of potential confounders.
Nevertheless, our study provides strong evidence that even in a relatively lean population increasing BMI during adulthood is associated with a significant increase in mortality. Our findings reinforce the need to consider potential methodological limitations in assessing the relationship between measures of adiposity and mortality.
Acknowledgments
This study was supported by research grants R01CA70867 and R01HL079123 from the National Institutes of Health. Dr. Zhang was supported by an NIH-sponsored Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program at Vanderbilt University (2K12HD043483-06).
Footnotes
Published version: http://www.nature.com/ijo/journal/vaop/ncurrent/abs/ijo200863a.html
Disclosure of conflicts of interests: None reported.
References
- 1.Manson JE, Bassuk SS, Hu FB, Stampfer MJ, Colditz GA, Willett WC. Estimating the number of deaths due to obesity: can the divergent findings be reconciled? J Womens Health (Larchmt) 2007;16(2):168–176. doi: 10.1089/jwh.2006.0080. [DOI] [PubMed] [Google Scholar]
- 2.Willett WC, Hu FB, Colditz GA, Manson JE. Underweight, overweight, obesity, and excess deaths. JAMA. 2005;294:551. doi: 10.1001/jama.294.5.551-a. [DOI] [PubMed] [Google Scholar]
- 3.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer M. Weight loss and mortality: what does the evidence show? PLoS Med. 2005;2(6):e181. doi: 10.1371/journal.pmed.0020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg JA. Correcting biases in estimates of mortality attributable to obesity. Obesity (Silver Spring) 2006;14(11):2071–2079. doi: 10.1038/oby.2006.242. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 8.Shu XO, Yang G, Jin F, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58:17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 9.Matthews CE, Shu XO, Yang G, et al. Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. Am J Epidemiol. 2003;158:1114–1122. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- 10.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FJ., Jr . Springer series in statistics. New York: Springer-Verlag; 2001. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. [Google Scholar]
- 12.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 13.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 14.Gelber RP, Kurth T, Manson JE, Buring JE, Gaziano JM. Body mass index and mortality in men: evaluating the shape of the association. Int J Obes (Lond) 2007;31(8):1240–1247. doi: 10.1038/sj.ijo.0803564. [DOI] [PubMed] [Google Scholar]
- 15.Baik I, Ascherio A, Rimm EB, et al. Adiposity and mortality in men. Am J Epidemiol. 2000;152(3):264–271. doi: 10.1093/aje/152.3.264. [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Hart CL, Hole DJ, Davey Smith G. Reverse causality and confounding and the associations of overweight and obesity with mortality. Obesity (Silver Spring) 2006;14(12):2294–2304. doi: 10.1038/oby.2006.269. [DOI] [PubMed] [Google Scholar]
- 17.Losonczy KG, Harris TB, Cornoni-Huntley J, et al. Does weight loss from middle age to old age explain the inverse weight mortality relation in old age? Am J Epidemiol. 1995;141(4):312–321. doi: 10.1093/aje/141.4.312. [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 19.Gu D, He J, Duan X, et al. Body weight and mortality among men and women in China. JAMA. 2006;295:776–783. doi: 10.1001/jama.295.7.776. [DOI] [PubMed] [Google Scholar]
- 20.Hu FB. Obesity and mortality: watch your waist, not just your weight. Arch Intern Med. 2007;167(9):875–876. doi: 10.1001/archinte.167.9.875. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Shu XO, Yang G, et al. Abdominal adiposity and mortality in Chinese women. Arch Intern Med. 2007;167(9):886–892. doi: 10.1001/archinte.167.9.886. [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 24.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 25.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 27.Casey VA, Dwyer JT, Berkey CS, Coleman KA, Gardner J, Valadian I. Long-term memory of body weight and past weight satisfaction: a longitudinal follow-up study. Am J Clin Nutr. 1991;53(6):1493–1498. doi: 10.1093/ajcn/53.6.1493. [DOI] [PubMed] [Google Scholar]
- 28.Tamakoshi K, Yatsuya H, Kondo T, et al. The accuracy of long-term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord. 2003;27(2):247–252. doi: 10.1038/sj.ijo.802195. [DOI] [PubMed] [Google Scholar]
- 29.Rao C, Yang G, Hu J, Ma J, Xia W, Lopez AD. Validation of cause-of-death statistics in urban China. Int J Epidemiol. 2007;36(3):642–651. doi: 10.1093/ije/dym003. [DOI] [PubMed] [Google Scholar]