Abstract
The anti-apoptotic Bcl-2 protein has the remarkable ability to prevent cell death from several noxious stimuli. Intriguingly, Bcl-2 overexpression in one cell type has been reported to protect against cell death in neighboring non-Bcl-2 overexpressing cell types. The mechanism of this “trans” protection has been speculated to be secondary to the release of a cytoprotective factor by Bcl-2 overexpressing cells. We employed a series of adoptive transfer experiments in which lymphocytes that overexpress Bcl-2 were administered to either wild type mice or mice lacking mature T and B cells (Rag-1-/-) to detect the presence or absence of the putative protective factor. We were unable to demonstrate “trans” protection. However, adoptive transfer of apoptotic or necrotic cells exacerbated the degree of apoptotic death in neighboring non-Bcl-2 overexpressing cells (p≤0.05). Therefore, this data suggests that dying cells emit signals triggering cell death in neighboring non-Bcl-2 overexpressing cells, i.e. a “trans” destructive effect.
Keywords: Apoptosis, Sepsis, Lymphocyte, Bcl-2, Necrosis, Splenocyte
Introduction
Apoptosis is one of the most actively investigated areas of sepsis research. This is due to the fact that sepsis induces a profound apoptosis-driven loss of cells of both the innate and adaptive immune system [1-3]. Furthermore, it is well established in animal models that blocking lymphocyte apoptosis by a number of diverse methods improves survival in this highly lethal disorder. One particularly efficacious method is the overexpression of the anti-apoptotic Bcl-2 protein [4-7]. A surprising finding in studies involving apoptosis has been the observation that protection against cell death in a particular type of cell can confer protection in a different phenotypic cell. This type of “cross-protection” of adjacent cells has been reported by several groups, and has prompted some investigators to propose a “neighborhood” theory in which cell survival is dependent upon “cross talk” from neighboring cells [8,9]. Although several growth factors and cytokines act in an autocrine/paracrine fashion, it remains to be elucidated which factors are responsible for promoting cell survival in neighboring cells.
Several independent investigative groups have demonstrated the remarkable ability of the anti-apoptotic protein Bcl-2 to prevent sepsis-induced cell death [7-9]. Furthermore, overexpression of Bcl-2 in either T or B lymphocytes also protected against cell death in neighboring non-Bcl-2 over expressing cells [6,7]. For example, there was a decrease in sepsis-induced B cell death in mice whose T cells over expressed Bcl-2. In like fashion, there was a decrease in sepsis-induced T cell death in transgenic mice whose B cells over expressed Bcl-2. In accordance with our results, Iwata et al. reported that mice which were transgenic for Bcl-2 in their myeloid-derived cells had decreased sepsis-induced apoptosis in their intestinal epithelial cells [8]. The mechanism of this cross-protection is unknown but has been reported by other groups [5-10]. Iwata et al. speculated, however, that the protective action of Bcl-2 over expressing cells was via a “trans” effect, i.e., Bcl-2 over expressing cells released an unknown cytoprotective factor which prevented death in non-Bcl-2 over expressing cells of distinct and distant lineages.
In addition to the “trans-protective” theory as proposed by Iwata and colleagues, others have reported a “trans-destructive” property that dying cells have on their neighbors. That is, exposure to dying cells may lead neighboring cells to undergo a cascade of events leading to their own death. This bystander effect has been well described in the radiation literature. Studies showed that irradiated cells undergoing apoptosis release soluble factors that can cause mitochondrial changes and induce apoptosis in neighboring unirradiated cells [11-13]. Our laboratory has shown that adoptive transfer of apoptotic cells caused immune suppression and worsened survival in a murine model of sepsis [14]. This effect of apoptotic cells to induce death in their neighbors may explain why the non-Bcl-2 overexpressing neighbors of Bcl-2 overexpressing cells appear to be protected from apoptosis after a variety of injuries.
In the present study, we determined the presence or absence of a cytoprotective factor released by Bcl-2 over expressing cells as well as the presence or absence of a cytodestructive factor released by dying cells. To accomplish this aim we employed a series of adoptive transfer experiments in which lymphocytes that overexpress Bcl-2, or lymphocytes induced to die, were administered to either wild type mice or mice lacking mature T and B cells (Rag-1-/-) to detect the presence or absence of the putative protective/destructive factor.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory. Rag-1-/- (no mature B or T lymphocytes) mice on a C57BL/6 background were also purchased from The Jackson Laboratory. Transgenic mice overexpressing human Bcl-2 in both myeloid and lymphoid cells (H-2Kb promoter) on a C57BL/6 background were a gift from Dr. Irving L. Weissman (Stanford University) [15]. Animals were housed in the Washington University School of Medicine animal facility and all protocols were approved by the Washington University Animal Studies Committee (St. Louis, MO).
Culture Medium and Reagents
Very low endotoxin medium RPMI 1640 was used as culture medium. Histopaque®-1083 (Sigma-Aldrich) was used to isolate mononuclear cells. C57BL/6 wild type (WT) cells were distinguished from H-2Kb-Bcl-2 overexpressing cells by plasma cell membrane labeling with 5(6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Sigma-Aldrich) in the cytoprotective experiments. In the cytodestructive experiments, irradiated and necrotic cells were distinguished from WT cells with CSFE labeling. Apoptosis was quantified by two independent methods. A commercially available phycoerythrin-labeled terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) kit (Phoenix Flow Systems, Inc.) was used to detect DNA strand breaks, and, alternatively, staining for active caspase 3 with a primary rabbit anti-caspase 3 antibody (Cell Signaling Technology, Inc.) and a phycoerythrin-labeled donkey anti-rabbit secondary antibody was performed as previously described [5,10]. T and B cells were identified using cyanine 5-labeled anti-mouse CD3 and cyanine 5-labeled anti-mouse b220 antibodies, respectively (BD Pharmingen).
Ex Vivo / In Vitro Cytoprotective Experiments
Spleens were harvested from both naïve C57BL/6 mice (n=12) and naïve H-2Kb-Bcl-2 mice (n=12). Spleens were strained over 70μm nylon cell strainers. The resulting suspension of splenocytes was layered over Histopaque® and mononuclear cells were isolated via differential centrifugation. C57BL/6 mononuclear cells were labeled with CFSE. Labeled C57BL/6 mononuclear cells were then combined with unlabeled H-2Kb-Bcl-2 mononuclear cells in either a 1:1 ratio or a 1:5 ratio of WT to transgenic cells. 2×106 total cells were then reconstituted in 1ml of culture medium and plated in 12-well culture plates. Cells were then incubated for 8 hours with E. coli (4 × 106 CFU) contained within 0.02μM microwells. Nonadherent cells were then harvested, fixed in 1% paraformaldehyde, permeablized in 90% methanol, and prepared for flow cytometric analysis of cell death.
Adoptive Transfer of Splenic Mononuclear Cells – Cytoprotective Experiments
C57BL/6 (n=10) and H-2Kb-Bcl-2 (n=4) mice were sacrificed and splenic mononuclear cells were isolated and labeled as described above. Approximately 70×106 splenic mononuclear cells in a 1:1 ratio of labeled C57BL/6 to unlabeled H-2Kb-Bcl-2 cells were reconstituted in 400μl of sterile saline. The cell solution was then adoptively transferred to Rag-1-/- mice (n=10) via retro orbital injection and 96 hours were allowed for engraftment. After lymphocyte engraftment, polymicrobial sepsis was induced (vide infra). 24 hours after polymicrobial sepsis was induced, animals were sacrificed and splenocytes were harvested for flow cytometric analysis.
In a similar manner, C57BL/6 mice (n=20) were sacrificed and splenic mononuclear cells were isolated and labeled as described above. Approximately 70×106 of the labeled C57BL/6 mononuclear cells were reconstituted in 400μl of sterile saline and adoptively transferred to H-2Kb-Bcl-2 mice (n=5) and to C57BL/6 control mice (n=5) via retro orbital injection. Once again, 96 hours was allowed for engraftment to take place followed by induction of polymicrobial sepsis. 24 hours after the induction of sepsis, the animals were sacrificed and spleens were harvested for flow cytometric analysis of cell death.
Adoptive Transfer of Apoptotic and Necrotic Splenic Mononuclear Cells – Cytodestructive Experiments
In order to test the hypothesis that dying cells have a cytodestructive effect on neighboring cells, C57BL/6 (n=20) mice were sacrificed and splenic mononuclear cells were again isolated and labeled as described above. Splenocytes were divided into three groups—uninjured, apoptotic, and necrotic. Apoptosis was induced by γ-irradiation (20Gy). Necrosis was induced by three freeze-thaw cycles in a liquid nitrogen bath (-196°C) and a warm water bath (37°C). Approximately 70×106 splenic mononuclear cells, either uninjured, apoptotic, or necrotic, were reconstituted in 400μl of sterile saline. The cell solution was then adoptively transferred to naïve C57BL/6 mice (n=20) via retro orbital injection. Animals were sacrificed 24 hours later and spleens were harvested for flow cytometric analysis of cell death.
Induction of Polymicrobial Sepsis
The cecal ligation and puncture (CLP) technique as developed by Chaudry et al. [16] and modified by our laboratory was used to induce septic peritonitis as previously described [4-7]. In brief, mice were anesthetized and an abdominal incision was made. The cecum was eviscerated, ligated below the ileocecal valve, and punctured once with a 27 gauge needle. The cecum was then returned to its normal anatomical position and the incision was sutured with 4-0 silk suture. Sham mice had cecal manipulation only. Animals were sacrificed 20 hrs post CLP.
Detection and Quantification of Apoptosis
Flow Cytometry
Spleens were obtained at the time of animal sacrifice. Mononuclear cells were isolated, fixed, and permeabalized as described above. The characteristic forward and side scatter properties of lymphocytes were used to identify the lymphocyte gate as previously described [17]. The percentage of apoptotic cells within the lymphocyte gate was quantitated by both TUNEL and active caspase 3. C57BL/6 wild type cells were distinguished from H-2Kb-Bcl-2 transgenic cells and control cells by labeling with CFSE. Flow cytometric analysis (50,000-200,000 events/sample) was performed on FACScan (BD Biosciences) as previously described [5].
Statistical Analysis
Data reported are the mean ± SEM. Data were analyzed with the statistical software program PRISM (GraphPad Software). Normally distributed data was analyzed using one-way ANOVA with Tukey's multiple comparison post-test. Significance was accepted at p ≤ 0.05. Unevenly distributed data was analyzed using a Mann-Whitney test. Significance was accepted at p ≤ 0.05.
Results
Bcl-2 overexpressing splenocytes do not protect wild type lymphocytes from E. coli-induced apoptosis ex vivo or in vitro
Apoptotic lymphocyte death occurs following bacterial induced injury. Furthermore, lymphocytes that overexpress Bcl-2 show a dramatic reduction in apoptosis after bacterial induced injury [6,15,18,19]. However, co-incubation of Bcl-2 overexpressing splenocytes from H-2Kb-Bcl-2 mice (n=12) did not protect wild type lymphocytes (n=12) from sepsis-induced or radiation-induced apoptosis (Fig. 1). WT control B cells had 37.0 ± 16.2% E. coli-induced apoptosis as quantitated by TUNEL. When WT B cells were incubated with Bcl-2 overexpressing splenocytes in a 1:1 ratio, the WT lymphocytes demonstrated 28.2 ± 6.0% apoptosis. Similarly, WT B cells incubated with Bcl-2 overexpressing splenocytes in a 1:5 ratio demonstrated 24.6 ± 4.9% apoptosis. There was no statistical difference between these groups (Fig. 1A). WT T cells showed similar results after E. coli-induced injury. Control WT T cells demonstrated 14.1 ± 5.5% apoptosis versus 23.9 ± 12.1% and 30.6 ± 12.5% apoptosis in a 1:1 and 1:5 ratio with Bcl-2 overexpressing mononuclear cells, respectively (Fig. 1B).
Figure 1. Bcl-2 overexpressing splenocytes do not protect WT lymphocytes from E. coli-induced apoptosis in vitro.
A, WT B cells have greater apoptosis than Bcl-2 overexpressing B cells whether co-incubated in a 1:1 or 1:5 ratio (p<0.002). B, WT T cells have greater apoptosis than Bcl-2 overexpressing T cells whether co-incubated in a 1:1 or 1:5 ratio (p<0.05).
Wild type lymphocytes are not protected from sepsis-induced apoptosis after co-adoptive transfer with Bcl-2 overexpressing splenocytes into Rag-1-/- mice
To test the hypothesis that Bcl-2 overexpressing cells could prevent cell death in non-Bcl-2 overexpressing cells, we harvested splenocytes from naïve wild type C57B/6 mice and from Bcl-2 overexpressing H-2Kb-Bcl-2 mice. WT splenocytes were labeled with CFSE and combined with splenocytes from H-2Kb-Bcl-2 transgenic mice in a 1:1 ratio. This mixture of cells, as well as a control mixture of wild type spenocytes alone, was then adoptively transferred into Rag-1-/- mice (n=10) which were subsequently made septic by CLP. The septic Rag-1-/- mice were sacrificed 20 hrs later and spleens were harvested for quantitative assessment of lymphocyte apoptosis via flow cytometric analysis.
WT lymphocytes were not protected from sepsis-induced apoptosis. WT control splenocytes had 11.7 ± 2.7 % CLP-induced B cell apoptosis while WT splenocytes co-adoptively transferred into Rag-1-/- mice with Bcl-2 overexpressing splenocytes had 15.0 ± 3.0% CLP-induced B cell apoptosis as quantitated by TUNEL (p>0.05; Fig 2A). Likewise, WT control splenocytes had 20.5 ± 4.9% CLP-induced T cell apoptosis while WT splenocytes co-adoptively transferred with Bcl-2 over expressing splenocytes had 36.6 ± 14.0% CLP-induced T cell apoptosis as quantitated by TUNEL (p>0.05; Fig 2B). There was no statistical difference in apoptosis between WT cells alone or WT cells co-adoptively transferred with Bcl-2 overexpressing cells. Similarly, there was no protection in cell death if apoptosis was quantitated by active caspase 3 (data not shown).
Figure 2. Co-adoptive transfer into Rag-1-/- mice does not protect WT lymphocytes from CLP-induced apoptosis.

A, WT B cells were not protected from apoptosis when co-adoptively transferred with Bcl-2 overexpressing splenocytes into Rag-1-/- mice (p>0.05). Bcl-2 overexpressing B cells retained their innate resistance to apoptosis (p<0.01). B, WT T cells were not protected from apoptosis when co-adoptively transferred with Bcl-2 overexpressing splenocytes into Rag-1-/- mice (p>0.05). Bcl-2 overexpressing T cells retained their innate resistance to apoptosis (p<0.01).
Wild type lymphocytes are not protected from sepsis-induced apoptosis after adoptive transfer into Bcl-2 overexpressing mice
The experiments discussed above provide strong evidence that Bcl-2 overexpressing cells do not release a cytoprotective factor capable of protecting neighboring non-overexpressing lymphoid cells in both in vitro and in vivo settings. However, the possibility remains that Bcl-2 overexpressing mice, by virtue of their resistance to sepsis-induced lymphoid and/or myeloid apoptosis, create an overall in vivo environment conducive to the survival of neighboring non-Bcl-2 overexpressing cells. To test this possibility, we labeled splenocytes from WT mice and adoptively transferred them into both H-2Kb-Bcl-2 overexpressing mice (n=5) and wild type controls (n=5). These mice were subsequently made septic and spleens were harvested for flow cytometric analysis.
WT lymphocytes were not protected from sepsis-induced apoptosis after adoptive transfer into H-2Kb-Bcl-2 mice. WT control mice that received an adoptive transfer of WT splenocytes demonstrated 42.1 ± 1.4% apoptosis in B cells and 18.4 ± 4.4% apoptosis in T cells as quantitated by TUNEL. H-2Kb-Bcl-2 mice that received an adoptive transfer of WT splenocytes demonstrated 55.7 ± 3.7% apoptosis in B cells and 19.8 ± 5.7% apoptosis in T cells as quantitated by TUNEL (Fig 3A-B). In fact, there was significantly more death in lymphocytes when transferred into H-2Kb-Bcl-2 overexpressing mice—possibly secondary to a host versus graft phenomena. Similarly, there was no protection in cell death if apoptosis was quantitated by active caspase 3 (data not shown).
Figure 3. Co-adoptive transfer into Bcl-2 overexpressing mice does not protect WT lymphocytes from CLP-induced apoptosis.
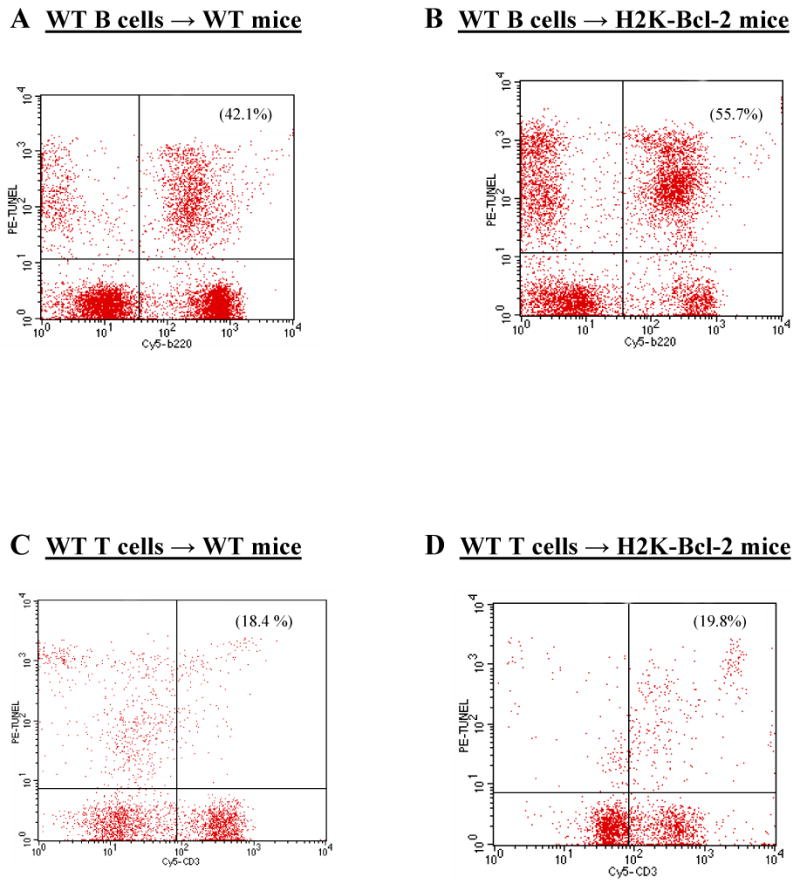
A-B, WT B cells are not protected from apoptosis when adoptively transferred into Bcl-2 overexpressing mice (p>0.05). C-D, WT T cells are not protected from apoptosis when adoptively transferred into Bcl-2 overexpressing mice (p>0.05).
Adoptive transfer of apoptotic or necrotic splenocytes increases lymphocyte apoptosis in recipient mice
After our previous experiments were unable to show a cytoprotective effect from Bcl-2 overexpressing cells, we turned our attention towards the possibility that dying cells have a cytodestructive effect on neighboring cells. Cell death can occur by two distinct pathways, i.e. apoptosis and necrosis. Apoptosis is a tightly regulated and highly conserved type of cell death. Necrosis, on the other hand, occurs in response to an acute injury and is characterized by cell and organelle swelling and rupture leading to an intense inflammatory reaction secondary to the release of proteases and other toxic lysosomal enzymes [20]. To test the possibility that either of these two types of cell death have cytodestructive bystander effects on neighboring cells, we adoptively transferred either apoptotic splenocytes or necrotic splenocytes into naïve recipient mice. Twenty four hours after the adoptive transfer, recipient mice were sacrificed and their spleens were harvested. Apoptosis of native recipient T and B lymphocytes was quantitated via flow cytometry.
WT lymphocytes from recipient mice had significantly increased levels of apoptosis after adoptive transfer of apoptotic and necrotic splenocytes. WT recipient B cells demonstrated 5.8 ± 1.1% apoptosis after adoptive transfer of uninjured control splenocytes. After adoptive transfer of apoptotic splenocytes, WT recipient B cells demonstrated 7.4 ± 1.9% apoptosis (p>0.05) and after adoptive transfer of necrotic splenocytes the WT recipient B cells demonstrated 8.8 ± 2.6% apoptosis (p≤0.05; Fig 4A-B). Likewise, WT recipient T cells demonstrated 7.7 ± 2.8% apoptosis after adoptive transfer of uninjured control splenocytes. However, after adoptive transfer of apoptotic splenocytes, WT recipient T cells demonstrated 11.6 ± 5.4% apoptosis (p<0.02) and after adoptive transfer of necrotic splenocytes the WT recipient T cells demonstrated 15.2 ± 8.7% apoptosis (p<0.04; Fig 4C-D) as quantitated by TUNEL. Similar significance was noted when apoptosis was quantitated by active caspase 3 (data not shown).
Figure 4. Adoptive transfer of dying cells increases apoptosis of native lymphocytes in recipient mice.
A, Necrotic splenocyte transfer induced native B cells to undergo apoptosis at a rate greater than controls (n=20; p≤0.05). Apoptotic splenocyte transfer caused a non-significant trend towards increased native B cell apoptosis. B, Transfer of either apoptotic or necrotic splenocytes induced native T cells in recipient mice (n=20) to undergo apoptosis at a rate greater than controls (p<0.02 and p<0.04, respectively).
Discussion
The major finding of the current study is the lack of a detectable trans-protective effect by Bcl-2 over-expressing cells on neighboring cells. Consistent with numerous prior investigations, Bcl-2 over-expressing lymphocytes were, in fact, protected from sepsis-induced apoptosis [6-8,10]. However, WT lymphocytes, whether co-incubated in-vitro with Bcl-2 overexpressing lymphocytes, adoptively transferred to Rag-/- mice in combination with Bcl-2 overexpressing lymphocytes, or adoptively transferred into transgenic Bcl-2 overexpressing mice, were highly susceptible to sepsis-induced apoptosis. In contrast to prior studies [4,8], these data suggest that Bcl-2 overexpressing cells do not have a trans-protective effect on neighboring cells. Given our inability to demonstrate a trans-protective effect of Bcl-2 on neighboring wild type cells, we postulated a second hypothesis.
We surmised that dying cells emit signals or release factors that trigger death in neighboring cells. A large percentage of cells in Bcl-2 transgenic mice will be protected from all death secondary to the anti-apoptotic effect of Bcl-2. Consequently, there would be much less death in neighboring non-Bcl-2 over-expressing cells. In order to test our theory, we employed an additional set of adoptive transfer experiments. In these experiments we adoptively transferred apoptotic, necrotic, or unmanipulated lymphocytes into naïve mice, then quantitated the degree of apoptosis in neighboring lymphocytes from the recipient animals. We found that adoptive transfer of either apoptotic or necrotic cells caused an increase in apoptosis in neighboring cells (p≤0.05). This is consistent with evidence in the radiation literature in which apoptotic cells have been found to release an apoptosis-inducing signal to viable neighboring cells [12-14,21]. Therefore, we believe that the data contained herein suggests that dying cells emit death-signals or release death-factors triggering apoptosis in neighboring cells.
In addition to the release of putative death signals, dying cells are profoundly immunosuppressive. They cause a shift from the pro-inflammatory (Th1) state to the anti-inflammatory (Th2) state, monocyte deactivation, and the development of anergy [1,21-23]. This immunosuppression leads to an inability of the host to clear primary infections as well as a propensity to develop secondary nosocomial infections, thereby worsening mortality [5]. We propose that it is this intense immune suppression, in conjunction with the release of death signals, which is responsible for worsened mortality in animal models of sepsis. We believe that Bcl-2 over-expressing animals, by virtue of their resistance to cell death, avoid this cascade of death factor release and immune deactivation in neighboring cells resulting in improved survival in sepsis. In summary, the data contained herein do not support the presence of a cyto-protective effect from Bcl-2 over expressing cells. However, both apoptotic and necrotic cells appear to have a cyto-destructive effect on neighboring cells. We feel that the ability of Bcl-2 over-expressing cells to avoid the cell death-induced cascade of apoptosis and immunosuppression may be an important mechanism in the overall survival benefit seen in Bcl-2 over-expressing mice.
Acknowledgments
This work was supported by National Institutes of Health Grants GM055194, GM044118, GM072808, GM66202, and GM00795-05
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 2.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 3.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 5.Schwulst SJ, Grayson MH, Dipasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic Monoclonal Antibody Against CD40 Receptor Decreases Lymphocyte Apoptosis and Improves Survival in Sepsis. J Immunol. 2006;177:557–565. doi: 10.4049/jimmunol.177.1.557. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Over expression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 8.Iwata A, Stevenson VM, Minard A, Tasch M, Tupper J, Lagasse E, Weissman I, Harlan JM, Winn RK. Over-expression of Bcl-2 provides protection in septic mice by a trans effect. J Immunol. 2003;171:3136–3141. doi: 10.4049/jimmunol.171.6.3136. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, O'Donnell D, Gordon JI. Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol. 1999;276:G677–686. doi: 10.1152/ajpgi.1999.276.3.G677. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 11.Mothersill C, Seymour C. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155:759–67. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Mothersill C, Seymour CB, Joiner MC. Relationship between radiation-induced low-dose hypersensitivity and the bystander effect. Radiat Res. 2002;157:526–32. doi: 10.1667/0033-7587(2002)157[0526:rbrild]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Maguire P, Mothersill C, Seymour C, Lyng FM. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat Res. 2005;163:384–90. doi: 10.1667/rr3325. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Chang KC, Grayson MH, Tinsley TW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domen J, Gandy KL, Weissman IL. Systemic over expression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 16.Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–592. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 19.Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer. 2000;83:1223–30. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 21.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 22.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]




