Abstract
We investigated the phenomenology of delirium severity measured with the Memorial Delirium Assessment Scale (MDAS) among 441 older patients (aged 65 and older) admitted with delirium in post-acute care. Using latent class analysis, we identified four classes of psychomotor-severity subtypes of delirium: hypoactive-mild, hypoactive-severe, mixed with hyperactive features-severe, and normal-mild. Among those with dementia (N=166), the hypoactive-mild class was associated with a higher risk of mortality (HR=3.98, 95% CI 1.76, 8.98). Among those without dementia (N=275), greater severity was associated with mortality, regardless of psychomotor features (hypoactive-severe, HR=1.99, 95% CI 1.02, 3.86; mixed with hyperactive features-severe, HR=1.98, 95% CI 1.08, 3.64) when compared to the normal-mild class. Our data suggest that instruments that measure delirium severity and psychomotor features provide important prognostic information and should be integrated into the assessment of delirium.
Keywords: Delirium, rating scales, post-acute care, latent class analysis
Delirium is an acute confusional state that is a common, preventable, and a life-threatening clinical syndrome. It has been estimated that the prevalence of delirium at hospital admission is between 14 to 24 percent, while after hospital admission the incidence of delirium ranges from 6 to 56 percent.1 Delirium is associated with many adverse outcomes, which include distress for the patients, spouses, caregivers, and nurses. Moreover, delirium is associated with increased length of hospital stay, morbidity, and mortality.1, 2 Despite overwhelming evidence for the clinical importance of recognizing and preventing delirium, the syndrome remains under-detected by clinicians.3-5
Currently, four psychomotor behavior subtypes of delirium are recognized: normal, hypoactive, hyperactive, and mixed. Hypoactive delirium is characterized by one or more of the following characteristics: slowing or lack of movement, paucity of speech with or without prompting, and unresponsiveness.6 Features of hyperactive behavior ranges from simple restlessness to constant movement and agitation.6, 7 Mixed forms manifest both hypoactive and hyperactive elements.6 The hypoactive subtype is least likely detected by clinicians8 when compared to the hyperactive or mixed subtypes. There is inconsistent evidence for the association between the under-detected hypoactive subtype of delirium and poorer health outcomes compared with the hyperactive, mixed, or no psychomotor disturbance groups.9 These findings are due in part to the limited number of (possibly under-powered)10 studies, which have simultaneously examined the underlying delirium severity of these psychomotor subtypes in relation to health outcomes.
Meagher and colleagues11-13 have conducted seminal work on the phenomenological study of delirium. They have shown the association between distinct symptoms of delirium with cognitive and non-cognitive features in adults.12 In another study, they found that severe cognitive symptoms in geriatric patients was a distinguishing feature between adult and geriatric delirium symptom profiles.11 Among 46 patients with delirium, aged 21-89, Meagher and colleagues found that the overall delirium severity and severity of delirium symptoms, based on the Delirium Rating Scale, differed significantly across motoric subtypes.13
The Memorial Delirium Assessment Scale (MDAS)6 is a reliable and valid measure of delirium severity that considers psychomotor activity. The MDAS was developed by Breitbart and colleagues6 as brief tool for assessing delirium severity in medically hospitalized cancer and acquired immunodeficiency syndrome (AIDS) patients. Since then, the MDAS has been used in different clinical and research settings and offers a short delirium severity index for both evaluating the efficacy of treatments or interventions and understanding the phenomenology of delirium in a specific population or setting.
We have recently completed a large prospective study of delirium in post-acute skilled nursing facilities. As part of this study, over 4000 new admissions to post-acute care were screened with a structured delirium assessment consisting of the Confusion Assessment Method (CAM)14, 15 for the diagnosis of delirium and the MDAS. Over 400 of these patients were found to have CAM-defined delirium and were followed longitudinally for six months, making this the largest cohort of delirious patients enrolled into a research study. Using this unique data source, the primary aim of this study was to examine whether the classic psychomotor subtypes (hypoactive, hyperactive, and mixed) reflect the underlying phenomenology of delirium severity using the entire MDAS. Our goal was to identify refined subtypes of psychomotor behavior distinguished by degrees of delirium severity. A secondary aim was to use the results from the subtypes identified to predict mortality risk over six months. Mortality is used to demonstrate the clinical predictive validity of these classes in our study. We hypothesized that each subtype would relate differently to mortality risk over six months.
Methods
Subjects
All 441 patients in this study had CAM-defined delirium at post-acute care (PAC) admission, between October 1, 2000 and December 31, 2003. Patients and their caregivers were recruited from a cluster-randomized clinical trial of a Delirium Abatement Program (DAP) at eight Boston area skilled nursing facilities and caregivers provided written informed consent. Methodological details of the DAP have been previously published.16 The inclusion criteria for the study were as follows: aged 65 and older, discharged directly from an acute-care medical or surgical hospitalization, English-speakers, resided within a 25-mile radius of the research site, and communicative prior to acute illness. Exclusion criteria included the following: significant hearing loss, admitted for terminal care (life expectancy less than 6 months), and end-stage dementia. We also excluded those with complete dependence for Activities of Daily Living (ADL)17 prior to hospitalization. Trained research assistants completed interviews within 5 days after admission. The protocol was approved by the Hebrew SeniorLife Institutional Review Board.
Study Measures
Delirium Severity Assessment
The MDAS6 was completed as part of the structured delirium assessment that was administered by trained research interviewers to each assenting patient on admission to the post-acute facility. The 15-20 minute assessment consisted of the Mini-Mental State Examination (MMSE),18 Digit Span forward and backwards,19 and Delirium Symptom Interview (DSI),20 an interview designed to measure 8 key symptoms of delirium. Using data from the MMSE, Digit Span, and DSI, trained research personnel scored the severity of delirium based on 10 symptoms described in the MDAS, each scored from 0 (None/Normal) to 3 (Severe) for a maximum score of 30. The 10 symptoms include reduced level of consciousness, disorientation, short-term memory impairment, impaired digit span, reduced ability to maintain and shift attention, disorganized thinking, perceptual disturbance, delusions, decreased or increased psychomotor disturbance, and sleep-wake cycle disturbance. Immediately after the psychomotor disturbance severity item, an additional observational question was to characterize the patient as “hypoactive,” “hyperactive,” “elements of both,” or “normal.” The responses were based on a query of nurses over the previous 24-hour period and the assessment by trained research assistants over the course of the interview Since pure hyperactive delirium was uncommon in this sample, those who were in the “elements of both” category were coded as having mixed with hyperactive features. Thus, for the current analysis, classification of psychomotor activity was separated into those with hypoactivity, those who are mixed with hyperactive features, and those with normal psychomotor activity.
Mortality
A secondary outcome was mortality at six months. There were three sources of information from which mortality status was derived, which included the National Death Index (NDI), medical records, and proxy interviews. When NDI information was not available for a subject (<2% of cases), the other two sources were used: 1) medical record information of patients who had died within the first 30 days of admission and 2) telephone interview with the proxy.
Covariates
We included several baseline patient sociodemographic characteristics as covariates in this study including age, gender, race/ethnicity, and educational achievement. We also adjusted for the following health measures in the analyses: comorbidity, dementia, and functional status. The Charlson Comorbidity Index (CCI) was completed with the caregiver.21, 22 We excluded dementia from the CCI score due to concern for collinearity. The MMSE 23 score, which was administered as part of the structured delirium assessment, was used to reflect the effects of both chronic cognitive impairment (if present) and of delirium. The Blessed Dementia Rating Scale (BDRS),24 a proxy-based instrument (0-28, 28 worst), was used to quantify cognitive impairment prior to hospitalization. Information on the patient's functional status prior to hospitalization, as identified by the caregiver, was obtained using the Katz Activities of Daily Living (ADL)17 scale, which scores functional dependence in eight areas: bathing, continence, dressing, feeding, toileting, transferring, walking, and grooming. ADL items ranged from 0 (total dependence) to 2 (independence), with a maximum total score of 16 (independent function).
Statistical Analyses
Latent class analysis (LCA) was used to categorize individuals based on their responses to a number of indicators or observed variables.25 Specifically, based on individuals' responses to all indicators, latent classes were assigned using posterior probabilities calculated by Bayes' Theorem and assuming conditional independence of the indicators.26 Each patient was assigned to the latent class to which the largest posterior probability was calculated. Detailed criteria for determining the best-fitting latent class model using Mplus v.527 is described elsewhere.25, 28 Briefly, the best-fitting model had the lowest values for the following criteria: Akaike Information Criterion (AIC), Log-likelihood (LL), Bayesian Information Criterion (BIC), and sample size-adjusted BIC (SABIC)=8795.0. We also used the Bootstrap likelihood ratio test (LRT) and Vuong-Lo-Mendell-Rubin LRT to determine the best-fitting model between two sequential LCA estimated models, with a higher p-value favoring the prior LCA model. For the clinical interpretation, we examined both the mean level and frequency of responses for each of the delirium severity indicators within each class.
Survival analysis was used to produce Kaplan-Meier survival curves and estimates. Cox29 proportional hazards analyses were also performed using the latent class (dummy) assignments with the normal-mild delirium group as the reference group, using STATA 10.0.30 An alpha level of .05 was used in all analyses to determine statistical significance. The predicted proportional hazards assumption was validated with observed values using log-likelihood and Kaplan-Meier survival plots.
Results
The sociodemographic and health characteristics of the sample are shown in Table 1. The mean age for the 441 patients was 84.1 years (SD 7.2; range, 65-104). The majority were older women, white, less than college educated, with modest functional needs prior to hospitalization. Within delirium patients, 32% had no psychomotor disturbance, based on the MDAS. Among those with hypoactivity (n=206), the frequency for the degree of severity were: 49% mild, 36% moderate, and 5% severe. The frequencies for degree of severity in hyperactive delirium patients (n=95) were 72% mild, 26% moderate, and 2% severe.
Table 1.
Sociodemographic and health Status of participants at post-acute facility admission (N=441).
| Sociodemographic | |
| Age (Mean (±SD)) | 84.1 (7.2) |
| Female (N (%)) | 285 (64.6) |
| White (N (%)) | 403 (91.4) |
| College education or higher (N (%)) | 93 (21.1) |
| Home residence before acute hospital admission, (N (%)) | 412 (93.4) |
| Health | |
| Dementia Present (N (%)) | 166 (37.6) |
| Charlson Comorbidity without dementia (Mean (±SD)) | 2.3 (2.4) |
| Activities of Daily Living (ADL) (Mean (±SD)) | 13.3 (3.3) |
| Mini-Mental State Examination Score (Mean (+SD)) | 12.5 (6.7) |
| Blessed Dementia Rating Scale (Mean (+SD)) | 7.4 (4.7) |
Abbreviation: SD = standard deviation.
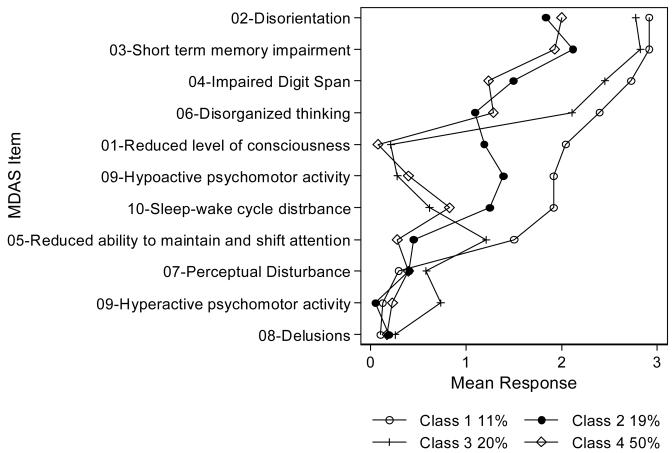
In the latent class analysis, the best-fitting model was the four-class model. Response profiles are illustrated in Figure 1. The four-class model had the most meaningful clinical interpretability and the lowest values for the information criteria and log-likelihood (AIC=8682.4, LL= −4218.2, BIC=9185.3, and SABIC=8795.0) while considering both the Bootstrap LRT (p<.001 for each class) and Vuong-Lo-Mendell-Rubin LRT, which showed the five-class model was a worse fit than the four-class model (p=0.9). For the clinical interpretation, we identified Class 1 as a hypoactive and severe delirium subtype; Class 2 as a hypoactive and mild delirium subtype; Class 3 as a mixed with hyperactive features and severe delirium subtype; and Class 4 as a normal psychomotor and mild delirium subtype. In addition, we found consistency in both the mean level of severity and frequency of responses (available upon request) for each of the delirium severity indicators within each class. The psychomotor activity question (MDAS item 9) were most helpful in separating the classes with the hypoactive psychomotor group forming two distinct classes (Class 1 and 2), the mixed with hyperactive features psychomotor group (Class 3), and the normal psychomotor group (Class 4). Along with the psychomotor subtypes, we found that there were consistent differences across the other delirium severity indicators (Figure 1). For example, Class 1 was labeled the hyper-severe subtype because the patients had a higher probability of experiencing higher mean levels of severity in eight out of the ten symptoms, relative to the other identified classes.
Figure 1. Response profile of four-class model with psychomotor (hypo/hyperactivity) categories.
Class 1: Hypo-Severe (N=48); Class 2:Hypo-Mild (N=85); Class 3: Hyper-Severe (n=86); Class 4:Normal-Mild (n=222)
This plot illustrates the mean severity rating (0-3) for 10 MDAS delirium symptoms (representing one symptom, [9] psychomotor activity, as two items, one indicating hypoactivity, another hyperactivity) with a separate line for each of four classes estimated from a latent class model. Percents in legend indicate the prevalence of class membership in the sample. Class 1 is named the hypoactive-severe class, class 2 is named the hypoactive-mild class, class 3 is named the mixed with hyperactive features-severe class, and class 4 is named the normal-mild class.
Survival analyses confirmed heterogeneity in the relationship of the classes to a clinically meaningful endpoint. The hypoactive-severe (Class 1) and hypoactive-mild (Class 2) demonstrated the lowest survival rates. The hypoactive–severe class had the worst survival rate than the hypoactive-mild class at 30 days (1 month), 110 days (close to 4 months), and 180 days (6 months). Between 30 and 110 days, the hypoactive-mild class had a lower survival rate than the hypoactive-severe class.
Table 2 shows the unadjusted and adjusted Cox proportional hazards ratios as Model 1 and Model 2, respectively. In Model 1, the hypoactive-severe and hypoactive-mild classes showed consistent results with the Kaplan-Meier analyses. However, with the normal-mild class as the reference group, the hypoactive-mild (Class 2) had a slightly higher significant hazards ratio than the hypoactive-severe (Class 1). After adjusting for the sociodemographic and health characteristics, the hypoactive-severe and hypoactive-mild classes, in respective order, were 1.67 (95% Confidence Interval (CI)=0.97, 2.87) and 1.62 (95% CI=1.05, 2.49) times more likely to die during the 6 month follow-up compared to those who had normal psychomotor behavior and mild delirium.
Table 2.
Cox Proportional Hazard Models for class membershihp predicting mortality at six months (180 days) with Class 4 (Mild-Normal) as reference (N=441)
| Model 1 (unadjusted) | |||
| Delirium Class | Hazards Ratio | 95% Confidence Interval | p-value |
| Class 1: Hypo-Severe (centered) | 1.90 | (1.14 , 3.16) | p<.05 |
| Class 2: Hypo-Mild (centered) | 1.98 | (1.30 , 3.02) | p<.001 |
| Class 3: Mixed with Hyperactive | |||
| Features-Severe (centered) | 1.37 | (0.87 , 2.17) | p>.100 |
| Class 4: Normal-Mild (centered, reference) | |||
| Model 2 (adjusted)† | |||
| Delirium Class | Hazards Ratio | 95% Confidence Interval | p-value |
| Class 1: Hypo-Severe (centered) | 1.67 | (0.97 , 2.87) | p>.05 |
| Class 2: Hypo-Mild (centered) | 1.62 | (1.05 , 2.49) | p<.05 |
| Class 3: Mixed with Hyperactive | |||
| Features-Severe (centered) | 1.48 | (0.91 , 2.39) | p>.100 |
| Class 4: Normal-Mild (centered, reference) |
Model 2 adjusts for age (centered at 65 years old), sex, race/ethnicity, college education (centered), Charlson Comorbidity without dementia, dementia status, and activities of daily living (ADL).
Upon stratifying the sample by dementia status, the identified classes remained the same, but we found significant effect modification of the association of psychomotor-severity classes with mortality. Among those without dementia, after adjusting for sociodemographic and health characteristics, the hypoactive-severe class and mixed with hyperactive features-severe class were associated with higher mortality (HR=1.99, 95% CI 1.02, 3.86), (HR=1.98, 95% CI 1.08, 3.64), respectively, when compared to the normal-mild class (Table 3). The hypoactive-mild class was not significantly associated with mortality among patients without dementia; however, this was the only class that was significantly associated with a higher risk of death at 6 months among those with dementia, (HR=3.98, 95% CI 1.76, 8.98). In sum, increased mortality was found in the hypoactive-severe and hyperactive severe classes in the non-dementia group; and in the hypoactive-severe class in the dementia group.
Table 3.
Cox Proportional Hazard Adjusted† Models for class membership predicting mortality at six months (180 days) with Class 4 (Mild-Normal) as reference for patients without dementia (N=243) and with dementia (n=198).
| No dementia | |||
| Delirium Class | Hazards Ratio | 95% Confidence Interval | p-value |
| Class 1: Hypo-Severe (centered) | 1.99 | (1.02 , 3.86) | p<.05 |
| Class 2: Hypo-Mild (centered) | 1.32 | (0.78 , 2.25) | p>.100 |
| Class 3: Mixed with Hyperactive | 1.98 | (1.08 , 3.64) | p<.05 |
| Features-Severe (centered) | |||
| Class 4: Normal-Mild (centered, reference) | |||
| Dementia | |||
| Delirium Class | Hazards Ratio | 95% Confidence Interval | p-value |
| Class 1: Hypo-Severe (centered) | 1.72 | (0.65 , 4.56) | p>.100 |
| Class 2: Hypo-Mild (centered) | 3.98 | (1.76 , 8.98) | p<.01 |
| Class 3: Mixed with Hyperactive | 1.15 | (0.51 , 2.61) | p>.100 |
| Features-Severe (centered) | |||
| Class 4: Normal-Mild (centered, reference) |
Models adjusted for age (centered at 65 years old), sex, race/ethnicity, college education (centered), Charlson Comorbidity without dementia, dementia status, and activities of daily living (ADL).
Discussion
There were four distinct delirium subtypes of delirium severity and psychomotor behavior based on the MDAS at admission in the post-acute care, which were hypoactive-severe, hypoactive-mild, mixed with hyperactive features-severe, and normal-mild. The hypoactive delirium classes had the worse survival rates, with the hypoactive-severe class having the lowest six-month survival rates of the classes. After adjusting for covariates, the hypoactive-mild class had a significantly higher likelihood of mortality at six-months than the normal-mild class. However, after stratifying the adjusted model by dementia, we found that the association of the delirium classes on mortality depends on the presence or absence of dementia. Among patients who did not have dementia, it was delirium severity rather than the motoric subtypes that was associated with higher risk of mortality at six months. Among those who had dementia, only the hypoactive-mild class was significantly associated with mortality at six months.
The overall mortality findings are consistent with the conclusions drawn by Kiely and colleagues31 with the hypoactive subtype having the highest mortality risk at 1-year and a statistically significant higher risk relative to the normal-mild group in post-acute care. The findings from this study further distinguish the psychomotor subtypes by severity of delirium for predicting mortality at six months. The presence of effect modification based on dementia status further reminds us of the importance of recognizing baseline cognitive symptoms, especially in older adults, when examining the phenomenology.11-13 In a systematic review, de Rooij and colleagues9 found the combination of the MDAS, Dublin Delirium Assessment Scale (DAS), and the new version of the DRS (DRSR-98) to be a reliable method of identifying clinical subtypes of delirium. While using all three instruments would likely refine the precision of the assessment, we found that using a single instrument, the MDAS, enabled us to identify meaningful subtypes of delirium based on overall severity and psychomotor characteristics.
This study is a step towards providing clearer clinical distinctions that can be made based on the psychomotor subtypes and severity of delirium using the MDAS. The MDAS provides an explicit description of the criteria for scoring that can be completed in 5 minutes, and integrates behavioral observations with objective cognitive testing. However, by just using the MDAS total score for delirium severity, clinicians are unable to detect the differences between the hypoactive and any hyperactive status. The findings from this study demonstrate the importance of examining the psychomotor subtype and the severity of delirium in predicting mortality. Since the distinction between psychomotor subtypes are already observed in the MDAS (item 9), clinicians need to take note of the specific psychomotor subtype as well as the MDAS total score of severity during the delirium assessment.
There are several strengths of this study that include the design and methodological features. The data was collected in a large prospective cohort study by trained research personnel with high inter-rater reliability.31 The death status was carefully linked and reviewed using several sources of available information. Another strength of this study is using latent class analysis, which is highly associated with a diagnostic approach for classifying heterogeneous groups based on individual characteristics.25, 32 Despite these considerable strengths, the data were collected from a single metropolitan region, which may limit the generalizability of these findings. However, the post-acute patients enrolled in this study are typical of those admitted to post-acute skilled nursing home facilities (SNFs) nationwide. Another limitation is that we did not use the DSM-IV criteria to establish a delirium diagnosis by a skilled clinician. However, we did use trained research interviewers who have undergone rigorous training, supervision, and monitoring by experienced delirium researchers. Finally, the lack of control patients without delirium is another limitation; however, our goal was to elucidate phenomenological subclasses within a group of patients with delirium.
The findings from this study confirm the fact that enhanced efforts are needed to detect mild delirium with hypoactive psychomotor behavior. Although there were nearly equal number of hypoactive-mild cases as there were mixed with hyperactive features-severe cases, the former group had the highest risk for mortality. It is possible that patients with mixed with hyperactive features-severe delirium are more disruptive and more easily recognized thereby receiving more intensive evaluation and treatment, thus resulting in lower mortality when compared to hypoactive-mild delirium patients. Our data reinforce the need to systematically assess patients for delirium at PAC admission, while considering dementia status. Among those with dementia, patients with the subtype of hypoactive with mild delirium, which is least likely to be recognized,8 are at increased risk of mortality. While among the non-demented, it was overall delirium severity, rather than psychomotor features, that was associated with death. Hence, the severe delirium classes with either hypoactive or mixed with hyperactive features psychomotor subtypes were predictive of increased risk for death among the non-demented.
Acknowledgement
Funded in part by grants from the National Institute on Aging, R03AG025262 (RNJ), R01AG17649 (ERM), K24AG00949 (SKI). Dr. Marcantonio is a Paul Beeson Physician Faculty Scholar in Aging Research. Dr. Inouye is supported by the Milton and Shirley F. Levy Family Chair.
References
- 1.Inouye SK. Delirium in older persons. New England Journal of Medicine. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 2.Bellelli G, Speciale S, Barisione E, Trabucchi M. Delirium subtypes and 1-year mortality among elderly patients discharged from a post-acute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007 Oct;62(10):1182–1183. doi: 10.1093/gerona/62.10.1182. [DOI] [PubMed] [Google Scholar]
- 3.Lawlor PG, Fainsinger RL, Bruera ED. Delirium at the end of life: critical issues in clinical practice and research. Journal of the American Medical Association. 2000 Nov 15;284(19):2427–2429. doi: 10.1001/jama.284.19.2427. [DOI] [PubMed] [Google Scholar]
- 4.Lawlor PG, Bruera ED. Delirium in patients with advanced cancer. Hematol Oncol Clin North Am. 2002 Jun;16(3):701–714. doi: 10.1016/s0889-8588(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 5.Fadul N, Kaur G, Zhang T, Palmer JL, Bruera E. Evaluation of the memorial delirium assessment scale (MDAS) for the screening of delirium by means of simulated cases by palliative care health professionals. Support Care Cancer. 2007 Nov;15(11):1271–1276. doi: 10.1007/s00520-007-0247-6. [DOI] [PubMed] [Google Scholar]
- 6.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of Pain Symptom Management. 1997;13(3):128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 7.Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004 Jan-Feb;12(1):7–21. [PubMed] [Google Scholar]
- 8.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001 Nov 12;161(20):2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij SE, Schuurmans MJ, van der Mast RC, Levi M. Clinical subtypes of delirium and their relevance for daily clinical practice: a systematic review. Int J Geriatr Psychiatry. 2005 Jul;20(7):609–615. doi: 10.1002/gps.1343. [DOI] [PubMed] [Google Scholar]
- 10.Marcantonio E, Ta T, Duthie E, Resnick N-M. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. Journal of the American Geriatrics Society. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 11.Leentjens AF, Schieveld JN, Leonard M, Lousberg R, Verhey FR, Meagher DJ. A comparison of the phenomenology of pediatric, adult, and geriatric delirium. J Psychosom Res. 2008 Feb;64(2):219–223. doi: 10.1016/j.jpsychores.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Meagher DJ, Moran M, Raju B, et al. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br J Psychiatry. 2007 Feb;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 13.Meagher DJ, O'Hanlon D, O'Mahony E, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000 Winter;12(1):51–56. doi: 10.1176/jnp.12.1.51. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 15.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008 May;56(5):823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003 May;58(5):M441–445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- 17.Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. Journal of the American Medical Association. 1963;185(12):914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Archives of General Psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. Psychological Corporation, A Harcourt Assessment Company; New York: 1989. [Google Scholar]
- 20.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. Journal of Geriatric Psychiatry and Neurology. 1992;5(1):14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996 Jan;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003 Apr 15;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 23.Smith MJ, Breitbart WS, Platt MM. A critique of instruments and methods to detect, diagnose, and rate delirium. J Pain Symptom Manage. 1995;10(1):35–77. doi: 10.1016/0885-3924(94)00066-T. [DOI] [PubMed] [Google Scholar]
- 24.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968 Jul;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 25.Muthén B, Asparouhov T. Item response mixture modeling: application to tobacco dependence criteria. Addict Behav. 2006 Jun;31(6):1050–1066. doi: 10.1016/j.addbeh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 26.McCutcheon AL. Latent Class Analysis. Vol. 64. SAGE Publications; Newbury Park: 2002. [Google Scholar]
- 27.Mplus Version 5.0 [computer program] Muthén & Muthén; Los Angeles: 19982008. Version. [Google Scholar]
- 28.Croudace TJ, Jarvelin MR, Wadsworth ME, Jones PB. Developmental typology of trajectories to nighttime bladder control: epidemiologic application of longitudinal latent class analysis. Am J Epidemiol. 2003 May 1;157(9):834–842. doi: 10.1093/aje/kwg049. [DOI] [PubMed] [Google Scholar]
- 29.Cox D. Regression models and life tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–200. [Google Scholar]
- 30.Stata [computer program] StataCorp; College Station, Texas: 2007. Version 10.0. [Google Scholar]
- 31.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007 Feb;62(2):174–179. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 32.Muthén B. Keynote Address--Latent Variable Hybrids: Overview of Old and New Models; Paper presented at: Center for Integrated Latent Variables Research; College Park, MD. University of Maryland; 2006. [Google Scholar]