Abstract
Exposure to chronic stress has been argued to produce maladaptive anxiety-like behavioral states, and many of the brain regions associated with stressor responding also mediate anxiety-like behavior. Pituitary adenylate cyclase activating polypeptide (PACAP) and its specific G protein-coupled PAC1 receptor have been associated with many of these stress- and anxiety-associated brain regions, and signaling via this peptidergic system may facilitate the neuroplasticity associated with pathological affective states. Here we investigated whether chronic stress increased transcript expression for PACAP, PAC1 receptor, brain-derived neurotrophic factor (BDNF), and tyrosine receptor kinase B (TrkB) in several nuclei. In rats exposed to a 7 day chronic variate stress paradigm, chronic stress enhanced baseline startle responding induced by handling and exposure to bright lights. Following chronic stress, quantitative transcript assessments of brain regions demonstrated dramatic increases in PACAP and PAC1 receptor, BDNF, and TrkB receptor mRNA expression selectively in the dorsal aspect of the anterolateral bed nucleus of the stria terminalis (dBNST). Related vasoactive intestinal peptide (VIP) and VPAC receptor, and other stress peptide transcript levels were not altered compared to controls. Moreover, acute PACAP38 infusion into the dBNST resulted in a robust dose-dependent anxiogenic response on baseline startle responding that persisted for 7 days. PACAP/PAC1 receptor signaling has established trophic functions and its coordinate effects with chronic stress-induced dBNST BDNF and TrkB transcript expression may underlie the maladaptive BNST remodeling and plasticity associated with anxiety-like behavior.
Keywords: Extended amygdala, Plasticity, Fear, Vasoactive intestinal peptide, PAC1 receptor, corticotropin-releasing hormone
Introduction
Stressors activate several physiological and behavioral systems to promote homeostasis. Many central nervous system (CNS) nuclei that mediate the response to stressors also mediate anxiety-like behavioral responding, and when stressor exposure is chronic, the sustained activation of these nuclei has been argued to lead to the maladaptive morphological and functional changes that underlie pathological affective states (Schulkin, et al. 1998; Vyas, et al. 2003; Pego, et al. 2008). Hence, chronic stress may produce anxiety disorders by promoting neuronal plasticity within these stress-responsive nuclei.
Activation of the bed nucleus of the stria terminalis (BNST) has been argued to mediate both stress-responding and anxiety-like behavioral responding to diffuse and/or unpredictable threat (Walker, et al. 2003). Stimulation of the BNST produces many physiological responses that are elicited by anxiogenic stimuli (Casada and Dafny 1991), anxiogenic pharmacological agents increase BNST neuronal activation (Singewald, et al. 2003), and BNST inactivation blocks many anxiogenic behaviors (for review, see (Walker, et al. 2003). Hence, alterations within the BNST have been argued to underlie anxiety disorders in humans (Walker, et al. 2003; Straube, et al. 2007). Consistent with this interpretation, chronic exposure to stressors or stress hormone increases anxiety-like behavior and enhances BNST dendritic branching, length and total BNST volume (Stout, et al. 2000; Vyas, et al. 2003; Pego, et al. 2008). Given that stressor exposure is a critical component in the etiology of many anxiety disorders, and that the BNST is a point of confluence between stress and emotion, these data suggest that stress-induced alterations in neuronal function and plasticity in the BNST may underlie some forms of chronic anxiety in humans. Despite provocative evidence, the mechanisms of BNST signaling/plasticity in stress-induced anxiety have not been elucidated.
Pituitary adenylate cyclase activating polypeptides (PACAP) have neurotransmitter and neurotrophic properties, and are also associated with the stress response (Arimura 1998; Sherwood, et al. 2000; Vaudry, et al. 2000). In the CNS, higher levels of PACAP and PAC1 receptor mRNA and immunoreactivity are expressed in stress-associated brain regions, including the hypothalamus, hippocampus, discrete regions of the amygdala, and the BNST (Hashimoto, et al. 1996; Jaworski and Proctor 2000; Hannibal 2002). PACAP signaling may modulate corticotropin releasing hormone (CRH) because PACAP-immunoreactive fibers innervate CRH neurons in the hypothalamic paraventricular nucleus (PVN) and BNST (Kozicz, et al. 1997; Legradi, et al. 1998). PACAP infusion into cerebral ventricles or PVN augments plasma corticosterone levels, activates PVN neurons, and increases PVN CRH expression (Agarwal, et al. 2005; Norrholm, et al. 2005). However, the role of PACAP in behavioral stress responding is unclear and has so far only been inferred from peptide or receptor knockout studies (Hashimoto, et al. 2001; Otto, et al. 2001b; Girard, et al. 2006). Moreover, only certain stressors regulate PVN PACAP expression (Hannibal, et al. 1995).
Here, we demonstrate that chronic stress selectively induces PACAP and PAC1 receptor transcript expression in the BNST, increases BNST brain-derived neurotrophic factor (BDNF) and TrkB receptor expression, and enhances anxiety-like behavior. Furthermore, PACAP infused into the BNST produces a long-lasting anxiogenic behavioral response.
Methods
Animals
Adult male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA), and were allowed to habituate in their home cages for at least one week before experimentation. Rats were single-housed, maintained on a 12 h light/dark cycle (lights on at 07:00 h), and food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Chronic variate stress
Stressed rats were exposed to a 7 day chronic variate stress paradigm. Rats were randomly assigned to control or chronically stressed groups, and for the latter, a single stressor was presented on each day (Table 1), including:
Table 1.
| Day | Stressor | Duration |
|---|---|---|
| 1 | Oscillation | 30 min |
| 2 | Swim | 5 min |
| 3 | Footshock | 5 sec (× 2) |
| 4 | Restraint | 60 min |
| 5 | Pedestal | 30 min |
| 6 | Swim | 5 min |
| 7 | Footshock | 5 sec (× 2) |
Oscillation stress
Rats were placed inside a plastic chamber 28 × 17 × 13 cm (L × W × H), that was secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ), and oscillated at low to medium speed for 30 min.
Forced Swim
Rats were placed in a cylindrical container 29 × 37 cm (D × H) that was filled with room temperature water to a depth that prevented the rat tail from touching the bottom. After 5 min of monitored swimming, rats were placed in a holding chamber for 30 min prior to being returned to their home cage.
Footshock
Rats were placed inside a Plexiglas conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 ×35 cm (L × W × H). After a 5 min acclimation period, two 1.0 mA 5 sec scrambled footshocks were delivered through the grid floor with a 1 min inter-trial interval.
Restraint
Rats were placed in a cylindrical restraining device 9 × 15 cm (D × H) for 60 min.
Pedestal stress
Rats were placed on an elevated platform 20 × 20 cm (L × W) that was 60 cm from the floor, for 30 min.
Surgical procedure
For intra-BNST injections, rats were anesthetized with isoflurane vapor (1.5 - 3.5%), and secured in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with “blunt” earbars. After a midline head incision, the skull was exposed and cleaned, and four screws were inserted to provide skullcap stability. Two stainless steel cannulae (26 gauge, PlasticsOne, Roanoke, VA) were lowered at a 20 degree angle toward the midline to a point just above the anterolateral BNST, using the following coordinates from Bregma in mm, AP = -0.3, ML = +3.8, and DV = - 5.3 from the surface of the dura. Once in place, a dental cement skullcap was formed to support the cannula. Once awake, rats were returned to their home cages for 7 day post-surgery recovery, during which all rats were observed and weighed daily.
Startle apparatus
Each acoustic startle stabilimeter chamber consisted of an 8 × 15 ×15 cm acrylic and wire-mesh cage with four 6.0 mm diameter stainless steel floor bars spaced 18 mm apart. The cage was suspended between compression springs within an acrylic frame and located within a 90 × 70 × 70 cm ventilated sound-attenuating cubicle. Chamber movement resulted in displacement of an accelerometer (Model U321AO2; PCB Piezotronics, Depew, NY), which was fixed to the bottom of the cage; the resulting voltage was proportional to the velocity of displacement. The analog output of the accelerometer was amplified (PCB Piezotronics, Model 483B21) and digitized on a scale of 0-10 V by an InstruNET analog to digital converter (GW Instruments, Model 100B; Somerville, MA) interfaced to a Macintosh G3 computer. Startle amplitude was defined as the maximal peak-to-trough voltage during the first 200 ms after the stimulus onset.
Startle responses were evoked by 50 ms white-noise bursts (5 ms rise-decay) generated by a Macintosh G3 computer sound file (0-22 kHz), amplified by a Radio Shack Amplifier (100 Watt; Model MPA-200; Tandy, Fort Worth, TX), and delivered through a high frequency speaker (Radio Shack Supertweeter; Tandy, Fort Worth, TX) located 5 cm from the back of each cage.
Quantititative PCR (QPCR) measures of transcript levels
After euthanasia, the brain was quickly sectioned using a rodent brain matrix (Ted Pella Inc.; Redding, CA), and brain regions were dissected with a brain punch set (Stoelting; Wood Dale, IL), and frozen on dry ice. The tissues were homogenized in Stat-60 total RNA/mRNA isolation reagent (Tel-Test “B”, Friendswood, TX) as described previously (Braas and May 1999; Girard, et al. 2002; Girard, et al. 2006; Braas, et al. 2007). The RNA (2 μg) was used to synthesize first strand cDNA using SuperScript II reverse transcriptase and random hexamer primers with the SuperScript II Preamplification System (Invitrogen, Carlsbad, CA) in a 20 μl final reaction volume. The same tissue regions from all rats were reverse transcribed simultaneously to obviate variability. Following the reverse transcriptase reaction, the cDNA samples were treated with RNase H to remove residual RNA. Real-time quantitative PCR methods and oligonucleotide primers were exactly as described previously (Girard, et al. 2002; Girard, et al. 2006; Braas, et al. 2007). The melting profiles for amplified DNA fragments were performed to verify unique product amplification in the quantitative PCR assays. For each target sequence, all samples from the same brain region were amplified together in the same assay to minimize variability. All data were normalized to 18S RNA levels; all assays were repeated 2 - 3 times to verify data reproducibility.
Experiment 1
We did not initially observe changes in baseline startle following chronic stress; hence, we reasoned that BNST-mediated anxiogenic behavioral changes might not be constitutively expressed but may need to be evoked by an anxiety-producing manipulation. Bright lights and handling increase startle amplitude, and these effects are dependent on BNST activity (Walker and Davis 1997; Walker and Davis 2002); therefore, we determined whether chronic variate stress is anxiogenic using the light-enhanced startle paradigm. In this paradigm, we were mindful that handling-induced elevations have also been reported, and shown to be mediated by BNST activity (Walker and Davis 1997).
Rats received stress or no stress as described above. On the 8th and 9th days, rats were tested using the light-enhanced startle procedure. In phase 1, all rats were tested for startle in the dark. Rats were removed from the startle chambers and placed in a holding cage for 5 min. Rats were then returned to the startle chambers for the phase 2 startle test. In phase 2, half of the rats were exposed to a bright light (8 W fluorescent bulb, Dark-Light) located behind each chamber. The other half remained in the dark (Dark-Dark). Phase 1 and 2 tests were repeated the next day so that all rats were tested in both the dark and light conditions. Data from two control rats were identified as outliers (> 2 SD from the mean) in the light-enhanced startle tests, and were excluded from the analysis.
Experiment 2
This experiment determined whether chronic variate stress increases PACAP, PAC1 receptors, BDNF, and/or TrkB receptor mRNA expression in brain regions associated with stress responding. In addition, rats were tested for changes in baseline startle amplitude induced by the chronic variate stress procedure. Rats were administered a baseline startle test in which they were placed in the startle chambers and allowed a 5 min acclimation to the chambers with a 60 db background noise. The response to 30 noise bursts (described above) that varied in intensity (95, 100, or 105 db) with a 30 sec inter-trial interval was then determined. After baseline startle testing, rats were returned to their home cages. For the next 7 days, stressed rats were treated with the chronic variate stress paradigm described above. On the 8th day, rats were returned to the startle chambers and tested as described above. Two hours after the startle test, rats were rapidly decapitated, and stress-related brain regions were processed for quantitative PCR.
Experiment 3
Experiment 3 was designed to determine whether intra-BNST PACAP injection is anxiogenic. Rats were implanted with BNST cannulae as described above. After one week, rats were administered 2 baseline startle tests on consecutive days as described in Experiment 1. Based on these baseline tests, rats were “matched” to experimental groups so that baseline startle amplitude did not differ between groups. Twenty-four hours after the second test, rats received a third identical baseline test. Immediately after this third test, rats were removed from the startle chambers, loosely restrained and infused bilaterally into the BNST with one of four doses of PACAP38 (0, 0.1 μg, 0.5 μg, 1 μg) in saline vehicle (0.5 μl per side). Infusion cannulae were left in place for 60 sec before removal. After infusion, rats were returned to the startle chambers, and following a 5 min acclimation period, rats were administered a 2nd startle test that consisted of 81 startle stimuli, which were identical to those described in the baseline tests. After the second test, rats were returned to their home cages.
Experiment 4
Experiment 3 determined that intra-BNST PACAP38 injection was anxiogenic. If BNST PACAP38 infusion enhanced BNST plasticity and function, then the anxiogenic effects might be expected to persist long after the initial infusion. Hence, rats were implanted with BNST cannulae and administered baseline startle testing as described above. After the third baseline test, rats received infusions of 1 μg PACAP38 in saline vehicle (0.5 μl per side) into the BNST and were subsequently tested for changes in baseline startle before being returned to their home cages as described in Experiment 3. One week after BNST PACAP38 infusion, rats were returned to the startle chambers and again administered the long version of the startle test (81 noise bursts).
Cannula verification
To verify cannula placements, rats were anesthetized and perfused transcardially with saline followed by 10% formalin. Brains were then removed, fixed in a 30% sucrose solution and sectioned on a cryostat for staining with cresyl violet. Cannula verifications were conducted under a light microscope.
Results
Experiment 1: Chronic variate stress is anxiogenic
Exposure to chronic stress has been shown to increase BNST neuroplasticity and facilitate anxiety-like behavior (Stout, et al. 2000; Vyas, et al. 2003; Pego, et al. 2008). To validate stress-induced anxiety in our chronic variate stress experiments, rats received stress or no stress as described above, and were tested using the light-enhanced startle paradigm (Walker and Davis 1997; Walker and Davis 2002).
As shown in Figure 1, overall, startle was enhanced in the presence of the light (F(1, 28) = 6.85, p = 0.014). However, rats with prior chronic stress exposure (n =16) showed enhanced startle responding compared to control rats (n = 14) in both the Dark-Dark (t(28) = 2.71, p = 0.011) and Dark-Light tests (t(28) = 2.49, p = 0.018). This likely reflects an exaggerated effect of incidental between-test handling which, like light-enhanced startle, reflects unconditioned BNST-mediated anxiety (Walker and Davis 1997). As in the previous experiment, we did not find an effect of stress treatment on baseline startle activity (average phase 1 startle amplitude; Mcontrol = 2.26, Mstress = 2.07, (t(28) = 0.424, p = 0.675) Among rats exposed to chronic stress, light did not appear to further increase anxiety-like behavior under these testing conditions (stress group Dark-Dark versus Dark-Light (t(15)=1.74, p = 0.102). Hence, in agreement with previous work (Walker et al, 1997), chronic stress elevated baseline startle amplitude both due to handling, as well as exposure to bright lights.
Figure 1. Chronic variate stress is anxiogenic.
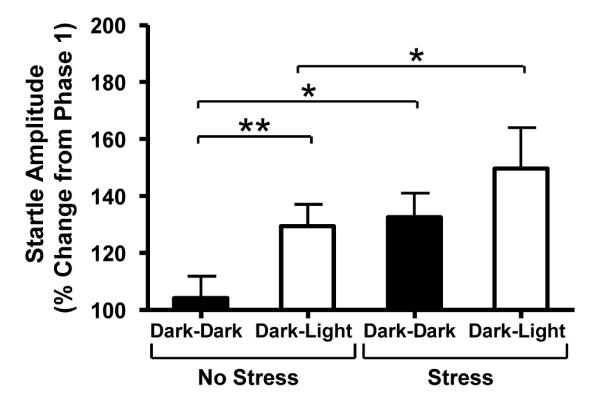
The startle responses of chronically stressed and control rats were measured in a dark environment (Phase 1). Half of the stressed and control animals were retested in the dark (Dark-Dark), while half were tested in bright light (Dark-Light). Stressed rats exhibited startle enhancement from phase 1 to phase 2 regardless of whether phase 2 testing was in the dark or in the light, indicative of a stress-induced enhancement of the anxiogenic effects of handling on baseline startle amplitude, which has been shown to be mediated by the BNST. * Two-tailed t-test p < 0.05, ** One-tailed t-test p < 0.05
Experiment 2: Chronic variate stress increases BNST PACAP, PAC1 receptor, BDNF, and TrkB receptor transcript expression
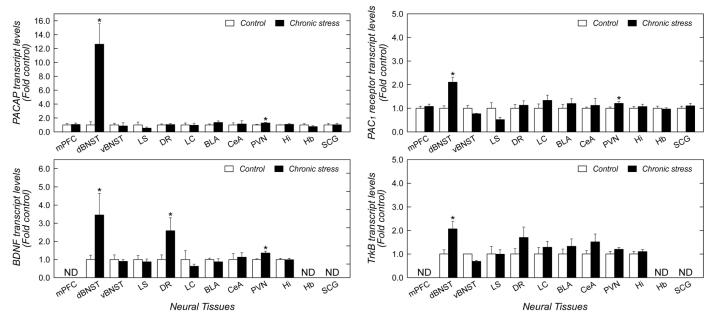
Among the 12 brain regions examined (see Figure 2), chronic variate stress increased PACAP transcript levels approximately 10-fold (p = 0.003) only in the dorsolateral BNST (dorsal aspect of anterolateral BNST) (dBNST, Figure 3). A smaller but significant 1.3-fold (p = 0.033) increase in PACAP mRNA was also apparent in the PVN of the hypothalamus; PACAP levels in the remaining stress-related brain regions were not different from non-stressed control tissues. To assess whether the changes were associated with increased receptor mRNA levels, the same cDNA templates were also assayed for PAC1 receptor expression. The stress-induced increase in dBNST PACAP expression was associated with a 2-fold (p = 0.001) increase in dBNST PAC1 receptor mRNA expression (Figure 3). The increase in dBNST PACAP/PAC1 receptor was unique as VIP, VPAC1 and VPAC2 receptor transcript levels in the same samples were not changed (data not shown); similarly, dBNST transcripts for CRH and NPY were also not different between stressed and control samples (data not shown).
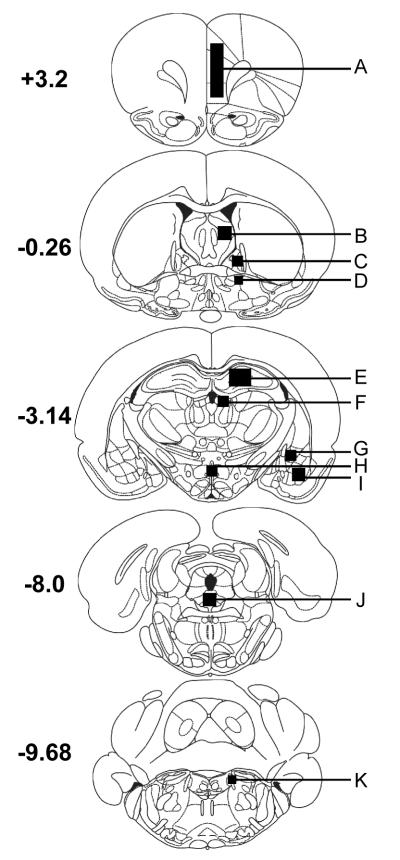
Figure 2. Location of tissue samples harvested for quantitative PCR analysis.
Adult male rats were exposed to chronic variate stress for 7 days as described in the text and the indicated brain regions were harvested for quantitative PCR analyses. Coordinates represent mm from Bregma. A. medial prefrontal cortex; B. lateral septum; C. dorsal aspect of the anterolateral BNST; D. ventral aspect of the anterolateral BNST; E. hippocampus; F. habenula; G. central amygdala; H. PVN; I. basolateral amygdala; J. dorsal raphe nucleus; K. locus coeruleus.
Figure 3. Chronic variate stress increases PACAP, PAC1 receptor and neurotrophin-related transcripts in the BNST.
Adult male rats were exposed to chronic variate stress for 7 days as described in the text and the indicated brain regions were harvested for quantitative PCR analyses. All tissue samples from each region were reverse transcribed at the same time with random hexamers to allow quantitation and normalization across samples against 18S RNA. n = 6 for each group; data represent mean fold-change ± SEM against tissue from control unstressed animals. Asterisk, significantly different at p < 0.05. mPFC, medial prefrontal cortex; dBNST, dorsolateral BNST; vBNST, ventrolateral BNST; LS, lateral septum; DR, dorsal raphe nucleus; LC, locus coeruleus; BLA, basolateral amygdala; CEA, central amygdala; PVN, hypothalamic paraventricular nucleus; Hi, hippocampus; hb, habenula; SCG superior cervical ganglion. ND, not determined.
Chronic stress facilitates BNST neuronal remodeling and functional plasticity, which have been suggested to contribute to chronic anxiety-like behavioral states (Stout, et al. 2000; Vyas, et al. 2003; Pego, et al. 2008). The mechanisms underlying this plasticity may be related to either PACAP function, which can stimulate fiber outgrowths, or to BDNF and TrkB neurotrophic signaling. Hence, we also analyzed for BDNF and TrkB transcript levels in this brain tissue. As shown in Figure 3, chronic variate stress increased BDNF and TrkB mRNA levels 3-fold (p = 0.012) and 2-fold (p = 0.015), respectively, in the dBNST when compared with non-stress control tissues. BDNF transcripts in a few select CNS regions were also significantly increased, including the dorsal raphe nucleus (3 - fold; p = 0.04) and the PVN (1.4 - fold; p = 0.009). TrkB transcripts in the dorsal raphe nucleus and PVN were not significantly elevated. Hence, the mechanisms underlying stress-induced BNST cytoarchitectural and functional plasticity may include PACAP and/or BDNF/TrkB signaling.
We did not find an effect of stress treatment on baseline startle activity in the absence of excessive handling or exposure to bright light (Mcontrol = 3.61, Mstress = 3.76, (t(10) = 0.106, p = 0.917).
Experiment 3: PACAP infusion into the BNST promotes anxiogenic behavior
High levels of PAC1 receptor expression have been observed in the BNST (Hashimoto, et al. 1996; Hannibal 2002). As chronic variate stress increased dramatically and selectively PACAP transcript expression in the dorsal aspect of the anterolateral BNST, PACAP signaling in BNST neurons may impact anxiety-like behavior. Hence, the anterior BNST of adult male rats were cannulated bilaterally and one of four doses of PACAP was infused into the BNST immediately prior to startle testing as described above. BNST injection sites are depicted in Figure 4. PACAP38 dose-dependently increased baseline startle amplitude (Figure 5), suggesting that the effect of BNST PACAP on anxiety-like behavior is receptor-mediated. Repeated Measures Analysis of Variance revealed an effect of PACAP dose (F(3,184) = 2.992, p = 0.05), no effect over trial blocks (F(8,184) = 1.117, p = 0.3539), and a reliable interaction between PACAP treatment and trial blocks (F(24,184) = 1.884, p = 0.0106). Bonferroni posttests revealed that 1 μg PACAP38 reliably increased baseline startle amplitude.
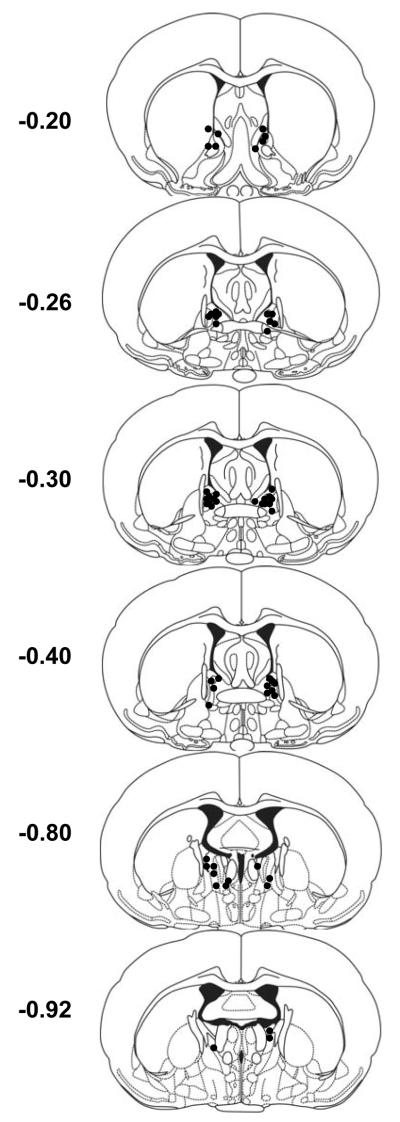
Figure 4. Experiment 3 BNST PACAP infusion sites.
Cannulae placements for rats that received 0, 0.1, 0.5 or 1 μg PACAP38 (0.5 μl/side) infusions into the BNST. Each circle represents the center of one injection. Coordinates represent mm from Bregma.
Figure 5. PACAP infusion into the BNST is anxiogenic.
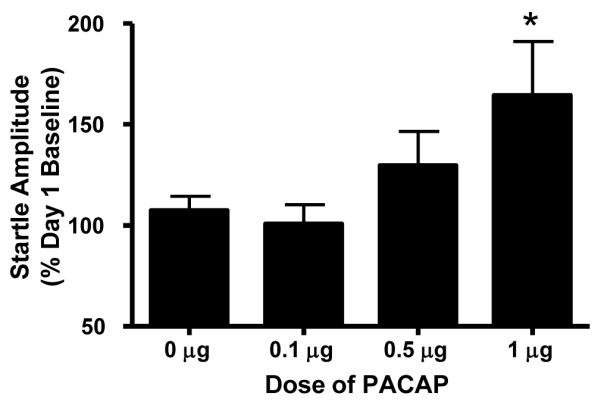
The BNST of adult male rats were cannulated bilaterally and the animals were tested for baseline startle for 2 days before PACAP38 injections (see Methods). Twenty-four hours later, the animals were baseline tested again before intra-BNST peptide infusion (0, 0.1 μg, 0.5 μg, 1 μg in 0.5 μl saline vehicle/side) and retesting. BNST PACAP38 dose-dependently increased startle amplitude. Data represent the mean response ± SEM to the last 9 startle stimuli. * Significantly different at p < 0.05.
Experiment 4: The anxiogenic effects of BNST PACAP infusion persist for 7 days
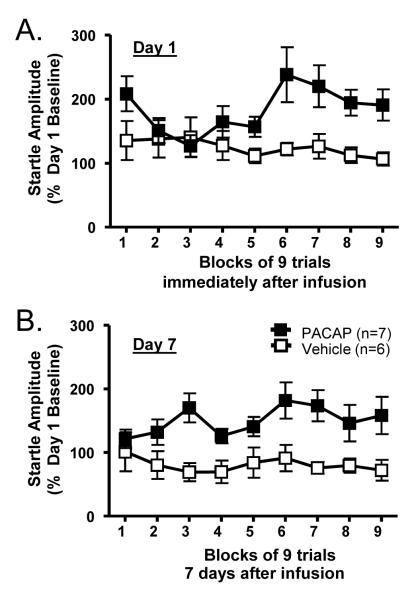
Experiment 3 determined that intra-BNST PACAP38 injection was anxiogenic. If BNST PACAP38 infusion enhanced BNST plasticity and function, then the anxiogenic effects might be expected to persist long after the initial infusion. Using procedures similar to those described for Experiment 3, 1 μg PACAP was infused into the BNST, and startle behavior was measured immediately after infusion and one week after infusion. BNST injection sites for Experiment 4 are depicted in Figure 6. As shown in Figure 7A, PACAP38 significantly increased baseline startle amplitude immediately after infusion, replicating the results from Experiment 3. Repeated Measures Analysis of Variance revealed a reliable effect of PACAP treatment (F(1,88) = 4.9, p = 0.0485). Interestingly, there was also an effect over trial blocks (F(8,88) = 2.125, p = 0.0415), and an interaction between PACAP treatment and trial blocks (F(8,88) = 3.135, p = 0.0036). Hence, unlike the results from Experiment 3, the anxiogenic effect observed immediately after PACAP injection increased over time. Compared to pre-injection baseline, BNST PACAP-injected rats continued to exhibit an elevation in baseline startle amplitude one week after PACAP38 injection (F(8,88) = 10.11, p = 0.0088). Once increased BNST neuroplasticity has been established there should not be any effect observed across trial blocks. Consistent with this, 7 days after injection, there was no effect over trial blocks (F(8,88) = 0.98, p = 0.4554) and no interaction between PACAP treatment and trial blocks (F(8,88) = 1.4, p = 0.2085). These data suggest that PACAP induced BNST neuroplasticity that produced a long-term elevation in anxiety-like behavior (Figure 7B).
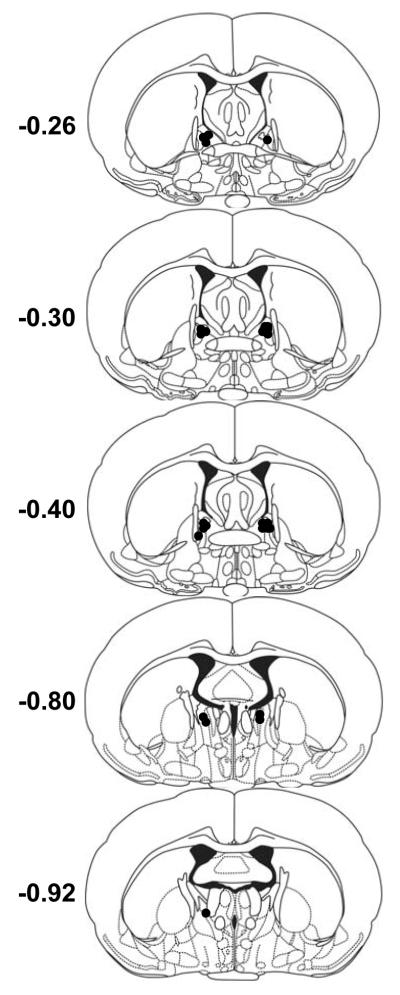
Figure 6. Experiment 4 BNST PACAP infusion sites.
Cannulae placements for rats that received 1 μg PACAP38 (0.5 μl/side) infusions into the BNST. Each circle represents the center of one injection. Coordinates represent mm from Bregma.
Figure 7. The anxiogenic effect of BNST PACAP infusion persists for 7 days.
The BNST of adult male rats were cannulated bilaterally and the animals were tested for baseline startle as described in Experiment 3. Rats were tested for baseline startle immediately after intra-BNST infusion with either 1 μg PACAP38 or vehicle (0.5 μl/side), and subsequently returned to their home cages. 7 days later, rats were returned to the startle chambers and tested again. Data represent startle amplitude after injections into the BNST, as a percent of pre-injection day 1 baseline. A. BNST PACAP38 increased startle amplitude immediately after infusion. Blocks represent the mean response to the last 9 startle stimuli. B. The increase in startle amplitude after a single PACAP38 injection was maintained 7 days later, suggesting plasticity changes within the BNST. Data represent mean ± SEM. * Significantly different at p < 0.05.
Discussion
Chronic stress selectively augmented PACAP expression 10-fold in the dorsal aspect of the anterolateral BNST when compared with non-stressed rats. PACAP transcript was also reliably elevated in the PVN, but did not differ from non stressed rats in other stress-associated brain regions including the basolateral amygdala, central amygdala, locus coeruleus, hippocampus, or ventral aspect of the BNST. Chronic stress also produced an anxiogenic profile on baseline responding in the light-enhanced startle test (see description below), and intra-BNST infusion of PACAP was anxiogenic in a dose-dependent manner that persisted for at least 7 days following PACAP infusion. These data suggest that chronic stress may selectively alter BNST function via a PACAP-dependent mechanism to produce enhanced anxiety; hence, the BNST PACAP system may represent an important therapeutic target for the treatment of anxiety disorders.
As mentioned above, the behavioral and catabolic responses to stressful stimuli are all coordinated by CNS nuclei such as the BNST, and the sustained activation of these regions has been argued to lead to maladaptive changes underlying pathological states (Schulkin, et al. 1998; Vyas, et al. 2003; Pego, et al. 2008). Hence, chronic stress may facilitate fear and anxiety disorders by promoting neuronal plasticity and/or remodeling within the BNST. Chronic stress has been shown to increase dendritic arborization in the BNST (Vyas, et al. 2003; Pego, et al. 2008); however, the mechanisms underlying this plasticity are still not well understood, but may include PACAP signaling. PACAP has well established trophic fiber outgrowth functions during development and in culture paradigms (Wolf and Krieglstein 1995; Gonzalez, et al. 1997; Lu and DiCicco-Bloom 1997; Lioudyno, et al. 1998; Nielsen, et al. 2004; Falluel-Morel, et al. 2005; Braas, et al. 2007), and PAC1 receptor-mediated activation of MAPK signaling pathways in the BNST may mediate the observed dendritic remodeling. Alternatively, PACAP has been shown to stimulate both BDNF and TrkB expression in central and peripheral neurons (Lu and DiCicco-Bloom 1997; Yaka, et al. 2003; Braas, et al. 2007); hence the changes in BNST neuronal cytoarchitecture (Vyas, et al. 2003; Pego, et al. 2008) and functional plasticity may be dependent on PACAP-stimulated BDNF and TrkB expression and neurotrophic signaling. Indeed, in addition to increasing PACAP transcript, chronic stress was associated with increased BDNF and TrKB expression in the BNST. Many studies have suggested that altered BDNF and TrkB expression may be related to behavioral disorders (Martinowich, et al. 2007); hence, the integrated effects of PACAP, BDNF and TrkB expression and function, through coordinated or sequential gene regulatory events, or via G-protein receptor crosstalk with Trk signaling, may enhance BNST plasticity and function to affect behavior. Regardless of mechanism, these studies show that altered PACAP expression and signaling in the BNST may mediate anxiety-related disorders after chronic stress.
Hypothalamic and extrahypothalamic CRH have been shown to mediate the endocrine, sympathetic, and behavioral stress response. Importantly, chronic stress has been shown to increase BNST CRH, and this increase has been argued to mediate an increase in anxiety-like behavior (Stout, et al. 2000). PACAP-immunoreactive fibers have been suggested to heavily innervate hypothalamic CRH neurons in the PVN and extrahypothalamic CRH neurons in the BNST (Kozicz, et al. 1997; Legradi, et al. 1998). PACAP expression in these two brain regions was elevated after chronic stress in the present study, and BNST PACAP injection enhanced baseline startle. CRH injection also has been shown to enhance baseline startle (Lee and Davis 1997); hence, PACAP may mediate increased plasticity and function specifically within BNST CRH neurons. The endogenous sources of the PACAP-containing axons in the BNST appear to originate in part from PACAP neurons in the PVN (Kozicz, et al. 1998). As specific regions of the BNST also project to the PVN, these results also support the suggestion that the hypothalamus and BNST may have reciprocal regulatory functions (Herman, et al. 2005). The observation that both PACAP and PAC1 receptor transcripts are increased in the same dBNST tissues suggests that local PACAP circuits within the BNST may be activated by chronic stressor exposure. However, as PVN PACAP mRNA was also increased by the same treatment, and the PVN projects to the BNST, hypothalamic PACAP afferents may have also contributed to the stress-induced increase in the mRNA for these peptides.
Studies examining the role of PACAP mediating the effects of stress have been limited. Early studies showed that some stressors increased PACAP mRNA expression in the hypothalamus while other stressors did not (Hannibal, et al. 1995). Intraventricular or PVN infusion of PACAP increased plasma corticosterone levels, stimulated hypothalamic c-fos and phosphorylated CREB immunoreactivity, increased PVN CRH mRNA levels, and altered grooming behavior and motor activity (Agarwal, et al. 2005; Norrholm, et al. 2005). Moreover, PAC1 receptor knockout mice demonstrated abnormal social and sexual behavior associated with pheromone processing (Nicot, et al. 2004) and impaired contextual fear conditioning (Sauvage, et al. 2000; Otto, et al. 2001a). Consistent with an anxiogenic role, PACAP and PAC1 receptor null mice demonstrate reduced anxiety-like behavior across several tests; null mice spent more time in the middle of an open field, more time in the open arms of the elevated-plus maze, showed a shorter latency to emerge from a covered cylinder, and increased exploration of novel objects (Hashimoto, et al. 2001; Otto, et al. 2001b; Girard, et al. 2006). Despite the anxiolytic effects observed in these knockout mice, the neuroanatomical mechanisms of PACAP action on anxiety-like behavior have not been identified.
The data presented here suggest that a primary locus for PACAP signaling in anxiety-like behavioral responding to stressor exposure is the BNST. This complicated brain region has been divided into as many as 30 subdivisions based on cytoarchitecture, chemoarchitecture and connectivity (Dong, et al. 2001). Interestingly, PACAP fibers target the dorsal aspect of the anterolateral BNST (Kozicz, et al. 1997), and this subdivision has been associated with anxiety-like responding (Walker, et al. 2003). While PACAP infusion into the anterolateral BNST was anxiogenic, this effect may have been mediated by spread into surrounding areas; however, chronic stress selectively increased PACAP transcript in this BNST subregion, suggesting that the dorsal anterolateral BNST mediates the anxiogenic effects of PACAP. Interestingly, chronic stress did not increase PACAP transcript in the anatomically-related central nucleus of the amygdala (CeA) or the basolateral amygdala (BLA). Connections between the BLA and CeA have been argued to mediate behavioral responding to threatening stimuli that are predictable and of short duration, whereas the BNST has been argued to mediate a more maladaptive response to unpredictable long-duration threat (Walker, et al. 2003). Hence, the prevention of chronic stress-induced increases in PACAP could selectively prevent the formation of pathological anxiety-like state, while leaving adaptive fear-responding intact, unlike many present pharmaco-therapies that modulate activity in both pathways.
While intra-BNST PACAP injection increased baseline startle amplitude, an anxiogenic behavioral change after chronic stress was only observed using the light-enhanced startle paradigm. Importantly, in this paradigm, chronic stress exaggerated startle responding in response to both light exposure and the incidental handling that occurred between experimental phases. These observations may suggest some complementary but mechanistic differences between the acute and chronic anxiogenic effects of PACAP in the BNST. The anxiogenic effects of acute PACAP infusion at relatively high concentrations may be mediated by rapid PAC1 receptor-dependent activation of BNST neurons. In contrast, the chronic effects of stress are likely associated with enhanced BNST neuroplasticity, which may be related to long-term induction of BNST PACAP expression and signaling. In fact, BNST neuroplasticity may be the mechanism underlying the persistent anxiety-like behavior 7 days after PACAP infusion. In these latter cases, evoked BNST stimulation, through incidental handling or light exposure, may be a necessary requisite to observe increases in anxiety-like behavior. In fact, animals were handled extensively prior to testing in both experiments, when PACAP was infused into the BNST and also in the light-enhanced startle paradigm following chronic stress. Hence, the anxiogenic effects of handling-enhanced startle were potentiated by both treatments. As previously reported (Walker et al., 1997), animal handling can enhance startle amplitude, and this anxiogenic effect is dependent on BNST activity. While it would be interesting to determine the effects of BNST PACAP injection in a light-enhanced startle paradigm, the large changes in baseline startle produced by BNST PACAP would make such an experiment difficult to interpret. Although other experiments may clarify these observations, the current studies nevertheless demonstrate that PACAP signaling and stress are behaviorally relevant and may share common pathways.
Interestingly, BDNF transcript was also reliably elevated in the dorsal raphe nucleus (DRN) following chronic stress. The activation of specific populations of DRN serotonergic neurons has also been argued to mediate anxiety-like responding (Lowry, et al. 2005), which may be mediated via projections from the DRN to the BNST (Phelix, et al. 1992; Commons, et al. 2003). Indeed, the behavioral changes associated with learned helplessness, which has been argued to model human anxiety disorders such as post-traumatic stress disorder (PTSD), are blocked by the inactivation of either the DRN or BNST (Maier, et al. 1993; Hammack, et al. 2004). Moreover, PACAP-containing neurons within the DRN target the dBNST, and represent an important source of PACAP input (Kozicz, et al. 1998). Hence, chronic stress-mediated changes in raphe BDNF expression may alter DRN morphology, function and synaptic connectivity within the DRN - dBNST pathway to mediate some anxiogenic effects of stressor exposure.
These data suggest that chronic stress dramatically increases BNST PACAP and is anxiogenic. Moreover, BNST PACAP produces a sustained increase in anxiety-like behavior. Hence, BNST PACAP may mediate the anxiogenic effects of chronic stress. We suggest that BNST PACAP may mediate stress-induced BNST neuroplasticity, and that this mechanism may underlie some human anxiety disorders. Because chronic stress seems to selectively increase PACAP within the BNST, BNST PACAP signaling may represent an important target for the treatment of anxiety disorders in humans.
Acknowledgments
This work was supported by grants HD27468 and NS37179 from the National Institutes of Health as well as startup funds from the University of Vermont. The use of the Molecular Biology Core Facility supported by National Institute of Health NCRR P20RR16435 is also gratefully acknowledged.
Role of Funding Source: This work was supported by grants HD27468 and NS37179 from the National Institutes of Health as well as startup funds from the University of Vermont. These institutions had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest: There are no conflicts of interest reported for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal A, Halvorson LM, Legradi G. Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Research Molecular Brain Research. 2005;138:45–57. doi: 10.1016/j.molbrainres.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Japanese Journal of Physiology. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. Journal of Biological Chemistry. 1999;274:27702–27710. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- Braas KM, Schutz KC, Bond JP, Vizzard MA, Girard BM, May V. Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:1856–1870. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–212. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Vaudry D, Aubert N, Galas L, Benard M, Basille M, et al. Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2637–2642. doi: 10.1073/pnas.0409681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, Braas KM, Razinia T, Vizzard MA, Ioffe Y, et al. Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. Journal of Neurochemistry. 2006;99:499–513. doi: 10.1111/j.1471-4159.2006.04112.x. [DOI] [PubMed] [Google Scholar]
- Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regulatory Peptides. 2002;109:89–101. doi: 10.1016/s0167-0115(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. Journal of Comparative Neurology. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Fahrenkrug J, Larsen PJ. Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology. 1995;136:4116–4124. doi: 10.1210/endo.136.9.7649120. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in NeuroPsychopharmacology & Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Research Developmental Brain Research. 2000;120:27–39. doi: 10.1016/s0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Research. 1997;767:109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. The source of origin of PACAP- and VIP-immunoreactive fibers in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Research. 1998;810:211–219. doi: 10.1016/s0006-8993(98)00692-1. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neuroscience Letters. 1998;246:145–148. doi: 10.1016/s0304-3940(98)00255-9. [DOI] [PubMed] [Google Scholar]
- Lioudyno M, Skoglosa Y, Takei N, Lindholm D. Pituitary adenylate cyclase-activating polypeptide (PACAP) protects dorsal root ganglion neurons from death and induces calcitonin gene-related peptide (CGRP) immunoreactivity in vitro. Journal of Neuroscience Research. 1998;51:243–256. doi: 10.1002/(SICI)1097-4547(19980115)51:2<243::AID-JNR13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lu N, DiCicco-Bloom E. Pituitary adenylate cyclase-activating polypeptide is an autocrine inhibitor of mitosis in cultured cortical precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR. The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Nicot A, Otto T, Brabet P, Dicicco-Bloom EM. Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Journal of Neuroscience. 2004;24:8786–8795. doi: 10.1523/JNEUROSCI.1910-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KM, Chaverra M, Hapner SJ, Nelson BR, Todd V, Zigmond RE, et al. PACAP promotes sensory neuron differentiation: blockade by neurotrophic factors. Molecular & Cellular Neurosciences. 2004;25:629–641. doi: 10.1016/j.mcn.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Das M, Legradi G. Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN) Regulatory Peptides. 2005;128:33–41. doi: 10.1016/j.regpep.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. Journal of Neuroscience. 2001a;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Research Molecular Brain Research. 2001b;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. European Journal of Neuroscience. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Serotonin-CRF interaction in the bed nucleus of the stria terminalis: a light microscopic double-label immunocytochemical analysis. Brain Research Bulletin. 1992;28:943–948. doi: 10.1016/0361-9230(92)90217-l. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Brabet P, Holsboer F, Bockaert J, Steckler T. Mild deficits in mice lacking pituitary adenylate cyclase-activating polypeptide receptor type 1 (PAC1) performing on memory tasks. Brain Research Molecular Brain Research. 2000;84:79–89. doi: 10.1016/s0169-328x(00)00219-9. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocrine Reviews. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. European Journal of Pharmacology. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacological Reviews. 2000;52:269–324. [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle paradigm. Biol Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light enhanced startle: futher pharmacological and behavioral characterization. Psychopharmacology. 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wolf N, Krieglstein K. Phenotypic development of neonatal rat chromaffin cells in response to adrenal growth factors and glucocorticoids: focus on pituitary adenylate cyclase activating polypeptide. Neuroscience Letters. 1995;200:207–210. doi: 10.1016/0304-3940(95)12116-l. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. Journal of Biological Chemistry. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]