Abstract
Scleroderma is a common autoimmune disorder with no effective therapy. Current concepts of scleroderma include the hypothesis that scleroderma results from excess conversion of endothelial cells to fibroblast like cells, called endothelial mesenchymal transformation. This process is thought to be mediated by cytokines including transforming growth factor beta (TGFb), which causes increased collagen synthesis, resulting in fibrosis, the hallmark of the disease. In vitro studies have hypothesized that rapamycin may be of benefit in scleroderma due to antagonism of collagen synthesis. Given that rapamycin has antiangiogenic activities, inhibits wound healing, and prevents the synthesis of collagen in vivo, we tried rapamycin in a patient with scleroderma. We observed rapid improvement in skin stiffness and mobility. Our results provide the rationale for larger clinical trials of rapamycin in scleroderma and other fibrotic disorders.
Introduction
SCLERODERMA (PROGRESSIVE SYSTEMIC SCLEROSIS) is a common connective tissue disorder with no effective therapy. Scleroderma results in significant morbidity due to stiffness and decreased range of motion, often resulting in profound disability. Mortality can occur in scleroderma as well, often due to pulmonary fibrosis and renal failure. The distinguishing feature of scleroderma is thickening and fibrosis of the skin, hence the name.
Previous treatment for scleroderma includes various immunosuppressive drugs, including prednisone, imuran, and cytoxan, as well as penicillamine. Newer drugs, namely tumor necrosis factor blockers, have been attempted, with mixed results. We report a case of scleroderma that was resistant to multiple therapeutic modalities, including etanercept, hydroxychloroquine, cellcept, and penicillamine. Given that rapamycin (sirolimus) is known to inhibit wound healing, and that scleroderma is thought to represent an aberrant wound healing phenomenon, we tried rapamycin in this patient. After initiating rapamycin, we observed a rapid diminution in skin tightness and increased range of motion. As of this writing, the patient has been maintained on oral rapamycin with stable disease. Our experience suggests the need for larger studies of this orally available drug for remission and maintenance of systemic scleroderma.
Case Report
The patient was initially seen in November 2001 at the University of Miami with a 5-month history of being diagnosed with progressive systemic sclerosis (PSS). At that time the diagnosis was made based on the clinical findings of proximal and acral induration, including sclerodactly, later confirmed by laboratory tests. Clinically, she had diffuse PSS characterized by a positive antinuclear antibody and SCL-70 antibody. At initial presentation, she was a 25-year-old black Haitian female, 3-months pregnant, who 9 months prior developed dyspigmentation. She denied gastrointestinal or respiratory symptoms. She presented with marked induration of both hands and arms associated with decreased range of motion; and with moderate induration of her face, chest, and legs. Additionally, she had multiple areas of depigmentation on her arms, face, and chest (Fig. 1). She was taking only prenatal vitamins and denied allergies. At that time, she had normal renal function. She began physical therapy; other therapy was withheld because of her pregnancy, although the use of prednisone was discussed with her OB/GYN.
FIG. 1.
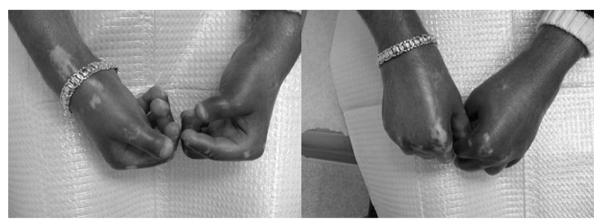
Patient with marked induration of both hands and arms associated with decreased range of motion multiple areas of depigmentation on her arms. Note that the patient gained the ability to clench her fist after rapamycin therapy.
After the patient delivered her baby in June 2002, she experienced subjective softening of her skin. After her pregnancy, further workup included an elevated IgG 6618 mg/dl (normal, 700–1600). She had a slightly elevated IgA 446 mg/dl (normal, 70–400) and an elevated total protein 11.6 g/dl, (normal, 6–8.5). She also had an elevated gamma globulin. 6.4 g/dl (normal, 0.5–1.6). Her blood count was significant for anemia with a hemoglobin of 9.3 g/dl; her hematocrit was 29%. She was seen by a rheumatologist who started penicillamine that was discontinued within several months because her condition was thought to worsen. The patient complained of Raynaud(s disease, as well as decreasing range of motion of her hands and mouth. At that time, in March 2003, she was started on Nifedipine 30 mg/day and Tagamet 400 mg twice daily. Her rheumatologist then started hydroxychloroquine 200 mg twice daily, prednisone 5 mg daily, and dovonex cream twice daily.
She continued these medications without improvement till April 2004. She was started on Etanercept 25 mg twice weekly. At that time, an SCL-70 was 5.54 (normal < 1.00), her ANA was 1:640 in a speckled pattern. Her hemoglobin was 10.5 g/dl with a hematocrit of 31.6%. Her renal function remained normal.
The patient was seen again in July 2004; during the interval her prednisone was increased to 7.5 mg. per day, but her hands were worsening with decreasing range of motion. She did not tolerate higher doses of prednisone. The hydroxychloroquine was discontinued, and both Minocycline 50 twice a day and Cellcept 1 gm twice a day were started. Repeat blood tests were unchanged and a rheumatoid factor was negative. A month later she returned to inform us that she was pregnant; all medications were stopped except the prednisone. After she terminated the pregnancy, she returned October 2004, when she restarted Cellcept, Minocycline, and Etanercept. She did not, however, tolerate the Cellcept. Upon return in December 2004, her blood pressure which had been always around 90/60 was 130/90. Her anemia was unchanged and she had normal renal function. Vasotec and Prevacid were added. Repeated EKGs were within normal limits. In a February 2005 follow-up, her total protein was 10 g/dl, albumin was 2.7 g/dl, with an elevated globulin 7.3 g/dl. She continued to worsen and finally she had (after 16 month) pulmonary function tests performed, demonstrating a restrictive and obstructive pattern with an FVC 50% predicted, FEV1 was 55% predicted, and a DLCO 43% predicted. A chest radiograph was normal. We added methotrexate 7.5 mg/ weekly. In May 2005, we discontinued the Etanercept, with the plan to start Humira that was never authorized by her insurance. She had worsened, and in June 2005 described her condition an 8 out of 10, with worsening of her hands and feet but denying dysphagia. Nifedipine 30 mg ×l was added. By October 2005, her hematocrit was 27%, which led to discontinuing the methotrexate and a referral to Hematology, which diagnosed anemia of chronic disease. A Rodnan skin score was performed, with a score of 30. Over the next 4 months, attempts were made to obtain Bosentan, Viagra, or Potaba for her, but failed, prior to her move to Georgia.
Upon her initial visit to Emory University School of Medicine, the patient was started on rapamycin 4 mg daily for 2 weeks, followed by a month of 4 mg rapamycin every other day, alternating with 2 mg every other day for 1 month. After 1 month of treatment, the patient experienced greatly decreased tightness and increased range of motion of her fingers and arms. Her hematocrit remained in the high 20s to low 30s range. Currently, she is being maintained on a dose of 1 mg rapamycin daily.
Discussion
Scleroderma is a multisystem disorder that is most well known for fibrosis and collagen deposition in the dermis, but affects multiple organ systems, leading to disordered esophageal motility, renal failure, and pulmonary fibrosis. Renal failure and pulmonary fibrosis may be life threatening, and require aggressive therapy with cytotoxic agents.1 Even in the absence of life-threatening symptoms, scleroderma can lead to severe morbidity due to limitation of motion and deformity. Thus, the need for additional modalities of therapy is urgent.
Cellular and animal model studies have led to an increased understanding of the pathophysiologic events seen in scleroderma.2-5 The cause of scleroderma remains unknown, but environmental features such as cytomegalovirus infection, vinyl chloride, and other agents have been implicated. Animal studies using the tight skin mouse, which has a duplication of the fibrillin-1 gene, implicates dysregulation of the transforming growth factor beta (TGFb) signaling pathway. Scleroderma-like features have been observed in graft vs. host disorder, and animals deficient in fli-1 manifest features of scleroderma.6,7 Interestingly, a phenotype of B cell hyperactivation has been described in scleroderma skin, and B cell immunodepletion results in amelioration of the tight skin mouse phenotype.8
We have previously demonstrated that rapamycin was of benefit in a patient with dermatomyositis,9 which like scleroderma, shares a potential B lymphocyte regulatory phenotype.10 Rapamycin has a potent effect on B lymphocytes, and has been used in combination with anti-CD20 antibodies (Rituximab) in the treatment of Epstein—Barr virus-induced lymphoproliferative disorder. B cells may also be a source of profibrogenic cytokines, including TGFb, interleukin-4, interleukin-13, and connective tissue growth factor (CTGF). Abrogation of B cell hyperactivity is a potential rationale for the use of rapamycin in scleroderma. A second rationale is the demonstration that rapamycin decreases the secretion of type 1 collagen, a primary component of fibrosis. Thus, rapamycin has a dual rationale for the treatment of scleroderma.
Multiple cytotoxic and immunomodulatory agents have been tried in scleroderma. These include prednisone, cyclophosphamide, mycophenolate, methotrexate, cyclosporine, TNF-a inhibitors, and most recently B cell depletion with anti-CD20 antibodies. Rapamycin is immunosuppressive but does not share the myelotoxicity of these inhibitors, nor the profound B cell immuodepletion of anti-CD20 antibody.
Acknowledgments
JLA was supported by the grant RO1 AR47901 and P30 AR42687 Emory Skin Disease Research Core Center Grant from the National Institutes of Health, a Veterans Administration Hospital Merit Award.
Footnotes
Disclosures Drs. Kirsner, Bhandarkar, and Arbiser and Mr. Fried have no conflicts of interest or financial ties to disclose.
References
- 1.Khanna D, Yan X, Tashkin DP, Furst DE, Elashoff R, Roth MD, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hse VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler R, Rothfield N, Metersky M, Clements RJ, Scleroderma Lung Study Group Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56:1676–1684. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 2.Connolly MK, McCalmont TH. Fetal DNA and cells in women with systemic sclerosis. N Engl J Med. 1998;339:771–772. doi: 10.1056/nejm199809103391113. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Gilliam AC. Animal models for scleroderma: an update. Curr Rheumatol Rep. 2002;4:150–162. doi: 10.1007/s11926-002-0011-3. [DOI] [PubMed] [Google Scholar]
- 4.Askew D, Zhou L, Wu C, Chen G, Gilliam AC. Absence of cutaneous TNFalpha-producing CD4+ T cells and TNFalpha may allow for fibrosis rather than epithelial cytotoxicity in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2007;127:1905–1914. doi: 10.1038/sj.jid.5700813. [DOI] [PubMed] [Google Scholar]
- 5.Aliprantis AO, Wang J, Fathman JW, Lemaire R, Dorfman DM, Lafyatis R, Glimcher H. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc Natl Acad Sci USA. 2007;104:2827–2830. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubo M, Czuwara–Ladykowska J, Moussa O, Markiewicz M, Smith E, Silver RM, Jablonska S, Blaszczyk M, Watson DK, Trojanowska M. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am J Pathol. 2003;163:571–581. doi: 10.1016/S0002-9440(10)63685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano Y, Czuwara J, Trojanowska M. Transforming growth factor regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem. 2007;282:34672–34683. doi: 10.1074/jbc.M703907200. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, Matsushita T, Matsushita Y, Horikawa M, Komura K, Takehara K, Sato S, Tedder TF. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tightskin mouse model for systemic sclerosis. Am J Pathol. 2006;169:954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadiminti U, Arbiser JL. Rapamycin (sirolimus) as a steroid-sparing agent in dermatomyositis. J Am Acad Dermatol. 2005;52(2 Suppl 1):17–19. doi: 10.1016/j.jaad.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi JT, Pergamenschikov A, McCalmont TH, Brown PO, Botstein D, Connolly MK. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci USA. 2003;100:12319–12324. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]


