Abstract
HIV-1 transmission worldwide is predominantly associated with heterosexual activity, and non-clade B viruses account for the most spread. The HIV-1 epidemic in Trinidad/Tobago and the Caribbean shares many features with such heterosexual epidemics, including a prominent role for coincident sexually transmitted diseases. This study evaluates the molecular epidemiology of HIV-1 in Trinidad/Tobago during a period when abrupt transition from homosexual to heterosexual transmission occurred in the absence of injecting drug use, concomitant with a rapid rise in HIV-1 prevalence in the heterosexual population. Of 31 viral isolates studied during 1987–1995, all cluster with subtype B reference strains. In the analysis of full env genes from 22 early seroconverters, the Trinidad isolates constitute a significant subcluster within the B subtype. The Trinidad V3 consensus sequence differs by a single amino acid from the prototype B V3 consensus and demonstrates stability over the decade of this study. In the majority of isolates, the V3 loop of env contains a signature threonine deletion that marks the lineage of the Trinidad HIV-1 clade B epidemic from pre-1984. No phenotypic features, including syncitium induction, neutralization profiles, and chemokine receptor usage, distinguish this virus population from other subtype B viruses. Thus, although the subtype B HIV-1 viruses being transmitted in Trinidad are genetically distinguishable from other subtype B viruses, this is probably the result of a strong founder effect in a geographically circumscribed population rather than genetic selection for heterosexual transmission. These results demonstrate that canonical clade B HIV-1 can generate a typical heterosexual epidemic.
Type 1 HIV was discovered in the early 1980s (1–3) and subsequently established as the etiologic agent of AIDS (4–6). In the United States, the HIV/AIDS epidemic has remained concentrated within so-called high-risk groups, including homosexual/bisexual men and i.v. drug users, with relatively slow expansion to other segments of the population (7). The majority of other countries in the Americas are experiencing similar low-level clade B HIV-1 epidemics with high prevalence rates in specific risk groups—the so-called concentrated HIV epidemic of the Joint United Nation's Programme on HIV/AIDS (UNAIDS) typology (8). This relatively slow spread is in contrast to the relatively rapid spread of subtype B into heterosexual populations of the Caribbean, where Haiti, Guyana, and the Bahamas had population HIV-1 prevalences exceeding 5%, or generalized epidemics, in 1999. This difference has been interpreted by some to imply that specific viral features may account for this development in the region, similar to clade specific epidemics among heterosexuals in Asia, specifically Thailand (9), and in Southern Africa (10).
The islands of Trinidad and Tobago comprise a single Caribbean nation with a population of 1.3 million (1996 midyear estimate). The HIV-1 epidemic in Trinidad underwent a demographic shift in the mid-1980s from predominantly homosexual/bisexual transmission to heterosexual spread (11). Since 1983, when the first cases in Trinidad were reported (12), more than 3,400 cases of AIDS have been documented through December 1998, including more than 200 children (13). Among gay men in Trinidad in 1983–1984, the strongest risk factor for HIV-1 infection was sex with a partner from North America, establishing the provenance of HIV-1 (14). The presence of efficient cofactors for spread into the heterosexual population, including high rates of concomitant sexually transmitted diseases (STDs) and high rates of sexual exposure, facilitated rapid dissemination of HIV-1 (15). The findings from studies conducted at the STD clinic in Port of Spain have allowed the development of an ethnographic model linking sex between older males, younger females, crack cocaine, sex for money, and STD. Injecting drug use is noticeably absent from this population, as in most other Caribbean populations. HIV-1 prevalence in STD clinic attendees increased from 3% in 1987–1988 to 13.6% in 1990–1991, providing evidence of an epidemiologic inflexion point in the Trinidad epidemic. HIV-1 incidence in the STD clinic population currently is estimated to be 2–3% per annum, with estimates as high as 6.9% in the most at-risk subgroup, men presenting with ulcerative STDs (16). Based on this epidemiologic progression, we postulated that closely related HIV-1 isolates would be harbored among individuals studied from Trinidad, and that specific viral or population features may be identified that associate with the period of rapid spread into the heterosexual population.
This analysis was undertaken as part of a series of HIV-1 vaccine preparedness studies (17) to define the genotypic and phenotypic characteristics of HIV-1 isolates from Trinidad and Tobago in relation to prototypic B strains as well as HIV-1 strains in other parts of the world.
Methods
Virus culture and isolation was performed on samples from the early homosexual epidemic and from heterosexual men and women through 1997. All specimens were collected after informed consent at the STD clinic in Port of Spain as part of a collaborative National Institutes of Health-funded research program.
Description of Populations and Sampling.
The centralized STD service is located in Port of Spain and is the single referral center for STDs and HIV-1 in the socialized national health system of Trinidad and Tobago. The patient population is drawn from the entire country (18). From 1983 to the present, we conducted a series of epidemiologic studies in this clinic population to describe the distribution and determinants of the HIV-1/AIDS epidemic. During the period for which these data were collected, approximately 6,000–8,000 individual visits per year occurred, of which approximately 40% attended for a new STD episode (12, 13). Most of the isolates described in this report (22 of 31) came from seroconverters ascertained early during primary infection by using p24 antigen screening of all consenting individuals attending the clinic.
Samples were collected and either processed on site at the Caribbean Epidemiology Center and then shipped to the National Cancer Institute (NCI) or shipped fresh to Duke University for processing, virus culture, lymphocyte subset analysis, and storage. For these studies, samples were accessed from fresh blood or from repositories at Duke University and the NCI. The samples were taken from 31 individuals, of which one was a homosexual male infected before 1983 (11) and collected in 1987, eight were prevalent samples from heterosexual males collected in 1990–1991, and the remaining 22 were heterosexual early seroconverters, including six females, collected during 1993–1997.
Preparation and Phenotypic Characterization of Low Passage Primary Virus Isolate Stocks.
Peripheral blood mononuclear cells (PBMCs) were prepared from venous blood obtained from HIV-1+ study subjects and seronegative donors by standard Ficoll-Hypaque density separation. Low passage primary virus isolates were prepared by coculturing 5 × 106 freshly prepared (or in some instances viably cryo-preserved) study subject's PBMCs with phytohemagglutinin (PHA)-activated seronegative donor PBMC in RPMI 1640 medium supplemented with 20% heat-inactivated FCS, 5% IL-2, and gentamycin. Twice weekly half of the culture supernatants were harvested and replaced with fresh medium alone (once per week) or with medium containing 107 PHA-activated activated donor PBMCs (once per week). HIV-1 p24 antigen levels were monitored in culture supernatants twice per week until two successive readings exceeded 30 pg/ml and were increasing. The cells then were pelleted and cocultured in 20–30 ml with 1–1.5 × 107 CD8+ cell depleted PBMCs from a pool of 10 seronegative donors that had been activated as follows. The PBMC pool of normal donors were activated for 3 days with a mixture of 50 ng/ml anti-CD3 and 100 ng/ml anti-CD28 antibodies in medium supplemented with 10% FCS, 20 units/ml recombinant IL-2 (Genzyme), penicillin, and streptomycin at 37°C in a humidified incubator. The PBMCs were depleted of CD8 T cells with anti-CD8-coated magnetic microspheres (Dynal) according to the manufacturer's recommendations. At daily intervals, the cells were pelleted, supernatants were harvested and filtered, and the cells were resuspended in fresh medium. Supernatants were monitored for the presence and amount of viral reverse transcriptase activity with a micro reverse transcription (RT) assay (19) to estimate virus replication. Supernatants containing significant amounts of viral RT activity were characterized with respect to infectious virus titer in a PBMC-based end-point dilution assay, and titers (expressed as TCID50/ml) were calculated according to Reed and Muench (20). Virus harvests possessing greater than 103 TCID50/ml were aliquotted and stored at −70°C until used.
Molecular Cloning of Primary env Genes.
Proviral DNA templates were prepared from frozen PBMC pellets of cocultures used to generate the primary virus isolates, except for samples QZ2226, QZ2269, QZ2291, QZ2313, QZ2432, QZ2551, and QZ2704, where proviral DNA templates were prepared directly from uncultured patient PBMCs. Cell pellets were lysed in a buffer containing 10 mM Tris, 1 mM EDTA, 0.001% SDS, and 0.5% Triton X-100 at pH 8.0. Proteinase K was added to a final concentration of 1 mg/ml, and the lysates were incubated at 60°C for 3 h, and then heated at 100°C for 10 min to inactivate Proteinase K. In some cases, the DNA was further purified by standard phenol-chloroform extraction techniques. The full-length env gene containing the gp160 ORF was amplified by PCR as follows: 0.5 μg of purified cellular DNA was used as the target for amplification of proviral env DNA by using a nested PCR-based approach. First-round amplification was performed with the E0 5′ primer (5′-TAGAGCCCTGGAAGCATCCAGGAAGTCAGCCTA) and the Nef 9023 3′ primer (5′-CATTGGTCTTAAAGGTACCTGAGGT), each at 0.1 μM final concentration. Two units/reaction of the eLONGase enzyme mix (GIBCO/BRL) was used with 200 μM dNTPs and 1.5 mM MgSO4. An initial denaturation for 3 min at 94°C preceded 40 amplification cycles performed as follows: denaturation for 1 min at 94°C, annealing at 57°C for 1 min, and extension for 5 min at 70°C. These cycles were followed by a final 10-min extension at 70°C. One microliter of the first-round reaction was amplified for 25 cycles in a second-round reaction using nested primers. Second-round primers were either the 5′ E00 primer (5′-AGA AAG AGC AGA AGA CAG TGG CAA TGA-3′) used with the 3′ MG20 primer (5′-TGC TGT ATT GCT ACT TGT GAG-3′), or the 5′ ENF primer (5′-AAA GAG CAG AAG ACA GTG GCA ATG AGAGTGAAGG) used in conjunction with either the 3′ ENR primer (5′-CAATCACACTACTTTTTGACCACTTGCCACCCAT) or the primer 3′ NL4–3-8917R primer (5′-AGGTCTCGAGATACTGCTCCCA). The concentrations of components and reaction conditions for the second round were identical to those described for the first-round PCR. In cases where only 660 bp of envelope sequence were generated, an envelope fragment was amplified by nested PCR by using conditions essentially as described above but with the 5′ primer MG-7 (5′-GTCAGCACAGTACAATGTACACAT-3′) and the 3′ primer MG-12 (5′-AATTGTCTGGCCTGTACCGTCAGCGT-3′) in the first-round reaction, and the 5′ MG-7 primer with the 3′ MG-10 primer (5′-CAC TTC TCC AAT TGT CCC TCA TATCTCCTCCT-3′) in the second-round reaction. Amplified env was gel-purified and recovered by using the QIAEX II gel extraction Kit (Qiagen, Chatsworth, CA), and cloned into the pcDNA3.1/V5/His-TOPO expression vector (Invitrogen).
Sequencing Cloned Full-Length env Genes and env Gene Fragments.
Cloned env genes were sequenced with an ABI 377 automated sequencer (Perkin–Elmer) by using the Taq Dye Terminator Cycle Sequencing Kit (ABI Prism) chemistries. All sequences were determined from both sense and antisense DNA strands to ensure the accuracy. Primers used to determine the env sequences were: ENF (5′-AAAGAGCAGAAGACAGTGGCAATGAGAGTGAAGG); E155R (5′-CTGTTCTACCATGTCATTTTTCCACATGT); E70 (5′-GGGATCAAAGCCTAAAGCCATGTGTAA); E90 (5′-CACAGTACAATGTACACATGGAAT); E145R (5′-AGCAGTTGAGTTGATACTACTGG); NL437500 (5′-AAGTAGGAAAAGCAATGTATGCCCCTCCCAT); HXB21299R (5′-ATGGGAGGGGCATACATT); E180 (5′-GTCTGGTATAGTGCAGCA); E55R (5′-GCCCCAGACTGTGAGTTGCAACAGATG); E220 (5′-TAACAAATTGGCTGTGGTATATAA); HXB28562R (5′-CTCGTTACAATCAAGAGTAAGTCTCTCAA); ENR (5′-CAATCACACTACTTTTTGACCACTTGCCACCCAT); T7 (5′-TAATACGACTCACTATAGGG); and PCR3.1 (5′-TAGAAGGCACAGTCGAGG).
An “R” at end of primer name indicates reverse primers. Primers residing in the cloning vector (T7 and PCR3.1) were used to sequence full-length env clones in the immediate 5′ and 3′ regions. Derived DNA sequences were initially analyzed and aligned by using software packages from Applied Biosystems.
Sequence Analysis.
Multiple alignments of the Trinidad envelope sequences were made with reference HIV-1 envelope sequences of each subtype (23). Gaps were inserted into the alignment as needed and those regions eliminated in the analysis. Phylogenetic analysis was performed and the consistency of the branching order of the phylogenetic trees was evaluated by using seqboot, dnadist, neighbor, consense, and dnapars modules of the phylip package (version 3.52c) (24, 25). Phylogenetic trees were constructed by using neighbor joining (26), and the stability of the nodes was assessed with the bootstrap value (24). Bootstrap values in excess of 70% were considered indicative of significant topological stability, i.e., that the topology is sufficiently stable for definitive assignment of isolates to particular groups or nodes (27). Nucleotide sequence accession numbers were AF279589–AF279597 and AF277054–AF277075.
A measure of variability in the C2-V5 envelope sequences was calculated by using the Kimura two-parameter method (28) of estimating pairwise genetic distances and compared with the genetic variability of other HIV-1 envelope sequences from distinct populations transmitting clade B HIV-1.
Results
Of 31 individuals from which isolates were studied, one was collected in 1987 from a gay man infected before 1983 (11), six were from women infected heterosexually between 1994 and 1997, and the remaining were from heterosexual males infected between 1988 and 1997. Of these 31 isolates, 22 full-length envelope sequences were generated from male and female seroconverters ascertained between 1993 and 1997. The remaining samples were acquired in cross-sectional studies. Table 1 illustrates these epidemiologic relationships.
Table 1.
Epidemiologic characterization of Trinidad isolates used for phylogenetic analysis
| Trinidad STD clinic | Homosexual prevalent | Heterosexual prevalent | Heterosexual seroconverters |
|---|---|---|---|
| Time period | 1987 sample infected before 1983 | 1987–1993 | 1993–1997 |
| N | 1 | 8 | 22 |
| Sex | Male | Male | 6 females |
| 16 males | |||
| Age (mean) | 30 yr | 40 yr | All - 33 yr |
| Females - 25 yr | |||
| Males - 36 yr | |||
| Population HIV | 1983 - 40% | 1987/88 - 3% | >13% in those with STD |
| Prevalence | 1987 - 63% | 1990/91 - 12% | |
| Risk factors | Sex with N.A. | Crack cocaine | New STD/GUD |
| Male | GUD | Crack cocaine |
N.A., North American; GUD, genital ulcerative disease.
Examination of the V3 loop sequences of the Trinidad isolates and comparison with the previously described Clade B Consensus (Table 2) showed that the Trinidad consensus V3 loop sequence is nearly identical to the previously determined B consensus sequence, differing only by a single amino acid deletion, and is highly conserved among and between individuals tested. A consistent feature of the Trinidad V3 loop sequences is the presence of a signature threonine deletion just C terminal to the crown of the loop. This feature was present in the earliest isolate studied, RH0011, the gay man infected before 1983 (11), and was present in the majority of isolates (>80%) from the succeeding 10 years. Of note, the arginine in the typical clade B crown of the V3 loop (GPGR motif) is not as common among Trinidad viruses (45%) as it is in clade B as a whole (76%) (23).
Table 2.
Comparison of Trinidad consensus V3 loop sequence to prototypic B
| B-subtype | CTRPNNNTRK | SIHIGPGRAF | YTTGEIIGDI | RQAHC |
|---|---|---|---|---|
| RH0011 | .......... | .......Q.. | .−........ | ..... |
| QZ2226 | .........R | ..P.....V. | .−........ | ..... |
| QZ2269 | .......... | .V.....K.. | .−........ | ..... |
| QZ2291 | .......... | ...L...A.. | F−........ | ..... |
| QZ2313 | .......... | G.P....SV. | .−........ | ..... |
| QZ2432 | .......... | G......G.. | .T..D..... | .K... |
| QZ2551 | .I........ | ........T. | .−........ | ..... |
| QZ2704 | .......... | .......SV. | .−........ | ..... |
| QZ4734 | .......... | ..N....... | .−........ | ..... |
| QZ6698 | ....S....R | ...F...A.. | .−........ | ..... |
| QH0016 | .......... | .....A.K.L | .−........ | ..... |
| QH0020 | .......... | ..P....SV. | .−........ | ..... |
| QH0060 | .........R | ...M...KV. | .−........ | ..... |
| QH0065 | .......... | G.T....SV. | .−........ | ..... |
| QH0132 | .........R | G..M...K.Y | F−........ | ..... |
| QH0136 | .........R | D.T.....V. | .−........ | ..... |
| QH0515 | .......... | ....EA.K.L | .−........ | ..... |
| QH0550 | .........R | D.A....KV. | .−......N. | ..... |
| QH0605 | .......... | G......KV. | C−....V... | ..... |
| QH0679 | .......... | .......... | .A......N. | ..... |
| QH0692 | ....G..... | .......... | .A..D..... | ..... |
| QH0705 | .......... | ...L.A...L | .−........ | ..... |
| QH0788 | .........E | ..N....... | .Y........ | ..... |
| QH0791 | .......... | G.T.....VS | .−....V... | ..V.. |
| QH0850 | .......... | ...L.A.K.L | .−........ | ..... |
| QH0864 | .........R | ...M...K.L | F−........ | ..... |
| QH0865 | .......... | ..P....... | .A......N. | ..... |
| QH0908 | .........R | GVT.....V. | .−...VT... | ..... |
| QH0944 | .I........ | .......... | .−..D..... | ..... |
| QH1116 | .......... | ...M.....L | .−........ | ..... |
| QH1420 | .......... | ...L.A...L | .−........ | ..... |
| Trinidad consensus | .......... | .......... | .−........ | ..... |
Trinidad samples are order chronologically from top to bottom. Deletions are represented by −.
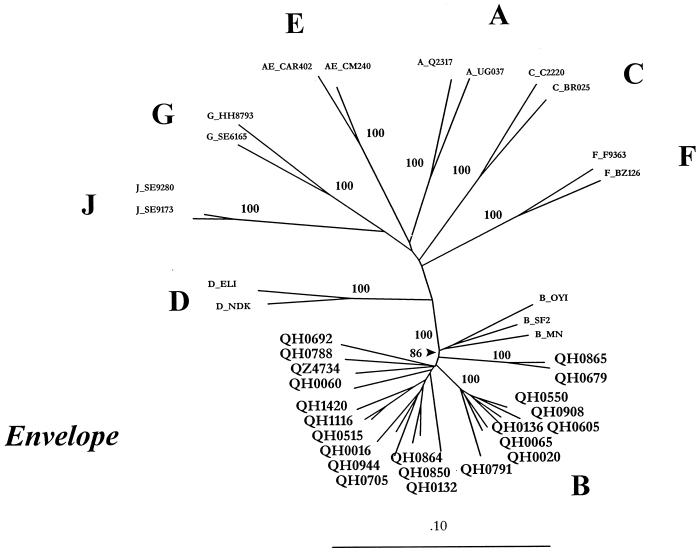
When we analyzed whole envelope sequences from the Trinidad isolates in alignment with representative isolates from all known clades, it was clear that all 22 isolates cluster within clade B of group M HIV-1 viruses, demonstrating 100% bootstrap with prototypic B sequences (23) (Fig. 1). In addition, these Trinidad isolates from seroconverters form a significant subcluster within clade B, with a bootstrap value of 86%. Within the Trinidad cluster are two subclusters of two (QH0865 & QH0069) and seven isolates (QH0550–QH0791) with bootstrap values of 100%, suggesting possible epidemiologically linked transmission. A distance scan of one of the Trinidad sequences, QH0065, with representatives of each known subtype, revealed no regions of recombination (data not shown).
Figure 1.
Phylogenetic analysis of whole envelope sequences from 22 Trinidad HIV-1 isolates. Trinidad isolates are represented by a Q designation, prototypic B clade isolates and representative isolates from other clades are designated. Two subclusters with 100% bootstrap values, (QH0865 and QH0679) and (QH0550, QH0908, QH0136, QH0605, QH0065, and QH0020) represent possible epidemiologically linked transmission.
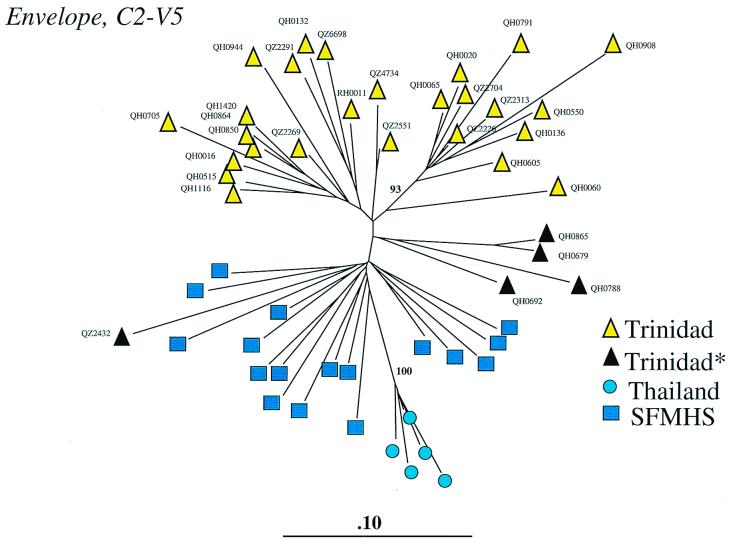
Fig. 2 depicts the phylogenetic analysis of the C2-V5 region from all 31 Trinidad sequences—22 seroconverters and nine seroprevalent cases. Alignment of 660 bp (corresponding to positions 6970–7637 of the HXB2 sequence; ref. 29) of envelope sequence with reference sequences from B subtypes from the United States and Thailand allowed the construction of this phylogenetic tree. The majority of Trinidad isolates form a diverse cluster within clade B, with certain isolates (n = 10) forming a significant subcluster (93% bootstrap value). The seven samples previously noted to form a subcluster in the full-length env analysis (Fig. 1) are included here, with the addition of three others. Four of five samples lacking the threonine deletion (QH0865, QH0679, QH0788, and QH0692) cluster together apart from the majority of isolates.
Figure 2.
Phylogenetic analysis of Trinidad HIV-1 C2-V5 sequences and comparison with clade B sequences from the San Francisco Men's Health Study (SFMHS) and Thailand heterosexuals/drug users. Black triangles designate five of 31 isolates lacking the signature threonine deletion in the V3 loop. Yellow triangles represent 26 of 31 isolates with the threonine deletion. RH0011 refers to a 1987 isolate from a gay man ascertained as seropositive in 1983. QH samples represent incident infections from 1993–1997, QZ samples represent prevalent cases from 1987–1993 except for QZ4374, which came from an incident case in 1993.
Although no specific demographic or epidemiologic feature, including age, sex, or the date of the isolate, was significantly associated with clustering in this analysis, we were able to demonstrate spatial relationships in certain clusters. Geographic location was most prominent, with one group of three isolates (QH0515, QH0864, and QH1116) living within a mile of each other.
We quantified the amount of genetic diversity in the Trinidad samples containing the signature deletion in the V3 loop by calculating the mean pairwise genetic distances between the sequences by using the Kimura two-parameter method (28). Overall, the mean genetic distance is 9.1% for the Trinidad samples, which compares to 8.1% for the early seroconverters in the San Francisco Men's Health Study (30) and 3.4% among the subtype B-infected Thais (31). As shown in Table 3, the mean interpatient distances have increased over calendar time from 7.1% in 1990 to 9.7% in 1995. This increase is approximately 0.5% per year in genetic expansion, a value that has been observed in other subtype B-infected populations. However, as observed in the Amsterdam cohort (32), no drift in the consensus sequence of the V3 loop was observed (Table 2).
Table 3.
Mean pairwise genetic distances of Trinidad HIV-1 “signature” C2-V5 sequences by year
| Year | Prevalent or incident | N | Pairwise distance | % Change |
|---|---|---|---|---|
| 1990 | P | 4 | 7.17 | |
| 1991 | P | 3 | 8.14 | 0.97 |
| 1993 | I | 5 | 8.11 | −0.03 |
| 1994 | I | 6 | 9.6 | 1.49 |
| 1995 | I | 7 | 9.7 | 0.1 |
| Totals | 25 | 9.12 | 2.53 in 5 years (0.5% per year) |
Samples containing the threonine deletion (N = 25) were used for this analysis, which calculates a summary measure per annum of inter-case sequence variability.
Discussion
HIV-1 is remarkable for its mutability, a source of resistance to antiretroviral agents, escape from immune surveillance, and a means of taxonomic classification of these viruses into clades or genetic subtypes. Available data on HIV-1 genetic subtyping from the Caribbean and South America are sparse, and comparisons are made difficult by the variety of techniques used. The majority of reports document the presence of HIV-1 subtype B (33–36), but there are also reports of up to 10% of subtype F in Brazil (37) and in isolated cases in Puerto Rican sex workers.‡‡ In Sao Paolo, subtype F has been isolated from injecting drug users, in contrast to both drug use and sexual transmission of subtype B (37). Brief reports on HIV-1 isolates from seroconverters from Barbados, prevalent positives from Cuba, and commercial sex workers from Honduras have documented that all these HIV-1 isolates fell within the North American/European type B variants (38–40), reflecting the possible routes of entry of HIV-1 into these locales.
The epidemic of HIV-1 in Trinidad and Tobago began with the first reported case of AIDS there in 1982. In 1983, the first of a continuing series of cross-sectional and prospective cohort studies was undertaken in the STD clinic in Port of Spain, and samples from this population form the basis of this analysis. By 1983, 40% of self-declared homosexual/bisexual men attending the STD clinic were HIV-1-infected, including RH0011, whose 1987 sample is the earliest in this analysis. The epidemic of HIV-1 as tracked in these cohorts documented that prevalence rose in the homosexual/bisexual population from 40% in 1983 to 67% in 1987. Among heterosexuals presenting with STDs the rate in 1987–1988 was 3% but dramatically increased to over 12% by 1990–1991 (15).
The 1987 sample has a V3 sequence that clearly falls in clade B, but with a deletion of a threonine residue on the right side of the loop that is present in over 80% of the subsequent isolates in this analysis. The consensus sequence derived from all 31 Trinidad isolates is almost identical to the prototype B sequence except for this “signature” threonine deletion. This deletion does not affect the charge of the protein, but its presence in over 80% of isolates, and over time, provides strong support for a founder effect in this population. The molecular epidemiologic data in this analysis demonstrate the close relationship of the virus identified from the homosexual man infected with HIV-1 before 1983 with all subsequent isolates from heterosexuals, including those with incident infections in the 1990s. These data are consistent with earlier epidemiologic findings that HIV-1 was introduced into this geographic locale via homosexual/bisexual activity between men from Trinidad and North America, with subsequent diffusion into the heterosexual population via a bisexual “bridge.” Subsequent discrete viral introductions into this population have occurred, but at a relatively low rate, and they have not acquired the epidemic force of the signature HIV-1.
The finding of significant subclustering of Trinidad sequences within clade B based on both C2-V5 and whole envelope sequencing raise issues unique to a geographically defined outbreak, such as occurs on islands. Moreover, in agreement with the epidemiologic data indicating a relatively recent (early 1990s) rapid increase in HIV-1 prevalence among heterosexual clinic attendees, the sequence data suggests that closely related isolates were harbored among the individuals studied, and that rapid dissemination of HIV-1 occurred in the mid- and late 1980s and early 1990s into the general population.
The magnitude of the bootstrap values on the analyses and the presence of a signature sequence are evidence of a genetic lineage in this viral population. The genetic diversity among the isolates from Trinidad is greater than that of early seroconverters in the San Francisco epidemic in the 1980s, as one would expect if the Trinidad epidemic in the mid-1990s was older than the U.S. epidemic in the 1980s. The genetic expansion of diversity between seroconverters over calendar time in a given population has been measured in the homosexual/bisexual population of Amsterdam over a 10-year period and consists of a mean of 0.4% per year (32). The genetic expansion seen in the Trinidad population between 1990 and 1995 is approximately 0.5% per year. This finding suggests that the diversification of the viral population in Trinidad and Tobago is occurring at a similar rate as has been observed elsewhere. As also seen in other populations, the consensus V3 loop of the Trinidad viruses has not drifted as a result of this expansion.
These analyses unequivocally document that all 31 Trinidad HIV-1 isolates cluster within clade B in envelope sequences. Unlike the United States, Europe, and Australia, where the main risk behavior for HIV-1 transmission remains homosexual activity and injecting drug use, the main risk behavior for HIV-1 transmission in Trinidad is heterosexual activity. Thus, our results demonstrate that in contrast to the inferences drawn by some investigators (21, 41), clade B strains of HIV-1 are capable of establishing a heterosexual epidemic and provide epidemiologic and genetic support for the observation that genital tract dendritic cells are susceptible to infection by both subtypes B and E (22). Analysis of the 22 full-length env genes from the seroconverter cohort indicates that these sequences form a significant subcluster within clade B. The subclustering of Trinidad sequences, together with the high prevalence of the unusual threonine deletion in the V3 loop, most likely reflects a founder effect in a geographically defined population. Such palmitate clusters are almost certainly consistent with a restricted geographic locale and a rapid epidemic curve.
It is interesting that no specific demographic or epidemiologic factor was statistically associated with genomic diversity in this population. These epidemiologic and molecular data suggest a second-wave epidemic of HIV-1 occurring in the mid- to late 1980s among sexually active heterosexuals. HIV-1 was most likely introduced in the late 1970s to early 1980s among gay/bisexual men through sexual contact with North Americans, then fueled by the confluence of a crack cocaine epidemic and secondary sexual exposure and concomitant STD. The uniform occurrence of this B subtype HIV-1 infection appears to result from an overwhelming founder effect from a North American homosexually transmitted virus. The Trinidad variant, with its signature deletion of a threonine residue in a position flanking the crown of the V3 loop, is possibly on its way to speciation. This may be an additional example of autochthonous transmission similar to the spread of subtype E in Thailand (9).
These findings have significant implications for further studies within this cohort, both for pathogenesis and vaccine evaluation. Understanding the natural history of infection in this setting will help to elucidate the biological and clinical properties of the Trinidad HIV-1. A major unanswered question in HIV vaccine research concerns the potential impact of viral clade diversity on vaccine efficacy. It is in this context that Trinidad provides an important resource for evaluating both clade homologous and heterologous vaccine candidates. Ongoing studies assessing heterologous neutralizing antibody activity will allow us to ascertain whether neutralizing antibody responses that develop in these individuals broaden to other genetically related but distinct viral isolates. The results of such studies will be highly informative for vaccine efforts directed at eliciting broadly reactive neutralizing antibodies and for understanding the role of more narrowly focused serotype vaccines.
Acknowledgments
We indebted to the patients who participated in these studies. We express our sincere appreciation to the research nurses and laboratory staff of the Medical Research Foundation of Trinidad and Tobago for their dedication and commitment to patient care and the goals of these research projects. We thank Dr. Dani Bolognesi for many insightful discussions and sustained support of this work. We extend our appreciation to the laboratory technicians at the Surgical Virology Laboratory at Duke University Medical Center for expert technical support and to Jacqueline R. Murphy at the Research Triangle Institute for excellence in study management. These projects were supported in part by National Cancer Institute Research Contracts CP-61022–21 and CP-40547, and National Institute of Allergy and Infectious Diseases Grants RO1-AI32393, RO1-AI40017, P30-AI28662, and PO1-AI40237.
Abbreviations
- STD
sexually transmitted disease
- PBMC
peripheral blood mononuclear cell
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF279589–AF279597 and AF277054–AF277075).
Flores, I., Moran, N., Alegria, M., Vera, M., Pieniazek, D., Janini, L. M., Bandez, C. I. & Yamamura, Y., Fourth Conference on Retroviruses and Opportunistic Infections, Jan. 22–24, 1997, Washington, DC, abstr. 163.
References
- 1.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 2.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, et al. Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe H W, Darrow W W, Echenberg D F, O'Malley P M, Getchell J P, Kalyanaraman V S, Byers R H, Drennan D P, Braff E H, Curran J W. Ann Intern Med. 1985;103:210–214. doi: 10.7326/0003-4819-103-2-210. [DOI] [PubMed] [Google Scholar]
- 5.Clumeck N, Robert-Guroff M, Van de Perre P, Jennings A, Sibomana J, Demol P, Cran S, Gallo R C. J Am Med Assoc. 1985;254:2599–2602. [PubMed] [Google Scholar]
- 6.Francis D P, Jaffe H W, Fultz P N, Getchell J P, McDougal J S, Feorino P M. Ann Intern Med. 1985;103:719–722. doi: 10.7326/0003-4819-103-5-719. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. Atlanta: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 8.Joint United Nation's Programme on AIDS. The Status and Trends of the HIV/AIDS Epidemics in the World MAP Provisional Report. Geneva, Switzerland: UNAIDS; 1998. [Google Scholar]
- 9.Weniger B G, Takebe Y, Ou C Y, Yamazaki S. AIDS. 1994;8:S12–S23. [PubMed] [Google Scholar]
- 10.Tarantola D, Schwartlander B. AIDS. 1997;11:S5–S21. [PubMed] [Google Scholar]
- 11.Bartholomew C, Cleghorn F R. Bull Pan Am Health Organ. 1989;23:76–80. [PubMed] [Google Scholar]
- 12.Bartholomew C, Raju C, Jankey N. West Indian Med J. 1983;32:177–180. [PubMed] [Google Scholar]
- 13.Public Health Laboratory, Ministry of Health. Report of the National Surveillance Unit. Port of Spain, Trinidad and Tobago: Government Printery; 1998. [Google Scholar]
- 14.Bartholomew C, Saxinger W C, Clark J W, Gail M, Dudgeon A, Mahabir B, Hull B, Cleghorn F R, Gallo R C, Blattner W. J Am Med Assoc. 1987;257:2604–2608. [PubMed] [Google Scholar]
- 15.Cleghorn F R, Jack N, Murphy J, Edwards J, Mahabir B, Paul R, Bartholomew C, Blattner W. AIDS. 1995;9:389–394. [PubMed] [Google Scholar]
- 16.Cleghorn F R, Jack N, Murphy J R, Edwards J, Mahabir B, Paul R, O'Brien T, Greenberg M, Weinhold K, Bartholomew C, et al. Am J Epidemiol. 1998;147:834–839. doi: 10.1093/oxfordjournals.aje.a009536. [DOI] [PubMed] [Google Scholar]
- 17.Paul R, Cleghorn F R, Jack N, Edwards J, Mahabir B, Murphy J, Blattner W, Bartholomew C. AIDS. 1994;10:S9–S10. [PubMed] [Google Scholar]
- 18.Queen's Park Counseling Center and Clinic. Annual Report of the Queen's Park Counseling Center and Clinic. Port of Spain, Trinidad and Tobago: Government Printery; 1995. [Google Scholar]
- 19.Chen C H, Weinhold K J, Bartlett J A, Bolognesi D P, Greenberg M L. AIDS. 1993;9:1079–1086. doi: 10.1089/aid.1993.9.1079. [DOI] [PubMed] [Google Scholar]
- 20.Reed L J, Muench H. Am J Hygiene. 1938;27:493–497. [Google Scholar]
- 21.Essex M, Soto-Ramirez L E, Renjifo E, Wang W K, Lee T Z H. Leukemia. 1997;3:93–94. [PubMed] [Google Scholar]
- 22.Pope M, Frankel S S, Mascola J R, Trkola A, Isdell F, Birx D L, Burke D S, Ho D D, Moore J. J Virol. 1997;71:8001–8007. doi: 10.1128/jvi.71.10.8001-8007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr J K, Foley B T, Leitner T, Salminen M O, Korber B, McCutchan F E. Database, Theoretical Biology and Biophysics. Los Alamos, NM: Los Alamos National Laboratory; 1999. , III, pp. 19–22. [Google Scholar]
- 24.Felsenstein J. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Felsenstein J. Cladistics. 1989;5:164–166. [Google Scholar]
- 26.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Hillis D M, Bull J J. Syst Biol. 1993;42:182–192. [Google Scholar]
- 28.Kimura M. Proc Natl Acad Sci USA. 1981;78:454–458. doi: 10.1073/pnas.78.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratner L, Fisher A, Jagodzinski L L, Mitsuya H, Liou R S, Gallo R C, Wong Staal F. AIDS Res Hum Retroviruses. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 30.McCutchan F E, Sanders-Buell E, Salminen M O, Carr J K, Sheppard W H. AIDS Res Hum Retroviruses. 1998;14:329–337. doi: 10.1089/aid.1998.14.329. [DOI] [PubMed] [Google Scholar]
- 31.Subbarao S, Limpakarnjanarat K, Mastro T D, Bhumisawasdi J, Warachit P, Jayavasu C, Young N L, Luo C C, Shaffer N, Kalish M L, et al. AIDS Res Hum Retroviruses. 1998;14:319–327. doi: 10.1089/aid.1998.14.319. [DOI] [PubMed] [Google Scholar]
- 32.Kuiken C L, Lukashov V V, Baan E, Dekker J, Leunissen J A, Goudsmit J. AIDS. 1996;10:31–37. doi: 10.1097/00002030-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Brindeiro R, Vanderborght B, Caride E, Correa L, Oravec R M, Berro O, Stuyver L, Tanuri A. Antimicrob Agents Chemother. 1999;43:1674–1680. doi: 10.1128/aac.43.7.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casseb J, Hong M A, Gonsalez C, Brigido L F, Duarte A J, Michael-Hendry R. J Med Biol Res. 1998;31:1243–1246. doi: 10.1590/s0100-879x1998001000002. [DOI] [PubMed] [Google Scholar]
- 35.Tanuri A, Swanson P, Devare S, Berro O J, Savedra A, Costa L J, Telles J G, Brindeiro R, Schable C, Pieniazek D, Rayfield M. J Acquired Immune Defic Syndr Hum Retrovirol. 1999;20:60–66. doi: 10.1097/00042560-199901010-00009. [DOI] [PubMed] [Google Scholar]
- 36.Galvao-Castro B, Couto-Fernandez J C, Mello M A, Linhares-de-Carvalho M I, Castello-Branco L R, Bongertz V, Ferreira P C, Morgado M, Sabino E, Tanuri A. Mem Inst Oswaldo Cruz. 1996;91:335–338. doi: 10.1590/s0074-02761996000300014. [DOI] [PubMed] [Google Scholar]
- 37.Sabino E C, Diaz R S, Brigido L F, Learn G H, Mullins J I, Reingold A L, Duarte A J, Mayer A, Busch M P. AIDS. 1996;10:1579–1584. doi: 10.1097/00002030-199611000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Roth W W, Levett P N, Hudson C P, Roach T C, Womack C, Bond V C. AIDS Res Hum Retroviruses. 1997;13:1443–1446. doi: 10.1089/aid.1997.13.1443. [DOI] [PubMed] [Google Scholar]
- 39.Rolo F M, Miranda L, Wainberg M A, Gu Z, Lobaina L, Noa E, Mato J, Machado F, Martin Z. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:123–125. [PubMed] [Google Scholar]
- 40.Renjifo B, Blackard J T, Klaskala W, Chaplin B R, Shah P, McLane M F, Barin F, Esparza J, Zclaya J E, Osmanov S, et al. Virus Res. 1999;60:191–197. doi: 10.1016/s0168-1702(99)00014-3. [DOI] [PubMed] [Google Scholar]
- 41.Soto-Ramirez L E, Renjifo B, McLane M F, Marlink R, O'Hara C, Sutthent R, Wasi C, Auewarakul P, Pena Cruz V, Chui D S, et al. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]